Abstract
Purpose
The metabolic cost of walking is greater in old compared to young adults. This study examines the relation between metabolic cost, muscular efficiency, and leg muscle co-activation during level and uphill walking in young and older adults.
Procedures
Metabolic cost and leg muscle activation were measured in young (22.3±3.6 years) and older adults (74.5±2.9 years) walking on a treadmill at six different slopes (0.0–7.5% grade) and a speed of 1.3 m·s−1. Across the range of slopes, ‘delta mechanical efficiency’ of the muscular system and antagonist muscle co-activation were quantified.
Main Findings
Across all slopes, older adults walked with a 13–17% greater metabolic cost, 12% lower efficiency, and 25% more leg muscle co-activation than young adults. Among older adults, co-activation was weakly correlated to metabolic cost (r=.233) and not correlated to the lower delta efficiency.
Conclusion
Lower muscular efficiency and increased leg muscle co-activation contribute to the greater metabolic cost of uphill slope walking among older adults but are unrelated to one another.
Keywords: aging, walking, efficiency, co-activation, gait
Introduction
Impaired mobility and reduced walking performance are closely associated with increased mortality among older adults [Studenski et al., 2011]. A subtle yet important characteristic of impaired walking performance is an increase in metabolic energy consumption. This greater metabolic cost of walking likely increases muscle fatigue during walking and may contribute to less participation in walking exercise among older adults. During level and uphill walking, older adults consume ~7–20% more metabolic energy to walk a given distance than young adults [Hortobagyi et al., 2011, Martin et al., 1992]. Among several factors, the metabolic cost of performing mechanical work; i.e. muscular efficiency is a key determinant of the metabolic cost of walking [Cavagna and Kaneko, 1977, Donelan et al., 2002]. Even though older adults perform a similar amount or even less external mechanical work during level and uphill walking than young adults [Franz et al., 2012, Mian et al., 2006, Ortega and Farley, 2007], whether they are performing that mechanical work as efficiently as young adults is uncertain. One factor that may contribute to lower mechanical efficiency of the muscular system during walking in older adults is increased co-activation of antagonist leg muscles [Mian, Thom, 2006].
In a variety of movements including walking, older adults use more co-activation of antagonist muscle pairs than young adults [Franz and Kram, 2012, Hortobagyi, Finch, 2011, Mian, Thom, 2006, Peterson and Martin, 2010]. When walking on level ground and at a 6.0% uphill slope, co-activation of antagonist leg muscles has been shown to be 30–50% greater in older adults and to have a low to moderate association to metabolic cost [Hortobagyi, Finch, 2011, Mian, Thom, 2006, Peterson and Martin, 2010]. Although these prior studies showed that metabolic cost and co-activation are greater in older walkers, they did not quantify the mechanical efficiency of the muscular system or its relation to co-activation of antagonist muscle. {Hortobagyi, 2011 #7312} Thus, it remains unclear how age-related changes in antagonist leg muscle co-activation during walking may be related to the mechanical efficiency and changes in metabolic energy consumption of the muscular system across a range of uphill slopes.
The purpose of the present study was to determine if the greater metabolic cost of level and uphill walking observed in older adults is related to reduced mechanical efficiently as a result of using greater co-activation of antagonist leg muscles than young adults. We hypothesized that across a range of slopes, older adults increase metabolic energy consumption more than young adults and thus perform mechanical work less efficiently during walking. We also hypothesized that antagonist leg muscle co-activation in older adults would be related to greater metabolic cost and reduced mechanical efficiency of walking. To test these hypotheses, we quantified metabolic cost, ‘delta mechanical efficiency’ of the muscular system [Gaesser and Brooks, 1975] and antagonist leg muscle co-activation as young and older adults walked at a constant speed up progressively steeper slopes. To our knowledge this is the first study to investigate muscular efficiency of steady-state level and uphill walking in older adults and its relation to leg muscle co-activation.
Methods
Participants
Thirteen healthy young adults (six male, seven female) and twelve healthy older adults (six male, six female) with no known orthopedic, neurological, or cardiovascular disease were recruited for this study. All subjects were physically active with no history falls in the year prior to participating in the study. Young and older subjects were similar in height, body mass, body mass index (BMI), and lean tissue mass (Table 1). Despite older subjects having greater body fat and a lower resting metabolic rate than young subjects, no subject characteristic other than age had a statistically significant influence on the dependent variables. All subjects gave their written informed consent before participating in the study. The University of Colorado Institutional Review Board approved this protocol.
Table 1.
Participant characteristics with statistics for a comparison of young versus older adults.
| Young (n=13) | Old (n=12) | |
|---|---|---|
| Age (years) | 22.3 ± 3.7 | 74.7 ± 3.1 |
| Height (m) | 1.76 ± 0.10 | 1.69 ± 0.09 |
| Leg length (m) | 0.90 ± 0.07 | 0.89 ± 0.05 |
| Body mass (kg) | 65.9 ± 9.3 | 66.8 ± 14.3 |
| Lean tissue mass (kg) | 50.8 ± 11.5 | 45.5 ± 10.2 |
| Body fat (% body mass) | 20.1 ± 6.8 | 28.0 ± 4.5* |
| Body Mass Index (kg/m2) | 21.2 ± 1.8 | 23.1 ± 2.9 |
| Standing metabolic rate (W kg−1) | 1.64 ± 0.28 | 1.39 ± 0.15* |
Values are mean ± SD.
Asterisk (*) indicates a significant age difference (p<.05).
Protocol
Subjects participated in two testing sessions. In the first session, we measured each subject’s height, leg length, and body composition. Subjects were then familiarized to level and uphill treadmill walking (0–7.5% grade) at the moderate speed of 1.3 m·s−1 for a minimum of 30 minutes [Wall and Charteris, 1981]. In the second session, each subject performed one resting trial and six walking trials in order for us to calculate delta efficiency and lower limb co-activation during walking across a range of slopes. In this session, we first measured resting metabolic rate as subjects stood quietly for seven minutes. Each subject then walked at 1.3 m·s−1 on a motorized treadmill (Model 18–60, Quinton Instruments, Seattle, WA, USA) at six different slopes (0.0, 1.5, 3.0, 4.5, 6.0, 7.5% grade) in randomized order as we collected metabolic, electromyography (EMG), and stride frequency data. The order of slopes was also counterbalanced across subjects.
For the walking trials, each subject performed one seven-minute trial per slope with a 3–5 minute rest period between trials. During the last three minutes of each seven-minute trial, we collected the rates of oxygen consumption (V̇O2) and carbon dioxide production (V̇CO2) using indirect calorimetry, leg muscle activation using EMG, and stride frequency. Average stride frequency was calculated from the time required to take 30 strides and was determined during the last 2 minutes of each trial.
Metabolic Energy Consumption
We measured sub-maximal steady-state V̇O2 (mlO2· min−1) and V̇CO2 (mlCO2· min−1) using open-circuit indirect calorimetry (Physio-Dyne Instruments CO., Quogue, NY, USA) to calculate metabolic power consumption. We calculated average metabolic power per kilogram body mass (W·kg−1) [Brockway, 1987] using the average V̇O2 and V̇CO2 for the last two minutes of each trial when the V̇O2 indicated that metabolic steady-state had been achieved. For each walking trial, we calculated net metabolic power consumption (W·kg−1) by subtracting the standing metabolic rate from gross metabolic rate during walking.
Delta Efficiency
We determined delta efficiency for each subject from the increase in net mechanical power output and the increase in net metabolic power consumption across the range of uphill slopes [Gaesser and Brooks, 1975]. Net mechanical power output represents the minimum power required to lift the body during uphill walking at a given speed and was calculated from the slope and speed of the treadmill using a standard equation [Brooks et al., 1996]. Thus, the six slopes (0.0, 1.5, 3.0, 4.5, 6.0, 7.5% grade) corresponded to six net mechanical power levels including 0.0, 0.19, 0.38, 0.57, 0.76, and 0.95 W·kg−1. We calculated each subject’s delta efficiency from the inverse slope of the regression line representing the relationship of mechanical power output to net metabolic power consumption across the range of slopes [Gaesser and Brooks, 1975]. Delta efficiency represents the mechanical power output achieved for each watt of metabolic power consumption expressed as a percent.
Electromyography (EMG)
To quantify the effects of age and uphill slope on muscle activity, we measured surface EMG signals (Noraxon, Scottsdale, AZ) using International Society for Electrophysiology and Kinesiology standard procedures [Merletti et al., 1999]. The skin over the lateral gastrocnemius (LG), soleus (SOL), tibialis anterior (TA), vastus medialis (VM), vastus lateralis (VL), biceps femoris (BF) muscles and lateral malleolus (ground electrode) of the right leg were shaved and abraded with electrode preparation gel prior to placing bipolar Ag/AgCl surface electrodes (10 mm diameter discs by Noraxon USA, Inc., Scottsdale, AZ) over each muscle belly. Two electrodes were place at the center of each muscle in parallel with muscle fiber orientation and at an inter-electrode distance of 20 mm. The EMG signals were collected at a rate of 1,000 Hz and pre-amplified with a gain of 1,700 (input impedance >100 MΩ, common mode rejection ratio >110 dB at 60 Hz). Using standard methods [Criswell and Cram, 2011], we verified that electrode impedance was less than 5000 Ω and that the cross talk between muscles was negligible. After data collection, raw EMG data was band-pass filtered (6th order Butterworth) to retain frequencies between 10 and 500 Hz.
For each trial, we processed ten consecutive strides of EMG data in two stages using a custom Matlab program (MATLAB, R2012b, MathWorks, Inc., Natick, MA). First, a temporal analysis was performed to determine when muscles were active in the stride cycle and, second, an amplitude analysis was performed to quantify the magnitudes of muscle activation and antagonist muscle co-activation.
For the temporal analysis, the onset, offset and duration of each EMG burst were determined using the Teager-Kaiser Energy Operator (TKEO) method described in prior studies [Solnik et al., 2008]. We also used the temporal EMG data to determine the time of overlap between the EMG bursts of the VM and BF, VL and BF, TA and SOL, and between TA and LG, respectively, in order to quantify the co-activation of these antagonist muscle pairs. For the amplitude analysis, we full-wave rectified the filtered EMG signals and calculated the root mean square (40 ms moving window) EMG amplitude (EMGRMS) across the stride cycle. For all walking trials, we normalized EMGRMS of each muscle relative to its peak EMGRMS amplitude during the level walking condition [Yang and Winter, 1984].
For this study, we quantified co-activation of agonist-antagonist muscle pairs of the thigh and shank segments using a co-activation index (CI) [Falconer and Winter, 1985, Peterson and Martin, 2010, Winter, 1990]. Using the normalized EMG, we calculated the CI of each muscle pair as the area of overlap (magnitude × duration) between the agonist and antagonist EMG signals as described in detail by Falconer and Winter [1985] (Fig. 1).
Figure 1.
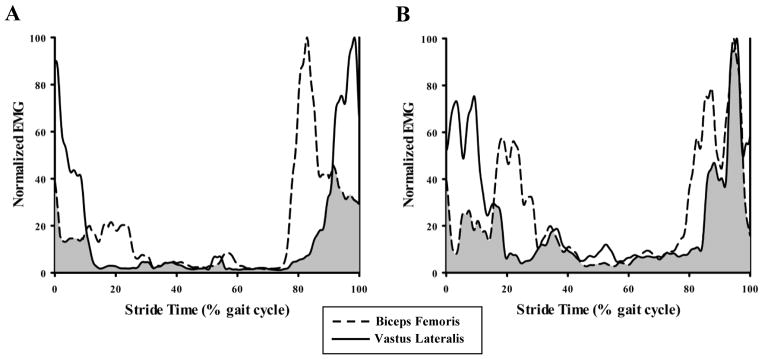
Typical surface electromyography (EMG) recorded during one stride of level walking at 1.3 m s−1 for (A) young and (B) older adults. Within each panel, traces represent normalized full-wave rectified, root mean square averaged (40 ms window) EMG profiles of vastus lateralis (solid line) and biceps femoris (hatched line) muscles. Co-activation index (CI) was quantified as the area of overlap between antagonist muscle pairs (shaded region) during each phase of the stride cycle.
To determine average co-activation levels across the shank and thigh segments, we averaged the co-activation indices of individual muscle pairs [Peterson and Martin, 2010]. Specifically, we calculated the co-activation of antagonist muscles across the shank (CIshank) as the average of the CI for the TA-SOL and TA-LG muscle pairs and the co-activation of antagonist muscles across the thigh (CIthigh) as the average of the CI for the VM-BF and VL-BF muscle pairs.
Statistics
The overall effect of age and slope (repeated-measure) on net metabolic power, co-activation, and stride frequency were tested using a two (age group) × six (treadmill slope) multivariate analysis of variance (MANOVA) with repeated-measures. For variables where main effects of age or age × slope interaction were observed, the effect of age at the different levels of slope was tested using Student’s t-tests with a Bonferroni correction. A Student’s t-test was also used to compare delta efficiency values between age groups. We used Pearson product-moment correlations to determine the strength of the relations between co-activation, metabolic power and delta efficiency. Statistical significance was defined as p < .05. All data are reported as mean ± SEM unless otherwise specified. All statistical analyses were performed using SPSS software (ver. 21.0, SPSS, Inc., Chicago, IL).
Results
Walking Energetics and Mechanical Efficiency
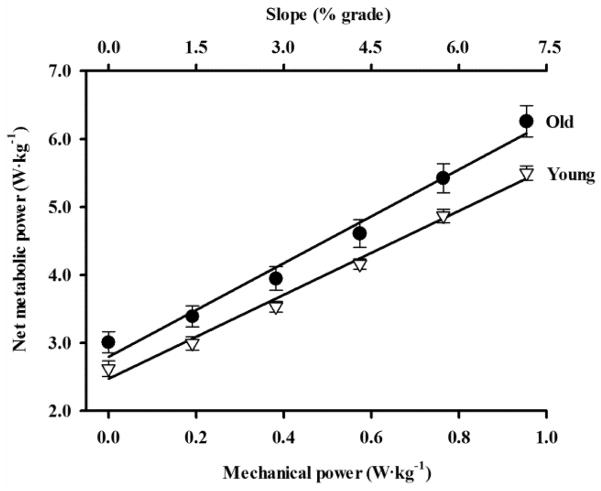
Across the range of slopes, older adults consumed metabolic energy 12% faster during walking than young adults (Fig. 2). For level walking, older adults had a 17% greater net metabolic power consumption (i.e. net metabolic cost) than young adults (p = .027). From level walking to the steepest slope, the absolute difference in net metabolic cost between young and older adults increased such that older adults consumed 0.39 W·kg−1 more metabolic energy than young adults when walking on the level slope (p = .019) and 0.76 W·kg−1 more metabolic energy than young adults when walking on the steepest uphill slope (p = .010). Across all slopes including level, older adults walked with a faster stride frequency (0.95 ± 0.02 Hz) than young adults (0.88 ± 0.01 Hz) (p = .031). Stride frequency did not change with uphill slope in either age group (p = .465).
Figure 2.
Net metabolic power consumption versus uphill slope (top x-axis) and mechanical power output required to lift the body up the hill (bottom x-axis) for young adults (open triangles) and older adults (filled circles) (mean ± SEM). Net metabolic power consumption was significantly greater for older adults than for young adults at each slope (p < .05). Lines are least squares regressions (r2 = .99 for young and r2 = .98 for older). “Delta efficiency” is calculated from the inverse of the slope of the regression line representing the relationship between metabolic power and mechanical power. Delta efficiency was significantly lower in older adults (29%) compared to young adults (33%) across the range of uphill slopes (p = .006).
As reflected by the inverse slope of the mechanical power vs. metabolic power relation (Fig. 2), older adults consistently performed mechanical work with a lower delta efficiency (29% ± 1%) than young adults (33% ± 1%) (p = .006). Between level walking and the steepest uphill slope, the mechanical power output increased by 0.95 W·kg−1. For this increase in mechanical power output, metabolic power consumption increased 13.0% more in older adults than in young adults (p = .031) and is reflected by the lower delta efficiency of walking observed in older adults.
Muscle Co-activation in Relation to Metabolic Cost and Efficiency
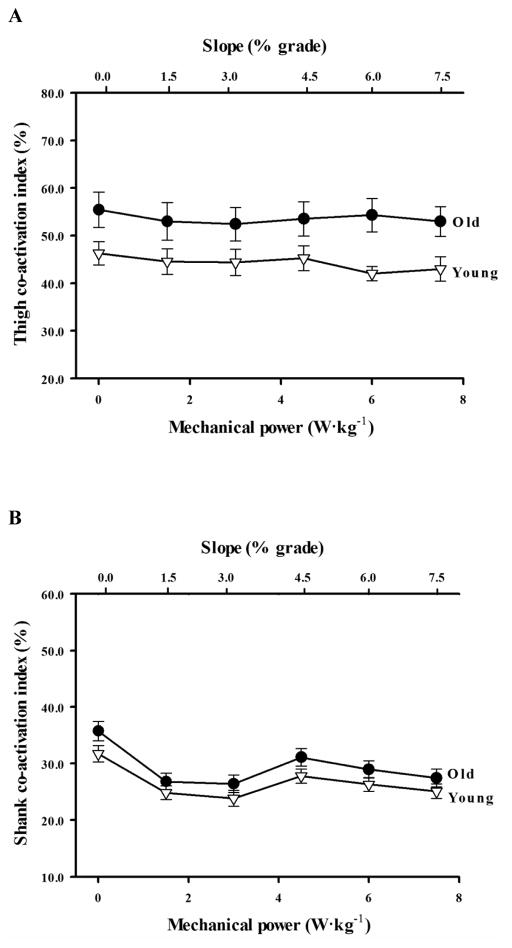
Across the range of slopes, older adults co-activated antagonist muscles of the thigh 25% more than young adults (p = .002, Fig. 3A). CIthigh did not change with slope in either the young or older adults (p =.701 and p = .638, respectively). Although co-activation about the shank was similar between young and older adults (CIshank = 28.1%, p = .927), both age groups reduced CIshank by an average 17% from level to uphill walking (p < .0001; Fig. 3B).
Figure 3.
(A) Thigh and (B) shank Co-activation Index (CI) versus slope (top x-axis) and mechanical power output required to lift the body up the hill (bottom x-axis) for young adults (open triangles) and older adults (filled circles) (mean ± SEM). Thigh CI was significantly greater across the range of uphill slopes in older adults compared to young adults (p = .002)
The net metabolic cost of walking was not correlated to co-activation about the shank or thigh when collapsed across age groups (p = .405 and p = .170, respectively). Since metabolic cost and co-activation were greater in older adults, we conducted separate correlational analyses for each age group. In older adults, CIthigh was significantly correlated to the metabolic cost of walking across the range of slopes (p = .026), but the strength of this positive relation was low (r = .233). In young adults, there was no correlation between metabolic cost and CIthigh across the range of slopes (p = .405). Moreover, there was no correlation between CIshank and metabolic cost in young or older adults (p = .133, p = .250, respectively). For both young and older adults, delta efficiency values were not correlated to either CIthigh or CIshank (p > .05).
Discussion
The purpose of this study was to determine whether the greater metabolic cost of level and uphill walking observed in older adults is related to performing mechanical work less efficiently due to using greater leg muscle co-activation than young adults. Our findings support our primary hypothesis that older adults have a lower delta efficiency of performing mechanical work and use greater antagonist leg muscle co-activation than young adults during steady-state level and uphill walking. However, our results also show that antagonist leg muscle co-activation is only weakly correlated to the greater metabolic cost of walking in older adults and does not explain their lower delta efficiency. Considering the modest difference in delta efficiency and the low correlation between metabolic cost and co-activation, these results suggest that the greater metabolic cost of uphill walking in older adults is likely the result several factors in addition to reduced mechanical efficiency and increase co-activation of lower limb muscles.
In our study, older adults consumed metabolic energy at a 9–17% faster rate than young adults across the range of slopes; a difference that is similar to that observed in prior studies [Hortobagyi, Finch, 2011, Martin, Rothstein, 1992, Mian, Thom, 2006, Peterson and Martin, 2010]. Although this is the first study to systematically investigate age-related difference in metabolic cost across a range of uphill slopes, Hortobagyi et al. [Hortobagyi, Finch, 2011] showed that older adults walking on a 6% uphill slope at a speed of 0.98 m·s−1 have a 7% greater metabolic cost than young adults; a value qualitatively similar to the 9–13% observed across a wider range of uphill slopes (1.5–7.5% grade) in the present study. Despite the greater total metabolic cost at each slope and the lower delta mechanical efficiency across the slopes, net metabolic power increased linearly with slope in both our young and older subject (R2=0.99 and 0.98, respectively); a finding that is agreement with other studies of the energetic cost of slope walking {Margaria, 1938 #657; Minetti, 1993 #241}.
Our study also shows that older adults co-activate antagonist muscle pairs of the thigh ~25% more than young adults across the range of slopes. Although using different methodologies, these results are consistent with past studies that show older adults use greater leg muscle co-activation compared to young adults during level, decline, and incline slope walking [Franz and Kram, 2012, Hortobagyi, Finch, 2011, Larsen et al., 2008, Mian, Thom, 2006, Peterson and Martin, 2010]. Most recently, Hortobagyi et al. [2011] shows that older adults co-activate the antagonist thigh muscles nearly twice as much as young adults during both level and 6.0% uphill slope walking. In contrast, Franz and Kram [Franz and Kram, 2012] found that healthy older adults use greater lower leg muscle co-activation than young adults but use a similar high level of antagonist thigh muscle co-activation during level and uphill slope walking (0–9%). Two reasons why the results between the various studies may differ is the fact that different methods have been used to calculate co-activation and different antagonist muscle pairs were evaluated. Although older adults appear to co-activate a variety of antagonist leg muscles more than their younger counterpart, the cause for the greater antagonist co-activation remains unresolved. The increase in leg muscle co-activation with age may be a compensatory mechanism for reduced sensory perception that improves stability during gait by increasing joint stiffness [Hortobagyi and DeVita, 2000, Hortobágyi et al., 2009, Larsen, Puggaard, 2008].
Although our results are in agreement with prior research that show older adults walk with lower efficiency and use greater co-activation [Mian, Thom, 2006], we found no correlation between co-activation levels and delta efficiency values in older adults walking. While Mian et al. [2006] investigated the relation between metabolic cost, efficiency, and co-activation in young and older adults walking on level ground, the present study investigated these relations across a range of slopes including level walking. Despite this methodological difference, our results show a similarly low but significant correlation between metabolic cost and thigh co-activation and a similar 13–17% lower efficiency of performing mechanical work in older walkers. This study further builds upon the results of Mian et al. [2006] in that we found no relation between greater co-activation levels and lower delta efficiency values in older walkers.
Because delta efficiency is calculated as the ratio of change in mechanical power output to change metabolic power consumption across multiple slopes, we predicted that co-activation would increase with the greater mechanical power demands of the steeper slopes based on the hypothesis that increased co-activation is a primary determinant of reduced delta efficiency of walking in older adults. However, our results clearly show that older adults use a similar high level of thigh muscle co-activation during walking across the range of slopes and that their lower delta efficiency values were not correlated to changes in CIthigh (r = .009). To our knowledge, this is the first study to show that the reduced walking efficiency in older adults is not related to increased co-activation. These results combined with our previous findings that show older adults perform a similar amount of work during walking [Ortega and Farley, 2007] suggest that the lower delta efficiency of walking in older adults may be more heavily influenced by factors other than co-activation such as reduced skeletal muscle efficiency [Amara et al., 2007]. Skeletal muscle efficiency (efficiency of converting O2 to muscular work) depends in part on mitochondrial coupling efficiency (energy conversion from oxygen uptake to ATP production). In human and rodent muscle, mitochondrial coupling efficiency is reduced by as much as 30–50% due to aging [Amara, Shankland, 2007, Marcinek et al., 2005]. The potential role of mitochondrial uncoupling in age-related decreases in walking efficiency is further supported by Conley et al. [Conley et al., 2007] who show that older adults with lower mitochondrial coupling efficiency also exhibited reduced cycling exercise efficiency compared to young adults. Interestingly, mitochondrial coupling efficiency may be improved with aerobic exercise [Conley, Jubrias, 2007]. Future studies should investigate whether aerobic exercise may improve walking efficiency and reduce metabolic cost by increasing mitochondrial efficiency.
While the greater metabolic cost of walking in older adults cannot be fully explained by their reduced efficiency, older adults may have a greater cost of generating force to support body weight during walking. In young adults, the metabolic cost of supporting body weight accounts for as much as 28% of the net metabolic cost of walking [Grabowski et al., 2005]. Stance limb geometry and the torque generated about the hip, knee, and ankle are key determinants of the cost of supporting body weight [Biewener et al., 2004]. Although the total torque required to support body weight appears to be similar for young and older adults, aging has been shown to cause a redistribution of torque generation from the ankle extensors to the hip extensors [DeVita and Hortobagyi, 2000]. This redistribution may increase the metabolic cost of supporting body weight as the hip extensors consume more metabolic energy than the ankle extensors to generate a given force due to their longer muscle fibers [Roberts, 2002]. Based on this evidence, we are currently investigating the relative cost of supporting body weight and its influence on the total metabolic cost of walking in young and older adults.
In conclusion, this study shows how aging affects the metabolic cost, muscular efficiency of performing mechanical work, and antagonist leg muscle co-activation during level and uphill walking. We find that metabolic energy consumption and co-activation of antagonist thigh muscles increase with age while muscular efficiency of performing mechanical work is reduced. Our results also show that increased antagonist leg muscle co-activation during level and uphill walking is weakly associated with a greater metabolic cost in older adult but does not explain their lower muscular efficiency. The lower muscular efficiency of walking in older adults is likely due to other factors such as mitochondrial uncoupling and a redistribution of power generation to the less efficient muscles of the hip. While reduced muscular efficiency and increase co-activation clearly contribute to the metabolic cost of walking, it remains unclear to what extent these factors account for the 10–20% greater cost of walking in older adults. Future studies need to determine how other physiological and mechanical factors such as increased aerobic fitness or the cost of generating force to support body weight may influence the metabolic cost of walking in older adults. Nonetheless, by providing a better understanding how muscle co-activation and muscle efficiency contribute to the greater metabolic cost of walking in older adults, the results of this study may help scientists and clinicians to further develop strategies aimed at neuromuscular rehabilitation and improving muscle efficiency as a means of improving mobility and independence among older adults [Dietz, 2009, Lanza and Nair, 2009, Miller, 1995].
Acknowledgments
Funding
This work was supported by National Institutes of Health (Grant numbers AG00279, M01 RR00051).
Biographies

Justus Ortega received a M.Sc. degree in kinesiology (2001) and Ph.D. in integrative physiology (2006) from the University of Colorado Boulder. He is currently a professor in the Department of Kinesiology at Humboldt State University and the Director of the biomechanics laboratory and North Coast Concussion Program. His research interest include the effects of aging and exercise on the energetics and mechanics of locomotion as well as the effects of concussion on cognitive function and motor control.

Claire T. Farley received a B.A. degree (1986) and Ph.D. in in Biology (1991) from the Harvard University. She was a professor in the Department of Integrative Physiology at the University of Colorado Boulder and the CO-director of the locomotion laboratory. Her research interests include the biomechanics of locomotion as related to (1) musculoskeletal springs in locomotion; and (2) biomechanical and musculoskeletal limitations to burst locomotion. She is currently retired and continues her study of animal behavior and movement in the Rocky Mountains of Colorado.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci U S A. 2007;104(3):1057–62. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener AA, Farley CT, Roberts TJ, Temaner M. Muscle mechanical advantage of human walking and running: implications for energy cost. J Appl Physiol. 2004;97(6):2266–74. doi: 10.1152/japplphysiol.00003.2004. [DOI] [PubMed] [Google Scholar]
- Brockway JM. Derivation of formulae used to calculate energy expenditure in man. Hum Nutr Clin Nutr. 1987;41(6):463–71. [PubMed] [Google Scholar]
- Brooks GA, Fahey TD, White TG. Exercise physiology: human bioenergetics and its applications. Mountain View, Calif: Mayfield; 1996. [Google Scholar]
- Cavagna GA, Kaneko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977;268(2):647–81. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Amara CE, Marcinek DJ. Mitochondrial dysfunction: impact on exercise performance and cellular aging. Exerc Sport Sci Rev. 2007;35(2):43–9. doi: 10.1249/JES.0b013e31803e88e9. [DOI] [PubMed] [Google Scholar]
- Criswell E, Cram JR. Cram’s introduction to surface electromyography. 2. Sudbury, MA: Jones and Bartlett; 2011. [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88(5):1804–11. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Dietz V. Body weight supported gait training: from laboratory to clinical setting. Brain Res Bull. 2009;78(1):I–VI. doi: 10.1016/S0361-9230(08)00410-3. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol. 2002;205(23):3717–27. doi: 10.1242/jeb.205.23.3717. [DOI] [PubMed] [Google Scholar]
- Falconer K, Winter DA. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr Clin Neurophysiol. 1985;25(2–3):135–49. [PubMed] [Google Scholar]
- Franz JR, Kram R. How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait Posture. 2012;37(3):378–84. doi: 10.1016/j.gaitpost.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Lyddon NE, Kram R. Mechanical work performed by the individual legs during uphill and downhill walking. J Biomech. 2012;45(2):257–62. doi: 10.1016/j.jbiomech.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol. 1975;38(6):1132–9. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol. 2005;98(2):579–83. doi: 10.1152/japplphysiol.00734.2004. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, DeVita P. Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J Electromyogr Kinesiol. 2000;10(2):117–26. doi: 10.1016/s1050-6411(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66(5):541–7. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobágyi T, Solnik S, Gruber A, Rider P, Steinweg K, Helseth J, et al. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture. 2009;29(4):558–64. doi: 10.1016/j.gaitpost.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. The American Journal of Clinical Nutrition. 2009;89(1):467S–71S. doi: 10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AH, Puggaard L, Hämäläinen U, Aagaard P. Comparison of ground reaction forces and antagonist muscle coactivation during stair walking with ageing. J Electromyogr Kinesiol. 2008;18(4):568–80. doi: 10.1016/j.jelekin.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569(2):467–73. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol. 1992;73(1):200–6. doi: 10.1152/jappl.1992.73.1.200. [DOI] [PubMed] [Google Scholar]
- Merletti R, Farina D, Hermens H, Freriks B, Harlaar J. European Recommendations for signal processing methods for surface electromyography. In: Hermans H, editor. SENIAM: European recommendations for surface electromyography. Gaithersburg, MD: Aspen Publishers; 1999. pp. 57–68. [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol Scand. 2006;186(2):127–39. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Miller RG. The Effects of Aging Upon Nerve and Muscle Function and Their Importance for Neurorehabilitation. Neurorehabil Neural Repair. 1995;9(3):175–81. [Google Scholar]
- Ortega JD, Farley CT. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. J Appl Physiol. 2007;102(6):2266–73. doi: 10.1152/japplphysiol.00583.2006. [DOI] [PubMed] [Google Scholar]
- Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture. 2010;31(3):355–9. doi: 10.1016/j.gaitpost.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Roberts TJ. The integrated function of muscles and tendons during locomotion. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):1087–99. doi: 10.1016/s1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Solnik S, DeVita P, Rider P, Long B, Hortobagyi T. Teager-Kaiser Operator improves the accuracy of EMG onset detection independent of signal-to-noise ratio. Acta of bioengineering and biomechanics / Wroclaw University of Technology. 2008;10(2):65–8. [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JC, Charteris J. A kinematic study of long-term habituation to treadmill walking. Ergonomics. 1981;24(7):531–42. doi: 10.1080/00140138108924874. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. 2. New York: John Wiley & Sons; 1990. [Google Scholar]
- Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65(9):517–21. [PubMed] [Google Scholar]