Abstract
During embryonic development, hemodynamic forces caused by blood flow support vascular remodeling, arterialization of luminal endothelium, and hematopoietic stem cell (HSC) emergence. Previously, we reported that fluid shear stress plays a key role in stimulating nitric oxide (NO) signaling in the aorta-gonad-mesonephros (AGM) and is essential for definitive hematopoiesis. We employed a Dynamic Flow System modified from a cone-and-plate assembly to precisely regulate in vitro exposure of AGM cells to a defined pattern of laminar shear stress. Here, we present the design of a microfluidic platform accessible to any research group that requires small cell numbers and allows for recirculation of paracrine signaling factors with minimal damage to nonadherent hematopoietic progenitors and stem cells. We detail the assembly of the microfluidic platform using commercially available components and provide specific guidance in the use of an emerging standard in the measurement of embryonic HSC potential, intravenous neonatal transplantation.
Keywords: Hemogenic endothelium, Hematopoietic stem cells, HSC, Biomechanical forces, Shear stress, AGM
1. Introduction
Accumulating evidence suggests that definitive hematopoietic stem cells (HSCs) derive from a specialized population of endothelial cells, termed hemogenic endothelium (1–7). These cells transiently appear in specific sites of the embryonic vasculature and undergo endothelial-to-hematopoietic transition (EHT) by specific, but relatively poorly understood, mechanisms (8). Recent studies have suggested that HSC maturation in the aorta-gonads-mesonephros (AGM) region is regulated by hemodynamic forces created by blood flow (9, 10). Fluid mechanical forces induce expression of hematopoietic markers and blood forming activity in explant culture, whereas cardiac mutants with impaired blood flow exhibit significant deficiency in definitive hematopoiesis (9, 10). Nitric oxide (NO) plays an important role in triggering signaling events downstream of biomechanical force, as interruption of NO production impairs blood formation and NO donor compounds are capable of partial restoration of hematopoietic activity in cardiac mutants (9, 10). However, how hematopoiesis is regulated by blood flow and whether other mechanosensitive pathways contribute to fate commitment remain critical questions in the field of developmental hematopoiesis.
Three mutually perpendicular hemodynamic forces exist within the vasculature (11). First, blood pressure exerts a hydrostatic force against the vessel wall. Second, vessel wall cells experience circumferential stretch imposed by hydrostatic pressure. Finally, blood flow exerts drag on the luminal wall and causes a frictional force between fluid and vessel surface cells, referred to as shear stress. Among these biomechanical forces, shear stress has been shown to promote hematopoiesis in mouse AGM and from embryonic stem cells (9, 10, 12). Studies of cellular response to shear stress can be challenging due to the specialized instrumentation required for generation of defined fluid flow intensities. Further, recirculation of culture medium is necessary for capture of paracrine signaling mechanisms stimulated by shear stress and translates to a substantial cost savings in reagents with prolonged culture. Peristaltic pumps fall short of meeting the demands of fluid management as they introduce refractory pulsatile patterns in shear stress and damage nonadherent cells that circulate through the roller mechanism. Here we describe a microfluidic laminar shear stress system capable of long-term recirculation of culture medium, accessible to any laboratory and reliant upon commercially available channel slides and one syringe pump. We describe use of the platform for several outcome measures and provide specific guidance in the use of neonatal transplantation, an emerging standard in functional assessment of embryonic HSCs.
2. Materials
The products and vendors listed here have been employed in our research; however, suitable alternatives may exist for microfluidic culture platforms and syringe pumps. Custom microfluidics could easily be adapted to the system with special attention to prevention of bubble formation.
2.1 AGM Culture
Mouse embryos
Accutase cell detachment solution (Stem Cell Research, catalog # 07920)
Trypan Blue Solution, 0.4% (Invitrogen/Life Technologies, catalog # 15250-061)
Sodium pyruvate, 100 mM (Invitrogen/Life Technologies, catalog # 11360-070)
MEM nonessential amino acids solution (Invitrogen/Life Technologies, catalog # 11140-050)
HEPES buffer solution, 1 M (Invitrogen/Life Technologies, catalog # 15630-080)
Penicillin/Streptomycin solution (VWR, catalog # 45000-652)
MyeloCult M5300 cell culture medium (Stem Cell Research, catalog # 05350). Add sodium pyruvate (5 ml), MEM nonessential amino acids (5 ml), HEPES (12.5 ml), and Penicillin/Streptomycin (5 ml) before use.
BD Falcon cell strainer, 70 μm mesh (Fisher Scientific, catalog # 08-771-2)
2.2 Laminar shear stress assembly
IBIDI VI0.4 six channel slide (IBIDI, catalog # 80606)
Matrigel Basement Membrane Matrix (BD Biosciences, catalog # 354234)
Fibronectin human protein, plasma (Invitrogen/Life Technologies, catalog # 33016-015)
PBS buffer, 500 ml (Invitrogen/Life Technologies, catalog # 10010-023)
Recombinant mouse SCF (mSCF), 10 ug/ml (PeproTech, catalog # 250-03)
Check valve 0.27 PSI CP (Qosina, catalog # 80184)
Three way stop cock, 3 female luer lock (Qosina, catalog # 99699)
Silicone tubing, 1/16 ID (Fisher Scientific, catalog # 1118915G)
Barbed T-connector, polypropylene, 1/16-inch (Cole Parmer, catalog # YO-06365-77)
Barbed Y-connector, PVDF (Cole Parmer, catalog # T30703-90)
Female Luer x 1/16 hose barb, PVDF (Cole Parmer, catalog # T45512-00)
Elbow Luer connector (IBIDI, catalog # 10802)
Syringe, 10 ml (BD, catalog # 309604)
Syringe pump PHD ULTRA 4400 with Remote (Harvard Apparatus, catalog # 703310)
2.3 Neonatal Transplantation
Hamilton syringe, TLL, 50 ml (FISHER, catalog # 14815-57)
30-gauge needle (FISHER, catalog # 14826F)
3. Methods
Carry out all procedures at room temperature unless otherwise specified.
3.1 AGM Tissue Dissociation
Carefully dissect out the AGM region from early mouse embryos (e.g. E10.5) in ice-cold PBS.
Remove excess PBS (see Note 1) and add 2–4 ml Accutase solution for AGM tissues from approximately 10 embryos.
Incubate tissues on a plate shaker for 20 min (see Note 2).
Gently pipette 5 times and shake for 3–5 more min.
After incubation, pipette 10 more times very gently to obtain a single cell suspension. Add 4 ml Myelocult medium and mix well.
Filter suspension through 70-μm nylon cell strainers.
Collect 10 μl of cell suspension and count live cells using trypan blue. Calculate total cell number.
Centrifuge cells at 900 × g for 5 min and resuspend in Myelocult medium at a final concentration of 25 × 106 cells/ml.
Seed cells into microfluidic slides or flow chambers immediately, as described below.
3.2 Laminar Shear Stress Assembly
-
Sterilize tubing and check valves for shearing before the experiment (see Note 3).
Setup for one laminar shear stress (LSS) channel requires the following materials:
One 8-inch tubing
Two 6-inch tubing
Six 3-inch tubing
Three T or Y connectors
Two elbow connectors
Four check valves
Two 3-way stopcocks
Six female luer x 1/16 hose barb connector
One 14 ml round bottom tube with cap
One 10 ml Syringe
One Harvard Apparatus syringe pump
Each low-flow control channel requires the following materials:
One 8-inch tubing
One 6-inch tubing
One female luer x 1/16 hose barb connector
Two elbow connectors
Prior to seeding cells, coat IBIDI slide channels with 50 μl of Matrigel matrix, diluted 1:20 in PBS (see Note 4). Incubate for at least 45 min at 37°C or room temperature. Wash channels twice with PBS.
Inject 30 μl of cell suspension into each channel of an IBIDI VI0.4 slide (see Note 5). Place the slide inside a 15 cm dish along with a 35 mm dish (no lid) filled with PBS to maintain humidity (see Note 6). Incubate at 37°C for 8 hrs to allow attachment to the culture surface.
Each channel requires a total volume of 10 ml Myelocult media containing 10 ng/ml mSCF for recirculation during application of LSS. Prepare an additional 3 to 5 ml of media with mSCF per channel to flush out nonadherent cells prior to starting fluidics.
Proceed with flushing of each channel by pipetting the corresponding channel volume into the slide inlet (see Note 7). Remove the excess media from the opposite end of the channel, but avoid removing all media and drying out the channel. Repeat flushing 2 more times. Afterwards, top off the channel and inlets with the media. When done, place the slide back in the incubator.
Set up all sterilized tubing and check valves under the hood while the slide is in the incubator warming (Fig. 1 and 2A). Assembly can be done on the incubator shelf if removed and placed under the hood. The two elbow connectors, which will connect to the slide through the inlets for one channel, must first be assembled to two female luer inlets of a 3-way stopcock (Fig. 2B). The third inlet of the stopcock is set to the “off” position.
Fill the reservoir tube with 10 ml myelocult-SCF media. Cut a small hole in the tube cap and insert the tubing ends into the reservoir (Fig. 2C). Apply parafilm around the cap and wrap carefully around the junction with the tubing. Secure the parafilm with tape, but be careful not to constrict the tubing, which would prevent media flow.
Hold the syringe by hand in a vertical position and repeatedly infuse and withdraw to fill the tubing assembly. Several repetitions will remove all bubbles from the assembly and syringe. Once this is done, set the syringe plunger to the 5 ml mark (see Note 8).
Move the slide into the hood and secure it to a flat surface. Clamp the tubing behind the elbow connectors with binder clips (see Note 9; Fig. 2D). Remove the 3-way stopcock and connect the elbow connectors to the inlets of the slide. Secure the junctions and then remove the clamps.
Check the tubing end inside the reservoir. Make sure it is immersed in media and not in contact with the bottom surface of the tube (Fig. 2C).
Secure the syringe in the syringe pump (Fig. 2E). Place the entire assembly inside the tissue culture incubator. If the incubator shelf was placed in the tissue culture hood, the entire LSS assembly and syringe pump can be placed on top and transferred into the incubator.
Connect the syringe pump to the Harvard Apparatus PHD ULTRA 4400 Remote, if using a remote system.
Set up a shearing program. Set the syringe pump force at 50%. Select the correct syringe characteristics by brand and size of the syringe. The infuse/withdrawal run program is listed as “Autofill”. Set the “Set Mode” to INF/WD. Under “Set Rates”, the INF and WD will be the same rate. For 5 dyne/cm2 on the IBIDI VI0.4 slide, the rate is 2.84 ml/min. Under “Set Volume per Cycle” enter the same volume for “Set Rates” (e.g. 2.84 ml) (see Note 10). Shearing time can be set to the number of cycles or the period of the time, so under “Set Total Volume of # Cycles” select Cycles or Time. Enter the cycle number or shearing time.
A low flow control can be set up by directly connecting a syringe containing medium to one inlet and a flow-out tubing to the other. Insert the tubing into a receiving tube to collect flow-through medium. Set the same pump force and syringe characteristics. Set the “Set Mode” to INF. For 0.001 dyne/cm2 on the IBIDI VI0.4 slide, the rate is 600 nl/min.
Press “Start” on the syringe control screen to start shearing and low flow control. Wait 10 min after the run starts and check the tubing assembly to make sure there are no leaks, bubbles, or errors on the pump.
Following application of shear stress, collect the cells by Accutase treatment and analyze by standard functional and phenotypic methods such as colony formation in methylcellulose, flow cytometry, gene expression profiling, or transplantation (described below).
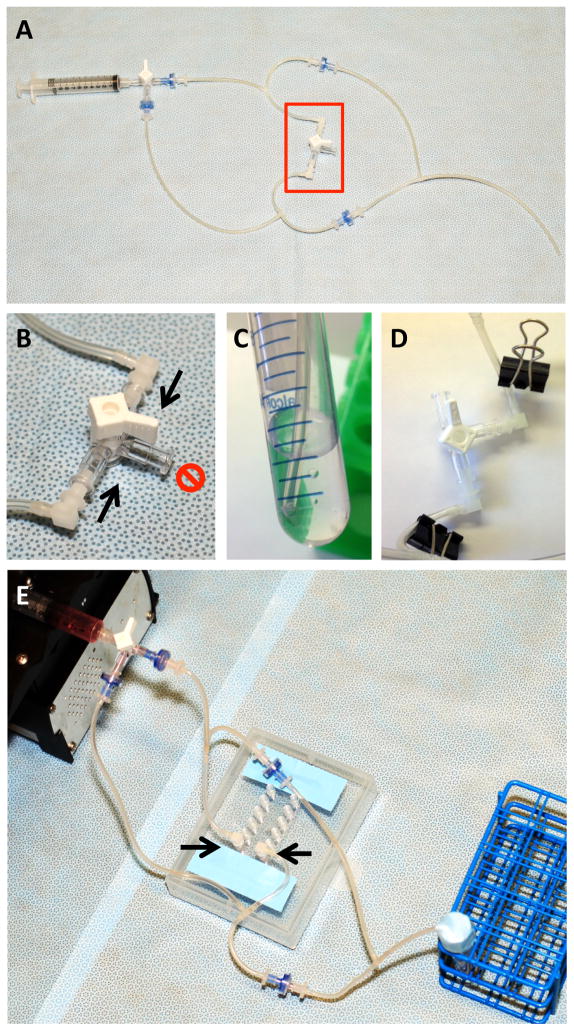
Figure 1.
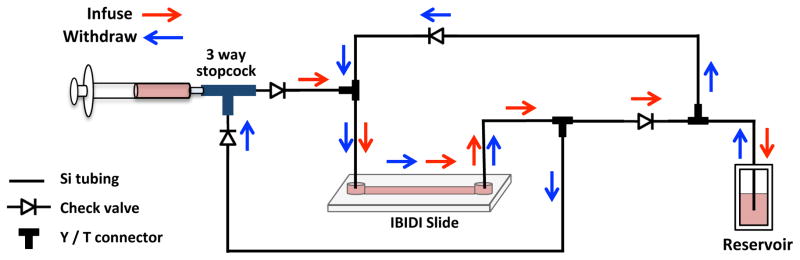
Schematic diagram of the microfluidic unidirectional shear stress system using a single syringe.
Figure 2.
Laminar shear stress tube assembly. A. Overview of the tube and check valve assembly. The second 3-way stopcock (red box) is temporary and serves as a “placeholder” for the future site of the slide. B. Two elbow connectors (arrows) are assembled to two female luer inlets of a 3-way stopcock before connecting to a slide. The third inlet (red “NO” symbol) is closed. C. Ensure the tubing in the media reservoir is close to but not in contact with the bottom of the 15 ml tube. D. To attach slide, first clamp the tubing on either side of the stopcock, then remove the stopcock. E. Connect the tube assembly to a slide by inserting the elbow connectors (arrows) into the two inlets of a channel (a 6-channel IBIDI VI0.4 slide is depicted).
3.3 Neonatal Transplantation
Set up the male and female(s) that are to provide the neonates 3 weeks exactly from the day of transplantation (see Note 11).
-
Sublethal neonatal myeloablation is typically done the day after birth (see Note 12). Approximately 3 hrs before transplantation, remove the mother from neonates, transfer pups into an irradiator dish and irradiate with dosing listed below. Put pups back into the cage, sprinkle with bedding, then reintroduce the mother. Change gloves if handling multiple adults and/or neonatal litters.
NSG neonates: 100 rads
Rag2−/− Common Gamma−/− neonates: 250 rads
C57Bl6 and B6.SJL (BoyJ): 300 rads
Dissociate cells from slides by Accutase treatment.
Run cells through a 70-μm strainer.
Resuspend cells in PBS volume sufficient for 15 μl per pup (see Note 12). Add a dose for an additional pup because volume can be “lost” in the syringe or during handling.
Remove the mother into a clean cage (see Note 13).
Place one or two pups on ice. Carefully observe them for color and movement. They are ready for injection when they appear slightly purple and are nonresponsive to handling or toe pinch. The facial vein is easiest to target when purple in color (see Note 14).
Inject 15 μl via the superior temporal vein of the face with a Hamilton syringe and 30-gauge needle (Fig. 3). Use care to remove air bubbles from the solution, which could result in immediate death if injected (see Note 15).
Transfer pups onto gentle heat. Cold stainless steel under the pups should be insulated by a heating pad (on LOW setting) or other material that can absorb heat from the heat lamp. Pups will regain pink tone and be very mobile. Be careful that they do not move away into a dangerous area (see Note 16).
Accumulate all the pups in the recovery area under the heat lamp, and then transfer together back into the cage. Sprinkle the pups with bedding to mask odors from gloves, and move the mother back in to join them.
Analysis of peripheral blood chimerism can be performed by flow cytometry beginning 5–6 weeks after transplantation, depending upon size and condition of pups, and should be monitored long-term (12–20 weeks) for assessment of stem cell function.
Figure 3.
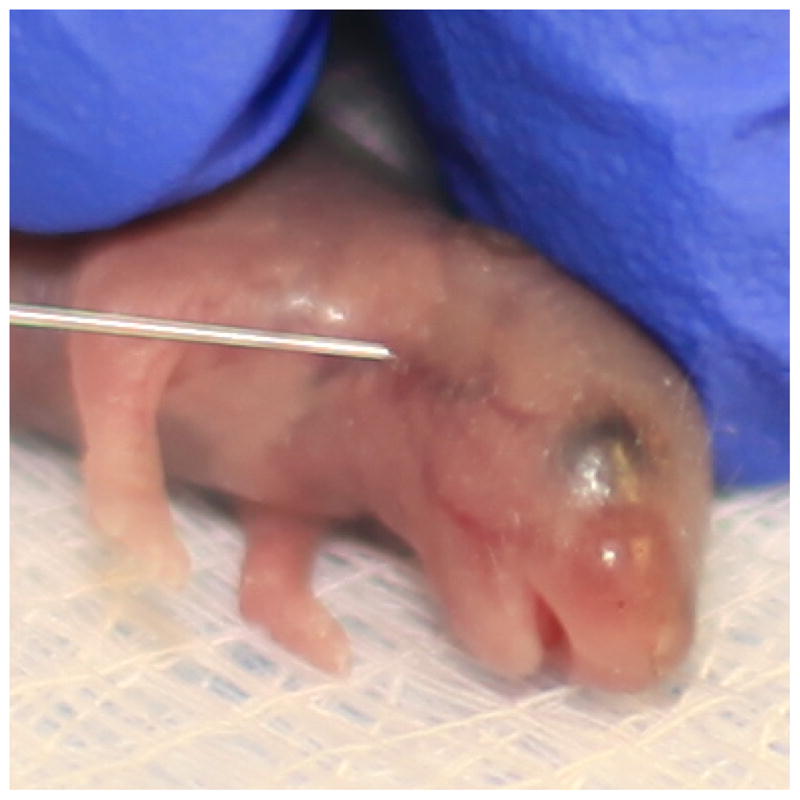
Facial vein injection of a neonatal mouse. The superior temporal vein highlighted by the needle tip serves as the best target for injection of the cell suspension.
Acknowledgments
This work was funded by grants from the American Society of Hematology, State of Texas Emerging Technology Fund, and National Institutes of Health to P.L.W.
Footnotes
Residual PBS buffer can be left in the tube as it does not have negative impact on dissociation efficiency.
Incubation time is important. Do not exceed 25 min, as cells may be damaged with excessive enzymatic digestion.
Be sure to sterilize tubing, connectors, 3-way stopcocks, and check valves. Tubing and all connectors are autoclavable up to 121° C for 20 min. The check valves and 3-way stopcocks are sterilized by Ethylene Oxide (EtO) gas.
Slide channels and flow chambers need to be coated first and washed with media to encourage cells to quickly adhere. A common coating solution includes matrigel (1: 20 dilution) and fibronectin (100 μg/ml) and must be freshly prepared in PBS on ice.
Please refer to IBIDI product manual for all information about the slides, medium volumes, and shearing parameters.
This provides moisture for the slides to prevent evaporation and drying of the channel.
Flushing the channels aids in removal of floating cells and nonadherent clumps of erythrocytes that should be removed prior to application of flow.
It is crucial that all syringes are set at the same mark.
This prevents leakage of media from the tubing.
Each cycle is equal to one full infusion and withdrawal, and lasts 2 min. Thus, for one hour of LSS, 30 cycles are required. The rate table for shear stress is available in the IBIDI product manual.
Adult transplantation can be used in lieu of neonatal transplantation, though emerging evidence suggests that the neonatal environment may provide a more sensitive readout of early hematopoietic stem and progenitor cells (13).
Transplant is least challenging the day after birth. As the pups age, injections become more difficult.
Transplant 0.5 to 2 million cells per neonate, depending upon the cell source and frequency of engraftable cells anticipated in the cell suspension. Trace amounts of serum are not detrimental but should not be added to the PBS for resuspension, as this could cause inflammatory response.
Remove the mother from the area so that she is unaware of the handling of her pups. This includes sight, smell, and sound.
If delays are encountered, transfer pups to heat and start over after full recovery. Over-chilled pups may be difficult to inject due to vessel constriction and may not revive. Each pup can tolerate cooling two times, if necessary. If a pup has been on ice over 15 min, transfer to heat.
Two aspects are critical during the facial injection procedure: 1. positioning of the pup, and 2. minimal movement of the hand while pushing the plunger. The beveled edge of the needle should face upward away from the pup. Try to secure the skin of the head to resist the needle as it enters the vein. If skin is stretched too tightly, the vein will “disappear”. If the needle is well placed in the vein, the vein will clear (red disappears) as cell suspension is injected. If the needle misses the vein, a fluid bubble will form during injection. Engraftment has been observed even when the vein is missed.
Warming the pups after immobilizing/anesthetizing on ice is critical. Care should be taken to avoid scorching pups under the heat lamp, and they should be warmed gently from above and below. Circulating water warming pads are particularly well suited to this application, as they provide gentle, uniform heat.
References
- 1.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–36. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 3.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–5. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–11. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–20. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 6.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–5. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 7.Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116:909–14. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- 8.Swiers G, Rode C, Azzoni E, de Bruijn MF. A short history of hemogenic endothelium. Blood Cells Mol Dis. 2013;51:206–12. doi: 10.1016/j.bcmd.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–5. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–48. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation. 2010;17:164–78. doi: 10.1111/j.1549-8719.2010.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe RP, Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng. 2013;110:1231–42. doi: 10.1002/bit.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–44. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]