Abstract
In this work we explore a semi-mechanistic model that considers cortisol’s permissive and suppressive effects through the regulation of cytokine receptors and cytokines respectively. Our model reveals the proactive role of cortisol during the resting period and its reactive character during the body’s activity phase. Administration of an acute LPS dose during the night, when cortisol’s permissive effects are higher than suppressive, leads to increased cytokine levels compared to LPS administration at morning when cortisol’s suppressive effects are higher. Interestingly, our model presents a hysteretic behavior where the relative predominance of permissive or suppressive effects results not only from cortisol levels but also from the previous states of the model. Therefore, for the same cortisol levels, administration of an inflammatory stimulus at cortisol’s ascending phase, that follows a time period where cytokine receptor expression is elevated ultimately sensitizing the body for the impending stimulus, leads to higher cytokine expression compared to administration of the same stimulus at cortisol’s descending phase.
Keywords: Cortisol suppressive effects, cortisol permissive effects, cytokine circadian rhythms, inflammatory response
1. Introduction
Inflammation is a critical component of body’s response to a variety of harmful stimuli such as infection and trauma. Under normal circumstances, the bi-directional flow of information between immune and neuroendocrine systems removes the pathogen or repairs the damaged tissue and restores homeostasis [1]. The principal peripheral effectors of the neuroendocrine system are glucocorticoids that are regulated by the hypothalamic-pituitary-adrenal (HPA) axis, and the catecholamines norepinephrine/epinephrine which are secreted by the sympathetic nervous system [2]. Mainly due to their immunosuppressive actions, glucocorticoids (cortisol in humans) have been regularly utilized for the treatment of autoimmune diseases and inflammatory disorders [3, 4]. Glucocorticoids induce their anti-inflammatory action through suppressing the production of numerous pro-inflammatory mediators (cytokines) such as IL-1 (interleukin-1), IL-2, IL-3, IL-6, and IFN-γ (interferon-γ) which are dangerous in excess [5, 6]. Along with their immunosuppressive role, it has long been suggested that they enhance the response to external stressors rather than solely limiting it [7]. Therefore glucocorticoids have been shown to up-regulate the expression of cytokine receptors [8-12] sensitizing the target cells to an upcoming stimulus. Interestingly, these opposing glucocorticoid effects do not cancel each other out, but are rather providing an optimal defense mechanism [13]. Investigation of the dynamics giving rise to glucocorticoids permissive and suppressive actions could provide insight into the emergent dynamics of response to stress.
Glucocorticoids exert their genomic effects through two types of receptors: type I (mineral corticoid receptor, MR), and type II (glucocorticoid receptor, GR) that after binding to glucocorticoid ligand, they translocate to the nucleus where they interact with specific promoter regions named glucocorticoid responsive elements (GREs) to activate appropriate hormone-responsive genes [14-16]. Since the affinity of MR to cortisol is much higher compared to that for GR [17], it has been hypothesized that lower cortisol levels mediate downstream effects mainly through MR while at higher cortisol concentrations binding to GR dominates [18, 19]. In the context of immunity and inflammation, lower cortisol levels have been further shown to act proactively, thus enhancing resistance to infection [20, 21] while suppressive actions are a characteristic of higher glucocorticoid levels [7].
We have previously presented a number of in silico studies of acute inflammation [22-27]. In the present work we further explore cortisol’s dynamic behavior taking into consideration its inducing effect on pro-inflammatory cytokine receptors aiming to elucidate the balance between its suppressive and permissive effects. Particularly in the work herein, cortisol’s permissive effects represent the MR-mediated induction of cytokine receptors whereas cortisol’s suppressive effects represent the GR mediated suppression of cytokines. Furthermore, we account for circadian rhythmicity present both at the single immune cell level (periphery) by peripheral clock genes (PCGs) as well as at the systemic level of hormonal secretion.
Our model describes cortisol’s antagonistic effects during the course of day. Permissive effects are accentuated during the dark (rest) period where the body is building its defense for the impending activity phase whereas during the light (active) period immunosuppressive characteristics of cortisol are denoted [7]. Thus we predict that acute LPS administration at night results in higher levels of cytokines compared to LPS administration at morning time. Furthermore, our model indicates that increased cytokine receptor expression during the night, leads to a more potent inflammatory response when acute stimulus is administered at cortisol’s rising phase compared to its descending phase even for the same cortisol values. This hysteretic behavior further illustrates cortisol’s preparative role for either sensitizing or desensitizing the body.
2. Materials and Methods
2.1. Modeling circadian rhythms at the systemic and peripheral level
2.1.1. Cortisol and glucocorticoid/mineralocorticoid receptors pharmacodynamics
The overall model is depicted in Figure 1. At the systemic level we considered the daily secretion of cortisol (F) using the “two rates” model [24, 25, 28], where a zero-order production term (RF) is set to two different values simulating the increased cortisol production at morning and the lower production at the rest of the day (Equation 1). In Equation 1, mod represents the remainder (modulo operation) of the division of time (t) with 24.
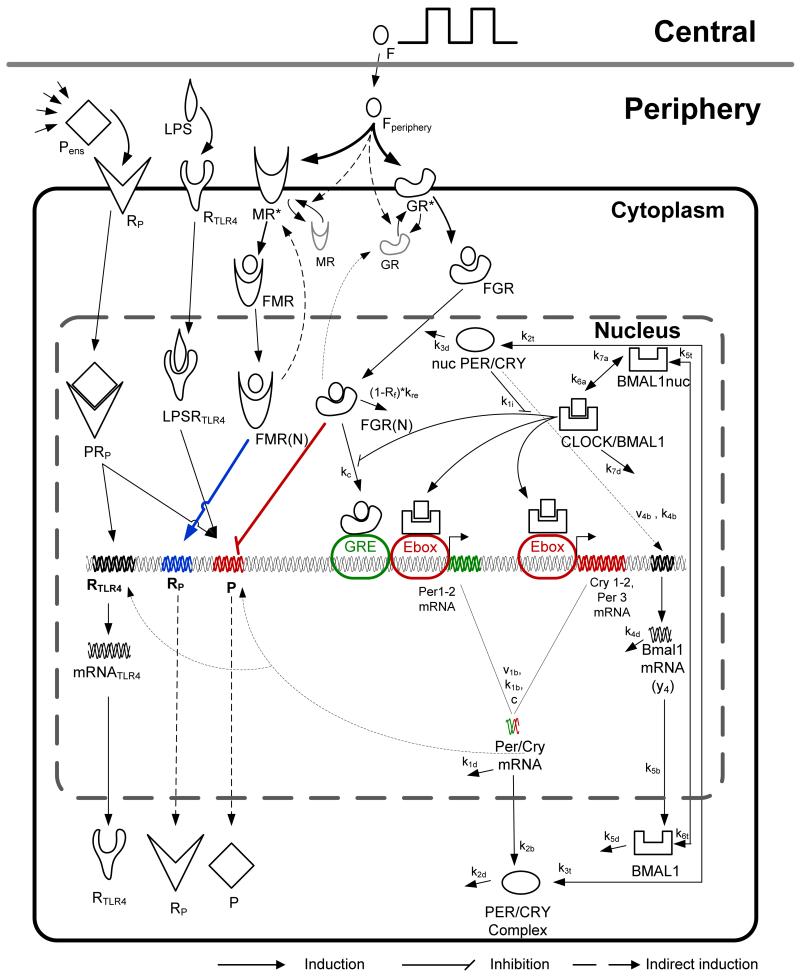
Figure 1.
Schematic figure of the model. After its rhythmic secretion, cortisol reaches peripheral cell level where it binds to the active form of its two receptors, mineralocorticoid (MR*) and glucocorticoid (GR*) receptors. Cortisol-mineralocorticoid receptor complex (FMR) after its translocation to the nucleus (FMR(N)) induces the expression of cytokine receptors (cortisol’s permissive effects denoted by the blue bold line) whereas cortisol glucocorticoid receptor (FGR) after its translocation to the nucleus (FGR(N)) inhibits the transcription of cytokines (cortisol’s suppressive effects denoted by the red bold line). Cytokine rhythmicity is also regulated by PCGs network through Per/Cry mRNA. PCGs network incorporates the positive and negative feedback loop among Per, Cry, Bmal1 clock genes and CLOCK/BMAL1 heterocomplex [36].
Subsequently, cortisol reaches peripheral cells (Equation 2) where it diffuses into their cytoplasm, and binds to the active forms of its two receptors (MR*c and GR*c). Similar to the model of [29] we hypothesize that cortisol activates, though phosphorylation, the two receptors [30, 31] rendering them active and able to bind cortisol (Equations 3 and 6). Following binding, the two glucocorticoid complexes (FMRc Equation 4, and FGRc Equation 7) translocate into the nucleus (FMR(N)c Equation 5, and FGR(N)c Equation 8) and ultimately binds to the GRE at the promoter regions of target genes (Per/Cry, cytokine receptors and cytokines)[32].
| (1) |
| (2) |
Mineralocorticoid receptor
| (3) |
| (4) |
| (5) |
Glucocorticoid receptor
| (6) |
| (7) |
| (8) |
Subscript c denotes the level of single peripheral cell. In order to account for the different compartment at the peripheral level, we assumed a transient compartment model (Equation 2) [33] using a mean transient time of τ=15 min [34]. We further assumed that the phosphorylation/dephosphorylation reactions of glucocorticoid and mineralocorticoid receptors (Equations 3 and 6) are governed by Michaelis-Menten kinetics [29]. Finally, in accordance with the theoretical model of [7, 13] we assumed a dissociation constant of cortisol for GR (KF,GR) equal to 30 (Equation 6) and for MR (KF,MR) equal to 0.5 (Equation 3). We further assumed similar reaction kinetics for the two receptors binding and translocation to the nucleus (Equations 4-5, and 7-8). Table 2 provides further information on variable notation.
Table 2.
Notation of model’s variables
| # | Notation | Variable |
|---|---|---|
| 1 | F | Cortisol at the systemic level |
| 2 | Fper,c | Cortisol that reaches peripheral cells |
| 3 | MR*c | Active form of mineralocorticoid receptor |
| 4 | FMRc | Cortisol-mineralocorticoid receptor complex |
| 5 | FMR(N)c | Cortisol-mineralocorticoid receptor nuclear complex |
| 6 | GR*c | Active form of glucocorticoid receptor |
| 7 | FGRc | Cortisol-glucocorticoid receptor complex |
| 8 | FGR(N)c | Cortisol-glucocorticoid receptor nuclear complex |
| 9 | Per/CrymRNA,c | mRNA of Per/Cry genes |
| 10 | PER/CRYc | Protein of Per/Cry gene |
| 11 | nucPER/CRYc | Nuclear protein of Per/Cry |
| 12 | BmalmRNA,c | mRNA of Bmall |
| 13 | BMAL1c | Protein of Bmall gene |
| 14 | nucBMAL1c | Nuclear protein of Bmall gene |
| 15 | CLOCK/BMAL1c | CLOCK/BMAL1 transcription factor |
| 16 | mRNAP,c | Pro-Inflammatory cytokine mRNA |
| 17 | Pc | Pro-Inflammatory cytokine protein |
| 18 | mRNARp,c | Pro-Inflammatory cytokine receptor mRNA |
| 19 | RP,c | Pro-Inflammatory cytokine receptor protein |
| 20 | PRP,c | Pro-Inflammatory cytokine- receptor complex |
| 21 | Pens | Ensemble levels of Pro- Inflammatory cytokines |
| 22 | LPS | Lipopolysaccharide (LPS) |
| 23 | mRNATLR4,c | mRNA of TLR4 receptor |
| 24 | RTLR4,c | Protein levels of TLR4 |
| 25 | LPSRTLR4,c | LPS-TLR4 receptor complex |
2.1.2. Peripheral clock genes dynamics
The molecular machinery of peripheral cells that is responsible for circadian time keeping includes a family of genes named clock genes which through transcriptional, translational a and post-translational feedback loops maintain circadian expression rhythms [35]. Our model incorporates the positive and negative feedback loop among Per, Cry, Bmal1 clock genes and CLOCK/BMAL1 heterocomplex. In particular, Per and Cry genes (Equation 9) form a negative feedback module since their proteins (PER/CRY, Equation 10), translocate to the nucleus (nuc PER/CRY, Equation 11) where they inhibit the CLOCKBMAL1 mediated transcription of their genes (Equation 1, denominator) while mediating an “accessory” positive feedback loop by indirectly inducing indirectly the expression of Bmal1 gene (Equation 12) that its receptor BMAL1 (Equation 13) after translocating to the nucleus (nuc BMAL1, Equation 14) and forming its active form (CLOCK/BMAL1, Equation 15), promotes the transcription of Per/Cry genes (Equation 1, numerator of first term) [36].
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
It is by now well established that rhythmicity in peripheral tissues is originated from the cell autonomous oscillatory activity of peripheral clock genes (PCGs) [35]. These peripheral oscillators are entrained by systemic signals carrying the photic information and as such they are in synchrony with the environment. In-vitro experiments in rat-1 fibroblasts have indicated that individual cells can maintain rhythmicity even without entrainment but become desynchronized and fall out of phase ultimately presenting a blunted ensemble amplitude [37, 38]. Furthermore, experiments in human peripheral blood mononuclear cells (PBMCs) and fibroblasts [39-41] have indicated that glucocorticoids can be particularly interesting putative entrainers of PCGs. In the present work, in accordance with our previous modeling efforts [42], we incorporate the entrainment of peripheral immune cells by cortisol by considering the binding of glucocorticoid/receptor complex to the GRE present at the promoter region of Per and Cry clock genes (Equation 9, last additive term) [43, 44]. By this we aim to further investigate the translation of PCGs rhythmicity to pro-inflammatory cytokine expression [45].
2.1.3. Modeling circadian rhythms of pro-inflammatory cytokines and cytokine receptors
Pro-inflammatory cytokines, such as interferon γ (IFN-γ), interleukin 1 (IL-1), or tumor necrosis factor α (TNF-α), exhibit distinct circadian rhythmicity with peak during the early morning periods [46, 47]. Cortisol has been recognized as a critical driver of circadian cytokine secretion [48]. In particular, experimental evidence suggests that cortisol mediated repression of cytokine expression is reduced by glucocorticoid receptor antagonist [49-52], further illustrating a GR mediated cytokine inhibition. Therefore, FGR(N) mediated inhibition of the expression of a cytokine’s mRNA, (mRNAP,c Equation 16), is simulated as an indirect response that further considers the saturation of cortisol receptor complex (cortisol’s suppressive effects). Cytokine circadian rhythms persist even in absence of systemic cues due to the presence of local peripheral clocks that are mediated by PCGs [45]. Therefore, the cytokine production is further regulated by Per/Cry to express the peripheral (autonomous) regulation of cytokine secretion. Finally, we simulate the “autocatalytic” role of proinflammatory cytokines [53-55] by further considering cytokine mediated induction of expression of mRNAP (Equation 16). After transcription, mRNAP,c further translates to its corresponding cytokine (Pc, Equation 17).
Cortisol’s mediated upregulation of cytokine receptors mRNA (mRNARp, c, Equation 18) was also modeled via an indirect response model where the nuclear component of cortisol/mineralocorticoid receptor complex, FMR(N), regulates the transcription of mRNARp (cortisol’s permissive effects). Following translation, the cytokine receptor (RP, Equation 19) binds to cytokine ligand forming cytokine/cytokine-receptor complex (PRP,c, Equation 20) which feeds back to mRNAP. The ensemble cytokine levels, Pens, were assumed to follow dynamics accounting for secretion of cytokines from the ensemble of peripheral immune cells with a simple degradation term (Equation 21). Compared to P that represents secretion of P at the cellular level, Pens simulates pro-inflammatory cytokines at the systemic level of peripheral blood.
| (16) |
| (17) |
| (18) |
| (19) |
| (20) |
| (21) |
The response of the system to an acute inflammatory challenge is based on our previously published model of human endotoxemia. Administration of low doses of endotoxin (lipopolysaccharides, LPS) in healthy individuals evokes signs and symptoms characteristic of systemic inflammation, making it a practical experimental model of systemic inflammation in humans [56]. LPS regulates the production of inflammatory mediators by binding to its Toll-Like receptor 4 (TLR4). This provides a controlled model of TLR4 agonist-induced systemic inflammation which has been used to study how inflammation activates physiological system (hormonal release, neural activity) as well as how exogenous treatment can modulate inflammation (hormone treatment, vagal stimulation). Based on recent experiments, our current work considers the cell autonomous circadian rhythmicity of TLR4 which is induced indirectly by PCGs [45] (Equation 23).
| (22) |
| (23) |
| (24) |
| (25) |
The binding of LPS to its receptor, TLR4, (Equations 22-24) induces the indirect transcription of the mRNA of pro-inflammatory cytokines (P) (Equation 16). We simulate the inhomogeneity of the peripheral cells by accounting for a population of (1000) cells. Intercellular variability is introduced by uniformly varying the parameters of single cell variables by 5% of their original value for each cell (Equations 3-20 and 23-25). Parameters of Equations 3,6,16-21 have been estimated based on cytokine and cortisol experimental data [47]. All the other model parameters have been adopted from our previous work. All the parameters of the model are presented in Table 1.
Table 1.
List of parameters used in the model
| # | Parameter | Value | Units | Description/Reference |
|---|---|---|---|---|
| 1 | tF1 | 4.62 | h | Start time of when cortisol production is heightened/[24] |
| 2 | tF2 | 12.1 | h | End time of when cortisol production is heightened/[24] |
| 3 | kin,Fen | 0.85 | ng/mL/hr | Base production rate of F/[24] |
| 4 | kFen,P | 0.35 | 1 | Strength of indirect stimulus on F by Pens/[24] |
| 5 | kout,F | 2.10 | 1/hr | Clearance rate of F/[24] |
| 6 | kin,RF1 | 2.60 | ng/mL/hr | Circadian production rate of F/[24] |
| 7 | τ | 0.15 | hr | Delay for the transportation of cortisol signal to periphery/[34] |
| 8 | kMR,pr | 0.56 | 1/hr | Base transcription rate of MR/Estimated |
| 9 | MRT | 1.35 | 1 | Total MR concentration/Estimated |
| 10 | KMR,pr | 0.13 | 1 | Michaelis constant for MR production/Estimated |
| 11 | kMR,deg | 0.58 | 1/h | Degradation rate for MR/Estimated |
| 12 | kb,MR | 0.00329 | l/nmol/hr | Degradation rate for cortisol/mineralocorticoid receptor binding/[16] |
| 13 | kr,MR | 0.001 | 1/h | Ratio of mineralocorticoid receptor recycled*rate of recycle/Estimated |
| 14 | kb,GR | 0.00329 | l/nmol/hr | Degradation rate for cortisol/mineralocorticoid receptor binding/[16] |
| 15 | kr,GR | 0.001 | 1/h | Ratio of mineralocorticoid receptor recycled*rate of recycle/Estimated |
| 16 | KMR,deg | 1.39 | 1 | Michaelis constant for degradation of MR/Estimated |
| 17 | kGR,pr | 1.18 | 1/hr | Base transcription rate of GR/Estimated |
| 18 | GRT | 1.81 | 1 | Total GR concentration/Estimated |
| 19 | KGR,pr | 0.74 | 1 | Michaelis constant for GR production/Estimated |
| 20 | kGR,deg | 1.52 | 1/h | Degradation rate for GR/Estimated |
| 21 | KGR,deg | 1.05 | 1 | Michaelis constant for degradation of GR/Estimated |
| 22 | kc | 0.004 | 1/hr | Coupling strength//Estimated |
| 23 | v1b | 9 | nM/hr | Maximal rate of Per/Cry transcription/ [36] |
| 24 | k1b | 1 | nM | Michaelis constant of Per/Cry transcription/ [36] |
| 25 | k1i | 0.56 | nM | Inhibition constant of Per/Cry transcription/ [36] |
| 26 | c | 0.01 | nM | Concentration of constitutive activator /[36] |
| 27 | p | 8 | Hill coefficient of inhibition of Per/Cry transcription/ [36] |
|
| 28 | k1d | 0.12 | 1/hr | Degradation rate of Per/Cry mRNA/ [36] |
| 29 | kc | 0.004 | 1/hr | Coupling constant /[36] |
| 30 | k2b | 0.3 | 1/nM/hr | Complex formation rate of Per/Cry mRNA /[36] |
| 31 | q | 2 | No. of PER/CRY complex forming subunits /[36] |
|
| 32 | k2d | 0.05 | 1/hr | Degradation rate of cytoplasmatic PER/CRY/ [36] |
| 33 | k2t | 0.24 | 1/hr | Nuclear import rate of the PER/CRY complex /[36] |
| 34 | k3t | 0.02 | 1/hr | Nuclear export rate of PER/CRY complex /[36] |
| 35 | k3d | 0.12 | 1/hr | Degradation rate of the nuclear PER/CRY complex/ [36] |
| 36 | v4b | 3.6 | nM/hr | Maximal rate of Bmal1 transcription /[36] |
| 37 | k4b | 2.16 | nM | Michaelis constant of Bmal1 transcription/ [36] |
| 38 | r | 3 | Hill coefficient of activation of Bmal1 transcription /[36] |
|
| 39 | k4d | 0.75 | 1/hr | Degradation rate of Bmal1 mRNA/ [36] |
| 40 | k5b | 0.24 | 1/hr | Translation rate of BMAL1 /[36] |
| 41 | k5d | 0.06 | 1/hr | Degradation rate of cytoplasmatic BMAL1 /[36] |
| 42 | k5t | 0.45 | 1/hr | Nuclear import rate of BMAL1 /[36] |
| 43 | k6t | 0.06 | 1/hr | Nuclear export rate of BMAL1/ [36] |
| 44 | k6d | 0.12 | 1/hr | Degradation rate of nuclear BMAL1/ [36] |
| 45 | k6a | 0.09 | 1/hr | Activation rate of nuclear CLOCK/BMAL1/ [36] |
| 46 | k7a | 0.003 | 1 /hr | Deactivation rate of CLOCK/BMAL1 /[36] |
| 47 | k7d | 0.09 | 1/hr | Degradation rate of CLOCK/BMAL1 /[36] |
| 48 | kmRNAP,in | 7.3 | 1/h | Base transcription rate of mRNAP |
| 49 | k P,LPSRTLR4 | 59.81 | 1 | Rate of LPSR mediated transcription of mRNAP/Estimated |
| 50 | kfr | 1.07 | 1 | Maximum rate of FGR(N) mediated suppression of mRNAP/Estimated |
| 51 | Kfr | 1 | 1 | Michaelis constant for FGR(N) mediated suppression of mRNAP/Estimated |
| 52 | kpc | 0.35 | 1 | Rate of Per/CrymRNA mediated transcription of mRNAP/Estimated |
| 53 | kmRNAP,out | 2.89 | 1/hr | Degradation rate of mRNAP/Estimated |
| 54 | kin,P | 0.29 | 1/hr | Translation rate of P/Estimated |
| 55 | kout,P | 1.06 | 1/hr | Degradation rate of P/Estimated |
| 56 | k mRNARP,in | 0.61 | 1/hr | Base transcription rate of mRNARP/Estimated |
| 57 | kfr,2 | 0.8 | 1 | Maximum rate of FMR(N) mediated transcription of mRNARP/Estimated |
| 58 | Kfr,2 | 0.5 | 1 | Michaelis constant for FMR(N) mediated transcription of mRNARP/Estimated |
| 59 | k mRNARP,out | 0.19 | 1/hr | Degradation rate of mRNARP- /Estimated |
| 60 | kin,Rp | 1.11 | 1/hr | Translation rate of RP/Estimated |
| 61 | kd | 0.14 | 1/hr | P-Rp binding rate/Estimated |
| 62 | kout,Rp | 0.26 | 1/hr | Dissociation rate of RP/Estimated |
| 63 | kout,PRp | 1.30 | 1/hr | Dissociation rate of PRP/Estimated |
| 64 | kLPS,1 | 4.5 | 1/hr | Growth rate of LPS/[24] |
| 65 | kLPS,2 | 6.79 | 1/hr | Clearance rate of LPS/[24] |
| 66 | kLPS,3 | 0.0914 | 1 | Base transcription rate of mRNATLR4/[24] |
| 67 | kmRNA,TLR4 | 1.74 | 1 | Rate of PRp mediated transcription of mRNATLR4/[24] |
| 68 | kLPS,4 | 0.32 | 1/hr | Decay rate of mRNATLR4/[24] |
| 69 | ksyn | 0.02 | 1/hr | Translation rate of TLR4/[24] |
| 70 | k2 | 5.04 | 1/hr | Dissociation rate between LPS and TLR4/[24] |
| 71 | k1 | 3 | 1/hr | Binding rate between LPS and TLR4/[24] |
3. Results
Our in-silico study aims to investigate the circadian interplay of cortisol’s permissive and suppressive balance of effects as well as its implications with respect to acute stress. In order to evaluate cortisol’s antagonistic effects we consider the actions of pathway through the mineralocorticoid and glucocorticoid receptors in the model shown in Figure 1.
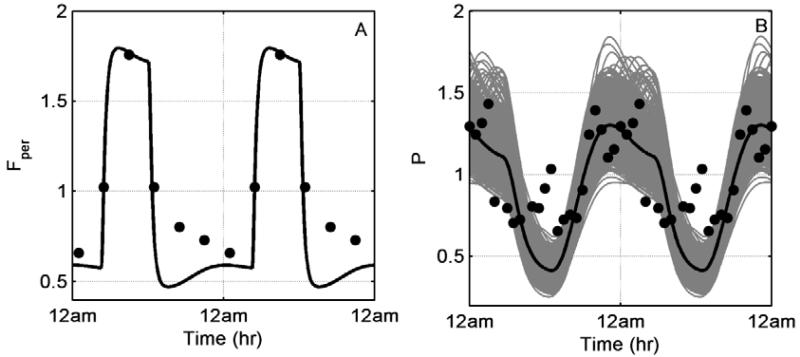
Figure 2 depicts the homeostatic response of our model. In accordance with experimental data, cortisol levels peak at morning (Figure 2A) whereas pro-inflammatory cytokines maintain peak values later at night (Figure 2B). Grey lines of Figure 2B reflect single cell simulations while thick black line their average profile.
Figure 2.
Homeostatic responses of A: cortisol (Fper) and B: pro-inflammatory cytokines (P). Grey lines represent single cell profiles and thick black line denotes their average profile. Experimental data from healthy volunteers have been adopted from [47].
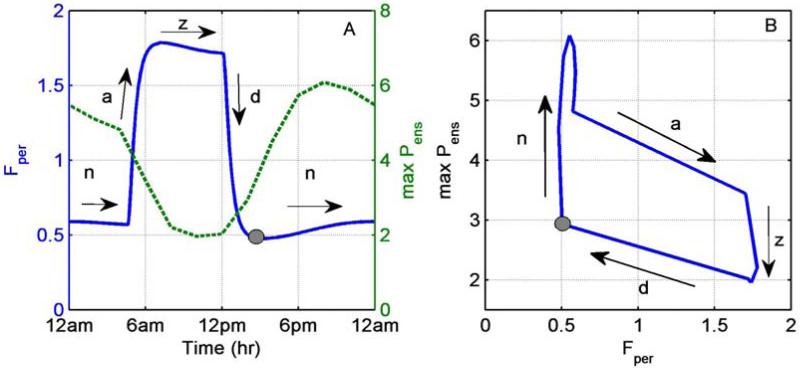
In order to explore the circadian dependence of the sensitivities of our model, we analyzed the response to an acute LPS stimulus at different times of day. We conducted a series of in silico experiments where LPS was injected at different times during the day and we recorded the maximum predicted levels of cytokines (denoted as max Pens) during the 24 h period post-injection. The LPS levels were assumed to induce an acute, self-resolving inflammatory response. Figure 3A depicts homeostatic cortisol rhythm (left panel, solid line) along with the maximum Pens level, while Figure 3B expresses a “phase plane”-like plot of the same data. It is important to realize that the figure depicts the level of cortisol at the time of the injection, whereas the maxPens denotes the maximum cytokine levels at some future time. A superficial reading of the figure may imply that the response is rather expected: the lower the cortisol’s level at the time of the injection of LPS, the higher the cytokine production, and vice versa. Closer examination, however, reveals a far more interesting and complex response.
Figure 3.
Administration of acute LPS at different times of day (TOD). A: Cortisol (Fper, solid line, left axis) and maximum ensemble pro-inflammatory cytokine levels (maxPens, dotted line, left axis) relative to time of day. B: Maximum ensemble pro-inflammatory cytokine levels (maxPens) relative to cortisol cortisol levels. Max Pens was calculated for the 24hr following LPS administration. a, d, z, and n denote cortisol’s ascending, descending, zenith and nadir levels respectively. Grey dot represents the start of nadir (n) phase.
Cortisol’s profile can roughly be separated in four domains. Periods where cortisol remains close to its nadir (n) or zenith (z) levels, and periods where cortisol is at its ascending (a) or descending (d) phase (Figure 3A, left panel solid line arrows). Generally, lowest responsiveness, expressed as low maximum Pens value, was observed at times where cortisol was indeed around its zenith (z) levels (Figure 3A, roughly from 6am to12pm). Figure 3B, however, indicates that even for the same cortisol values the response of the system upon an acute LPS stimulus can be different depending on whether cortisol is at its ascending (a) or descending (d) phases. Even during periods where cortisol is close to its zenith (z) or nadir (n) levels, Figure 3B also indicates significantly different sensitivities of the response relative to cortisol level.
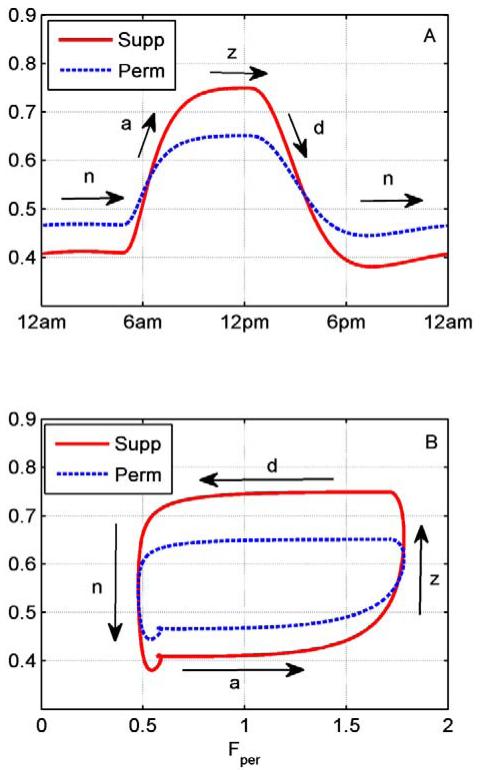
To further elaborate on the underlying dynamics leading to changes in Pens levels following acute LPS administration, Figure 4 depicts the permissive/suppressive effects of cortisol relative to time (Figure 4A) and cortisol levels (Figure 4B). Cortisol’s permissive effects quantify the cortisol’s mediated induction of cytokine receptor through mineralocorticoid receptor (, Equation 18) whereas the suppressive effects quantify the cortisol-mediated suppression of cytokine secretion through glucocorticoid receptor ( Equation 16). Figure 4A indicates that when cortisol is near its nadir (n) levels, the permissive effects are greater than the suppressive. As cortisol increases, moving to its peak levels (ascending phase), the suppressive effects begin to increase and eventually dominate leading to a switch near the zenith (z) levels where the suppressive effects dominate. On the other hand, as cortisol decreases while moving towards its circadian nadir levels (descending phase) the permissive effects begin to dominate until eventually reaching cortisol’s nadir (n) to where permissive effects are greater than suppressive. Figure 4B further illustrates the relative impact of cortisol’s permissive/suppressive effects as a function to cortisol levels.
Figure 4.
Permissive and suppressive effects of cortisol during the day. A: Cortisol’s permissive (perm, dotted line) and suppressive (sup, solid line) effects relative to time. B: Permissive and suppressive effects relative to cortisol levels (Fper).Permissive profile represents the cortisol mediated induction of cytokine receptors through mineralocorticoid receptor whereas suppressive, the cortisol mediated inhibition of cytokines through glucocorticoid receptor . a, d, z, and n denote cortisol’s ascending, descending, zenith and nadir levels respectively. Angle brackets denote average levels.
4. Discussion
The balance between cortisol’s suppressive and permissive effects is crucial for the maintenance of homeostasis as well as the response of the body to stress. Rhythmic hormonal and metabolic signaling between organs establishes proper phase relations among the various clocks and these rhythmic signals play a major role in immune [57-59] and metabolic functions[60] conferring adaptive advantages by means of anticipatory control mechanisms [61]. In this work, we examined the interplay between cortisol’s, seemingly, antagonistic effects by investigating cortisol mediated regulation of cytokine receptors and cortisol mediated inhibition of cytokines.
The time of day dependence of the immune response has been extensively studied in animal and human models and appears to be regulated both by central and peripheral circadian clocks that function autonomously [45]. In our model we considered the existence of circadian oscillation both at the central level of hormone secretion (Equation 1) as well as the peripheral level of immune cells (Equations 2-25). In particular, autonomous oscillations at the central level (cortisol, F) are described by a “two rates model” (Equation 1) with a zero-order production term (RF) simulating the distinct cortisol production levels during the day [28]. At the level of peripheral immune cells (denoted by the subscript c, Equations 2-20 and 23-25) autonomous oscillations are driven by the convolution of positive and negative feedback loops further regulating the rhythm of pro-inflammatory cytokines (mRNAp, Equation 16). In order to further account for rhythmicity at the tissue/organ level, we considered a representative population of 1000 cells where intercellular variability was introduced by varying the parameters of the equations representing peripheral cell level (Equations 2-20 and 23-25) by ±5% of their nominal value [62]. In contrast to our prior modeling efforts [24, 25], in the work presented herein we omitted several inflammatory parameters (i.e. anti-inflammatory cytokines, melatonin, epinephrine) that may also play a role in body’s response to acute stress. As the main focus of this work was the investigation of cortisol’s permissive/suppressive effects and its forward effects on pro-inflammatory cytokines, our main assumption is that we can use pro-inflammatory cytokines as a representative pro-inflammatory signal of body’s stress response, and cortisol as a representative anti-inflammatory hormone that inhibits the pro-inflammatory surge and restores homeostasis.
Figure 2 depicts the homeostatic response of our model at the population (thick black lines Figure 2A and Figure 2B) and single cell level (grey lines, Figure 2B). Introduction of intercellular variability leads to a distribution of phases, amplitudes and periods for each peripheral cell. We have previously [42] discussed that cortisol’s rhythmic secretion synchronize the population of peripheral cells in order to maintain a narrow distribution of periods and phases that is further mediated to cytokine rhythm (Figure 2B). The average of single cell responses is optimized in order to be in accordance with available experimental data (Figure 2A, 2B) [47]. In particular, parameters of the Equations 3,6,16-21 were estimated based on the available experimental evidence of cortisol and pro-inflammatory cytokines, whereas the rest of the parameters values were adopted from our previous publications [25, 42]. However, similar to our previous modeling efforts, the dynamics observed in this work do not represent parameter-specific responses but are rather indicative of dynamics present at a larger parameter regime and are highly based on model structure and underlying assumptions.
Experimental data suggest that circadian rhythms can influence the human response to an inflammatory challenge [45]. The work of [63] further demonstrated that diurnal variation of human’s susceptibility to endotoxin may be due to a suppression of the cytokine effects by glucocorticoids. Our model demonstrates that the time of day dependence of the inflammatory response is a broader function of the relative strength of the pro- and anti-inflammatory characteristics of cortisol and, likely, does not reflect a simple dose-response. Our time of day response to endotoxin dependence results (Figure 3A), are in agreement with experimental data of human endotoxemia showing that acute LPS administration at 12am results to a more pronounced inflammatory response and higher cytokine expression compared to LPS administration at 12pm [64]. Furthermore, our predictions lie in close proximity with our own work (unpublished data) showing that healthy volunteers challenged with endotoxin at 9pm present significantly higher secretion of cytokines compared to volunteers challenged with LPS at 9am. Furthermore, ex-vivo studies [48] also indicate that cytokine peak production occurs at night when cortisol level is lowest. Interestingly, Figure 3B illustrates that acute LPS administration at times when cortisol maintains same levels (i.e. Fper=1) can result in significantly different cytokine levels depending on if LPS is administered at cortisol’s ascending or descending phase. In particular, administration of LPS stimulus during cortisol’s ascending (a) phase results in higher cytokine secretion compared to LPS administration at cortisol’s descending (d) phase. Similar response is observed at cortisol’s nadir (n) and zenith (z) levels where despite cortisol’s constant levels, our model present significantly different inflammatory responses. Cortisol’s complex dynamics embedded in the ascending and descending phases has also been evaluated in the context of light induction both experimentally [65] as well as in silico in our recent work [66], where we demonstrate that a bright light stimulus at the rising phase of cortisol due to the reduced intensity of FRncentral negative feedback, leads to a more pronounced down-regulation compared to a stimulus at the descending phase. Administration of acute LPS stimulus may also affect circadian rhythmicity at the peripheral level [67]. In particular, experimental evidences suggest that in-vivo endotoxin suppresses clock gene expression [68] possible through the secretion of pro-inflammatory cytokines [69]. The present model does not include this interaction since our work explores mainly cortisol’s permissive/suppressive effects. However, we would wait that an LPS-mediated disruption of PCGs rhythmicity would have only a small impact on the expression of cytokines (Equation 16), since the main driver of cytokine rhythmicity is cortisol’s mediated downregulation of cytokine expression (Equation 16).
Cortisol’s “permissive” effects likely sensitizes immune cells to an upcoming challenge [70] and has been argued that this is mediated through an increase in the number of cytokine receptors [12, 71]. Recently, we demonstrated that 24 hr cortisol infusion in healthy individuals, as means to emulate periods of increased stress, enhanced expression of genes encoding for cytokine receptors [10]. This surge in cytokine and pattern recognition receptors had been further linked with priming effects of cortisol on the immune function. In the model presented herein, cortisol’s permissive effects are represented by the induction of pro-inflammatory cytokine receptors (Equation 18) and suppressive by the suppression of cytokines (Equation 16). Particularly, in accordance with experimental evidence [19] we investigated the scenario under which permissive cortisol effects are mediated through the nuclear compartment of cortisol-mineralocorticoid receptors (FMR(N)), while suppressive effects by cortisol-glucocorticoid receptor nuclear compartment (FGR(N)). Figure 4 shows the relative contribution of permissive and suppressive effects of cortisol during the day as quantified by the terms regulating the two effects in Equation 16 and 18( and respectively). At times when cortisol maintains its nadir (n) values, permissive effects are elevated compared to cortisol’s suppressive effects (Figure 4A). In particular, Figure 4B depicts that near cortisol’s nadir levels, there is a switch from a state where suppressive effects are greater (activity phase of previous day) to a state where permissive effects are greater. During this time of the day cortisol acts as a priming agent, sensitizing the organism in preparation for the activity phase. This further implies that LPS administration at consecutive times at cortisol nadir (n) levels will result in higher cytokine levels until a point where cortisol reaches nearly the end of its ascending (a) phase (Figure 3A right axis dotted line, Figure 3B). At cortisol’s ascending (a) phase, permissive effects still retain higher values compared to suppressive in contrast with cortisol’s descending phase where the adverse is true (Figure 4B). This hysteretic behavior where the previous states of the model regulate its response, leads to a higher response when LPS stimulus is introduced at times when cortisol is at its ascending (a) phase (Figure 3B, Figure 3A) compared to when cortisol is at its descending (d) phase even for the same cortisol levels. Hysteresis generally arises in systems that contain a positive feedback loop or a mutually inhibitory, double negative-feedback loop [72]. In our model this positive regulation is articulated through cortisol mediated induction of its own receptors (GR* and MR*, Equation 3 and 6). Hysteretic behavior is regularly seen in biological systems [73, 74] and it has been linked with tolerance against intracellular noise [75].
The physiological properties of mineralocorticoid/glucocorticoid receptors included in this model are based on the theoretical models of [7, 13]. Therefore, the mineralocorticoid affinity for cortisol has been modeled by a lower dissociation constant of cortisol/MR complex (KF,MR Equation 3 vs KF,GR Equation 6). Furthermore, in accordance with experimental evidence showing a higher secretion of GR under higher cortisol concentrations [7], we further assumed a higher maximum rate of GR (kF,GR Equation 6 vs kF,MR Equation 3).The different dissociation constants between the two cortisol’s receptors results to a faster saturation of mineralocorticoid receptor as cortisol reaches its zenith values (Figure 4A). On the other hand, glucocorticoid receptors will saturate slower but to a higher value. Therefore, nearing cortisol’s zenith we observe a switch from a state of higher permissive effects (mediated by mineralocorticoid receptor) to a state where suppressive effects (mediated by glucocorticoid receptor) dominates. This further reveals that although cortisol levels are same at its zenith, administration of LPS stimulus to consecutive times at zenith levels, results in lower maximum Pens levels.
5. Conclusions
Despite the classical view of glucocorticoids as an endocrine response to stress that generally prevents the stress activated stress reactions from overshooting, their time of day dependent role still remains controversial [7]. Through our modeling effort we aimed to explore the convolution of cortisol’s permissive and suppressive effects and their role in the body’s time of day dependent response to an acute stimulus. In order to investigate these effects, in accordance with experimental data we assumed that mineralocorticoid receptors mediate cortisol’s permissive role through regulating cytokine receptors while glucocorticoid receptors mediate cortisol’s suppressive role through suppressing cytokine transcription. Our model points towards a non-dose dependent cortisol effect. In particular, our results unveil the preparative role of cortisol that sensitizes the organism at times of day less likely to get infected (second half of cortisol’s nadir (n) and ascending (a) phase), in order to ultimately mount an efficient defensive response at times of day when it is more likely to react to a stimulus (second half of cortisol’s zenith (z) and descending phase).
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the financial support from NIH grant GM082974.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 3.Tsigos C, Chrousos GP. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am. 1994;23(3):451–66. [PubMed] [Google Scholar]
- 4.Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94(6):557–72. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234(4775):470–4. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987;164(5):415–22. [PubMed] [Google Scholar]
- 7.Sapolsky RM, Romero LM, Munck AU. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 8.Gottschall PE, et al. Glucocorticoid upregulation of interleukin 1 receptor expression in a glioblastoma cell line. Am J Physiol. 1991;261(3 Pt 1):E362–8. doi: 10.1152/ajpendo.1991.261.3.E362. [DOI] [PubMed] [Google Scholar]
- 9.Re F, et al. The type II “receptor” as a decoy target for interleukin 1 in polymorphonuclear leukocytes: characterization of induction by dexamethasone and ligand binding properties of the released decoy receptor. J Exp Med. 1994;179(2):739–43. doi: 10.1084/jem.179.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamisoglu K, et al. Effects of coupled dose and rhythm manipulation of plasma cortisol levels on leukocyte transcriptional response to endotoxin challenge in humans. Innate Immun. 2013 doi: 10.1177/1753425913508458. [DOI] [PubMed] [Google Scholar]
- 11.Snyers L, De Wit L, Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci U S A. 1990;87(7):2838–42. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akahoshi T, Oppenheim JJ, Matsushima K. Induction of high-affinity interleukin 1 receptor on human peripheral blood lymphocytes by glucocorticoid hormones. J Exp Med. 1988;167(3):924–36. doi: 10.1084/jem.167.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munck A, Naray-Fejes-Toth A. The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited. Mol Cell Endocrinol. 1992;90(1):C1–4. doi: 10.1016/0303-7207(92)90091-j. [DOI] [PubMed] [Google Scholar]
- 14.Pratt WB. Glucocorticoid receptor structure and the initial events in signal transduction. Prog Clin Biol Res. 1990;322:119–32. [PubMed] [Google Scholar]
- 15.Funder JW. Mineralocorticoids, glucocorticoids, receptors and response elements. Science. 1993;259(5098):1132–3. doi: 10.1126/science.8382375. [DOI] [PubMed] [Google Scholar]
- 16.Ramakrishnan R, et al. Fifth-generation model for corticosteroid pharmacodynamics: application to steady-state receptor down-regulation and enzyme induction patterns during seven-day continuous infusion of methylprednisolone in rats. J Pharmacokinet Pharmacodyn. 2002;29(1):1–24. doi: 10.1023/a:1015765201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arriza JL, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–75. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 18.Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43(1):1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 19.De Kloet ER, et al. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 20.Jefferies WM. Cortisol and immunity. Med Hypotheses. 1991;34(3):198–208. doi: 10.1016/0306-9877(91)90212-h. [DOI] [PubMed] [Google Scholar]
- 21.Jefferies WM. Mild adrenocortical deficiency, chronic allergies, autoimmune disorders and the chronic fatigue syndrome: a continuation of the cortisone story. Med Hypotheses. 1994;42(3):183–9. doi: 10.1016/0306-9877(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 22.Foteinou PT, et al. In silico simulation of corticosteroids effect on an NFkB-dependent physicochemical model of systemic inflammation. PLoS One. 2009;4(3):e4706. doi: 10.1371/journal.pone.0004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foteinou PT, et al. Modeling endotoxin-induced systemic inflammation using an indirect response approach. Mathematical Biosciences. 2009;217(1):27–42. doi: 10.1016/j.mbs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheff JD, et al. Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol. 2010;264(3):1068–76. doi: 10.1016/j.jtbi.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Scheff JD, et al. Modeling autonomic regulation of cardiac function and heart rate variability in human endotoxemia. Physiol Genomics. 2011;43(16):951–64. doi: 10.1152/physiolgenomics.00040.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheff JD, et al. Translational applications of evaluating physiologic variability in human endotoxemia. J Clin Monit Comput. 2013;27(4):405–15. doi: 10.1007/s10877-012-9418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheff JD, et al. Modeling physiologic variability in human endotoxemia. Crit Rev Biomed Eng. 2012;40(4):313–22. doi: 10.1615/critrevbiomedeng.v40.i4.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborty A, Krzyzanski W, Jusko WJ. Mathematical modeling of circadian cortisol concentrations using indirect response models: comparison of several methods. J Pharmacokinet Biopharm. 1999;27(1):23–43. doi: 10.1023/a:1020678628317. [DOI] [PubMed] [Google Scholar]
- 29.Tyson JJ, Chen KC, Novak B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol. 2003;15(2):221–31. doi: 10.1016/s0955-0674(03)00017-6. [DOI] [PubMed] [Google Scholar]
- 30.Frey FJ, Odermatt A, Frey BM. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. 2004;13(4):451–8. doi: 10.1097/01.mnh.0000133976.32559.b0. [DOI] [PubMed] [Google Scholar]
- 31.Sathiyaa R, Vijayan MM. Autoregulation of glucocorticoid receptor by cortisol in rainbow trout hepatocytes. Am J Physiol Cell Physiol. 2003;284(6):C1508–15. doi: 10.1152/ajpcell.00448.2002. [DOI] [PubMed] [Google Scholar]
- 32.Jin JY, et al. Modeling of Corticosteroid Pharmacogenomics in Rat Liver Using Gene Microarrays. Journal of Pharmacology and Experimental Therapeutics. 2003;307(1):93–109. doi: 10.1124/jpet.103.053256. [DOI] [PubMed] [Google Scholar]
- 33.Sun YN, Jusko WJ. Transit compartments versus gamma distribution function to model signal transduction processes in pharmacodynamics. J Pharm Sci. 1998;87(6):732–7. doi: 10.1021/js970414z. [DOI] [PubMed] [Google Scholar]
- 34.Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci. 2010;277(1688):1627–33. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 36.Becker-Weimann S, et al. Modeling feedback loops of the Mammalian circadian oscillator. Biophys J. 2004;87(5):3023–34. doi: 10.1529/biophysj.104.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagoshi E, et al. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119(5):693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Welsh DK, et al. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14(24):2289–95. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–7. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 40.Balsalobre A. Clock genes in mammalian peripheral tissues. Cell Tissue Res. 2002;309(1):193–9. doi: 10.1007/s00441-002-0585-0. [DOI] [PubMed] [Google Scholar]
- 41.Burioka N, et al. Dexamethasone influences human clock gene expression in bronchial epithelium and peripheral blood mononuclear cells in vitro. Chronobiol Int. 2005;22(3):585–90. doi: 10.1081/CBI-200062416. [DOI] [PubMed] [Google Scholar]
- 42.Mavroudis PD, et al. Entrainment of peripheral clock genes by cortisol. Physiol Genomics. 2012;44(11):607–21. doi: 10.1152/physiolgenomics.00001.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So AYL, et al. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences. 2009;106(41):17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto T, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280(51):42036–43. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 45.Keller M, et al. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences. 2009 doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrovsky N, Harrison LC. Diurnal rhythmicity of human cytokine production: a dynamic disequilibrium in T helper cell type 1/T helper cell type 2 balance? J Immunol. 1997;158(11):5163–8. [PubMed] [Google Scholar]
- 47.Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10(4):307–12. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 48.Petrovsky N, Harrison LC. The chronobiology of human cytokine production. Int Rev Immunol. 1998;16(5-6):635–49. doi: 10.3109/08830189809043012. [DOI] [PubMed] [Google Scholar]
- 49.Knudsen PJ, Dinarello CA, Strom TB. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol. 1987;139(12):4129–34. [PubMed] [Google Scholar]
- 50.Paliogianni F, Boumpas DT. Glucocorticoids regulate calcineurin-dependent trans-activating pathways for interleukin-2 gene transcription in human T lymphocytes. Transplantation. 1995;59(9):1333–9. [PubMed] [Google Scholar]
- 51.Amano Y, Lee SW, Allison AC. Inhibition by glucocorticoids of the formation of interleukin-1 alpha, interleukin-1 beta, and interleukin-6: mediation by decreased mRNA stability. Mol Pharmacol. 1993;43(2):176–82. [PubMed] [Google Scholar]
- 52.Kutteh WH, Rainey WE, Carr BR. Glucocorticoids inhibit lipopolysaccharide-induced production of tumor necrosis factor-alpha by human fetal Kupffer cells. J Clin Endocrinol Metab. 1991;73(2):296–301. doi: 10.1210/jcem-73-2-296. [DOI] [PubMed] [Google Scholar]
- 53.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1-3):184–7. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 55.Akira S, et al. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) Faseb J. 1990;4(11):2860–7. [PubMed] [Google Scholar]
- 56.Lowry SF. Human Endotoxemia: A Model for Mechanistic Insight and Therapeutic Targeting. Shock. 2005;24:94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- 57.Lee JE, Edery I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol. 2008;18(3):195–9. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paladino N, et al. Paying the circadian toll: the circadian response to LPS injection is dependent on the Toll-like receptor 4. J Neuroimmunol. 2010;225(1-2):62–7. doi: 10.1016/j.jneuroim.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Silver AC, et al. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36(2):251–61. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feillet CA, Albrecht U, Challet E. “Feeding time” for the brain: a matter of clocks. J Physiol Paris. 2006;100(5-6):252–60. doi: 10.1016/j.jphysparis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3(2):59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 62.Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16(5):581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- 63.Pollmacher T, et al. Diurnal variations in the human host response to endotoxin. J Infect Dis. 1996;174(5):1040–5. doi: 10.1093/infdis/174.5.1040. [DOI] [PubMed] [Google Scholar]
- 64.Alamili M, et al. Pronounced inflammatory response to endotoxaemia during nighttime: a randomised cross-over trial. PLoS One. 2014;9(1):e87413. doi: 10.1371/journal.pone.0087413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung CM, et al. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 2010;25(3):208–16. doi: 10.1177/0748730410368413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mavroudis PD, et al. Mathematical modeling of light mediated HPA axis activity and downstream implications on the entrainment of peripheral clock genes. Physiol Genomics. 2014 doi: 10.1152/physiolgenomics.00026.2014. in press. [DOI] [PubMed] [Google Scholar]
- 67.Mavroudis PD, et al. Systems biology of circadian-immune interactions. J Innate Immun. 2013;5(2):153–62. doi: 10.1159/000342427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haimovich B, et al. In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med. 2010;38(3):751–8. doi: 10.1097/CCM.0b013e3181cd131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cavadini G, et al. TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A. 2007;104(31):12843–8. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ingle DJ. Permissibility of hormone action; a review. Acta Endocrinol (Copenh) 1954;17(1-4):172–86. [PubMed] [Google Scholar]
- 71.Baker JB, et al. Dexamethasone modulates binding and action of epidermal growth factor in serum-free cell culture. Proc Natl Acad Sci U S A. 1978;75(4):1882–6. doi: 10.1073/pnas.75.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angeli D, Ferrell JE, Jr., Sontag ED. Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proc Natl Acad Sci U S A. 2004;101(7):1822–7. doi: 10.1073/pnas.0308265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pomerening JR, Sontag ED, Ferrell JE., Jr. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat Cell Biol. 2003;5(4):346–51. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 74.Barkai N, Leibler S. Circadian clocks limited by noise. Nature. 2000;403(6767):267–8. doi: 10.1038/35002258. [DOI] [PubMed] [Google Scholar]
- 75.Rao CV, Wolf DM, Arkin AP. Control, exploitation and tolerance of intracellular noise. Nature. 2002;420(6912):231–7. doi: 10.1038/nature01258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.