Abstract
Peyer’s patches are nodules that play a central role in intestinal immunity. Few studies demonstrate the relationship between the number of Peyer’s patches and intestinal polyps. Here we identify a statistically significant inverse correlation between the quantity of Peyer’s patches and of the development of intestinal polyps in ApcMin/+ mice, which are a useful model to clarify the role of Peyer’s patches in intestinal tumorigenesis. Using this model, we increased the number of Peyer’s patches using 0.1% and 1% corn husk arabinoxylan through feed. Intestinal polyp formation significantly decreased, concomitant with an increase in Peyer’s patches development (n = 12/group). In Aly−/−ApcMin/+ mice (negative control; no Peyer’s patches) there was no change in the amount of intestinal polyps (n = 10/group). Immune reaction following corn husk arabinoxylan treatment was measured by cytokine array. Increasing the number of Peyer’s patches decreased interleukin-17 production, which showed a dose dependent correlation with transcription factor/lymphoid enhancer-binding factor. This study identified a relationship between levels of Peyer’s patches and intestinal polyp formation, partly explained by the involvement of interleukin-17 production and β-catenin signaling in ApcMin/+ mice.
Keywords: Peyer’s patch, corn husk arabinoxylan, intestinal polyp, cancer prevention, ApcMin/+ mouse
Introduction
Colorectal cancer is the third most common malignant neoplasm worldwide and the first leading cause of cancer deaths (irrespective of gender) in Japan.(1) According to the Ministry of Health, Labour and Welfare, the colorectal cancer mortality rate has increased from 1955 to the present.(2–4) Thus, relationship between food and intestinal tumors has generated attention because it is proposed that this disease is not only caused by hereditary factors but also environmental factors like change in diet.
Nutritive and foreign substances, such as germs, simultaneously enter the intestinal tract. The gut immune system normally protects against foreign invasion.(5) Peyer’s patches (PP) are a central lymphoid organs of this immune response mechanism in the intestines, and several important reports demonstrate an association between PP and allergy, intestinal bacteria, etc.(6–9) However, few studies address whether or not PPs are linked to intestinal tumorigenesis and an underlying mechanism has not been elucidated.
The adenomatous polyposis coli (Apc) Min heterozygous knockout (ApcMin/+) mouse, lacking a functional Apc gene product, is a model of human familial adenomatous polyposis, which spontaneously generates numerous intestinal polyps.(10–12)
In this study, we observed an inverse relationship between the number of PP and intestinal polyps in ApcMin/+ mice. Therefore, we artificially increased the amount of PP in murine intestines using corn husk arabinoxylan (CHAX) as an adjuvant. We examined the effects of PP development on intestinal polyps following ingestion of CHAX in ApcMin/+ and Aly−/−ApcMin/+ mice, which have no PP in the intestine.(13) Furthermore, we tried to clarify the molecular mechanism for the prevention of polyp formation in the mice.
Materials and Methods
Mice and feed
Wild type (WT; C57B1/6J) mice and alymphoplasia knockout (Aly−/−, C57B1/6J) mice, were obtained from Clea Japan, Inc. (Tokyo, Japan). The ApcMin/+ (C57B1/6J) mice were supplied by Jackson Laboratories (Bar Harbor, Maine, State). The mice were maintained under specific pathogen-free conditions at the Animal Center of Nagasaki International University. (Nagasaki, Japan) Female ApcMin/+ and male Aly−/− mice were mated to obtain Aly+/−ApcMin/+ mice. The mouse feed was compounded with two different doses of CHAX (NIHON SHOKUHIN KAKO CO., LTD, Tokyo, Japan): 0.1 and 1% mixed (i.e., 0.2 and 2 g/kg (body weight)/day each). Preparation of CHAX is described elsewhere.(14) According to standard dietary intake guidelines provided by The Ministry of Health, Labour and Welfare, we calculated the appropriate dose for mice. We analyzed 10–12 mice in each feeding group: CHAX-free, 0.1, and 1% CHAX for each genotype (WT, ApcMin/+, and Aly−/−ApcMin/+). Approximately 100 mice were used in the ingestion experiment. Mice were sacrificed at 12, 24, and 32 weeks of age for the analysis of intestinal polyps and PP. All animal experiments were conducted according to the Guidelines for Animal Experiments in the Faculty of Pharmaceutical Sciences, Nagasaki International University.
Genotyping
Mouse tails were genotyped using a Genomic DNA Purification Kit (QIAGEN, Hilden, Germany) according to the manufacture’s protocol. Allele-specific polymerase chain reaction (PCR) primers for the Apc gene were produced according to previously published methods.(15) Forward and reverse primers for Aly−/− mice had complimentary annealing temperatures and a product length of 302 base pairs. Genomic DNA was used as a template for PCR to amplify fragments containing forward Aly_Wild 5'-CTGACATCCCGAGCTACTTCAACG, forward Aly_Mutant 5'-CTGACATCCCGAGCTACTTCAACA, and reverse 5'-GCCTAGGATCGGCCATTTTCTTCC primers using the AmpliTaq Gold 360 Master Mix (Applied Biosystems, California, CA). Touchdown PCR consisted of one cycle of 94°C for 9 min for the initial denaturation step. This was followed by 10 cycles each of denaturation at 94°C for 1 min, varying annealing conditions for 1 min, and extension at 72°C for 1 min. Annealing temperatures for the touchdown portion were as follows: starting annealing temperatures of 72°C decreasing by 0.5°C decrements per PCR cycle down to 67°C. Further 25 cycles consisted of the following: 94°C for 1 min, 68°C for 1 min, and 72°C for 1 min.
Counts of intestinal polyps and statistical analysis
Intestinal polyps and statistical analysis were examined according to previously published methods.(16) All statistical analyses were carried out using the GraphPad Prism 5 program. (GraphPad Software Inc., San Diego, CA) The correlation coefficient of the number of PP and intestinal polyps were analyzed using Pearson’s correlation coefficient. We analyzed of two-group by using Mann–Whitney t test, and three or more groups were analyzed simultaneously by using Dunnett Comparison test.
Cytokine array
Serum was collected from the heart when mice were dissected. We pooled serum samples from four WT mice fed with or without CHAX, respectively. Similarly, we pooled serum samples from ApcMin/+ or Aly−/−ApcMin/+ mice and we analyzed using Multiplex Suspension Array (Genetic Lab Corp., Hokkaido, Japan) The total number of samples was six, and the experiment was repeated two times using new samples.
Enzyme-linked immunosorbent assay (ELISA)
All serum samples were stored in a –80°C deep freezer until use. The levels of interleukin (IL)-17 were measured by IL-17 Mouse ELISA Kit (Abcam, Cambridge, UK), according to the manufacturer’s protocol. We measured fluorescence using a Model 680 Microplate Reader (Bio-Rad, Hercules, CA).
Quantitative real-time PCR analysis
All tissue samples from intestinal polyps of mice fed with or without CHAX were rapidly soaked in RNA later solution (Qiagen). Total RNA was isolated from tissue using the RNeasy Mini Kit (Qiagen). Complementary DNA (from reverse transcribed total RNA) and real-time PCR were examined according to previously published methods.(17) Primers for mouse cyclin D1 (5'primer-CCATGGAACACCAGCTCCTG and 3'primer-CGGTCCAGGTAGTTCATGGC) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 5'primer-TGTCAGCAATGCCTGCA and 3'primer-TTACTCCTTGGAGGCCATGT) were employed. The expression levels of cyclin D1 were normalized based on GAPDH levels.
Histology and immunohistochemistry
Small intestines were fixed, embedded, and sectioned as Swiss rolls for further immunohistochemical examination with the avidin–biotin complex immunoperoxidase technique and monoclonal mouse anti-β-catenin antibody (Ab; BD Transduction Laboratories, Franklin Lakes, NJ) at 100× dilution. The secondary Ab, biotinylated antimouse immunoglobulin G (Vector Laboratories, California, CA), was used at a 200× dilution. Staining was performed using avidin–biotin reagents (Vectastain ABC reagents; Vector Laboratories), 3,3'-diaminobenzidine and hydrogen peroxide, and the sections were counterstained with hematoxylin to facilitate orientation. As a negative control, consecutive sections were immunostained without exposure to the primary Ab.
Reporter assays
Caco-2 cells were plated at a concentration of 5 × 105 cells/well in 12-well plates and cultivated for 24 h. Furthermore, cells were transfected with pTOP or pFOP-Flash (Merck Millipore, Darmstadt, Germany) luciferase reporter plasmids (1.0 µg each) and Simian virus 40 (SV40)–RenillaLuc (pRL-SV40; 15:1 ratio) for 24 h. All samples were normalized for transfection efficiency using the SV40–RenillaLuc expression plasmid (Promega, Madison, WI) as a transfection control. Cells were harvested and lysed in 200 µl of lysis buffer 48 h after transfection. We measured four points using 20 µl of sample, and luciferase and Renilla luciferase activity were assayed using the Bright-Glo Luciferase Assay System and Renilla-Glo Luciferase Assay System respectively (Promega) using a GENios Microplate Reader (Tecan, Männedorf, Switzerland). Transfections were repeated three times.
After correction of measurements, we calculated the average of four measurements, and transcription factor/lymphoid enhancer-binding factor (TCF/LEF) activity was calculated by TOP divided by FOP. The relative transcriptional activity was calculated for the aforementioned value was divided by the measurement of the control cell.
Results
The number of PP correlates significantly with the number of intestinal polyps in ApcMin/+ mice over 24 weeks in age
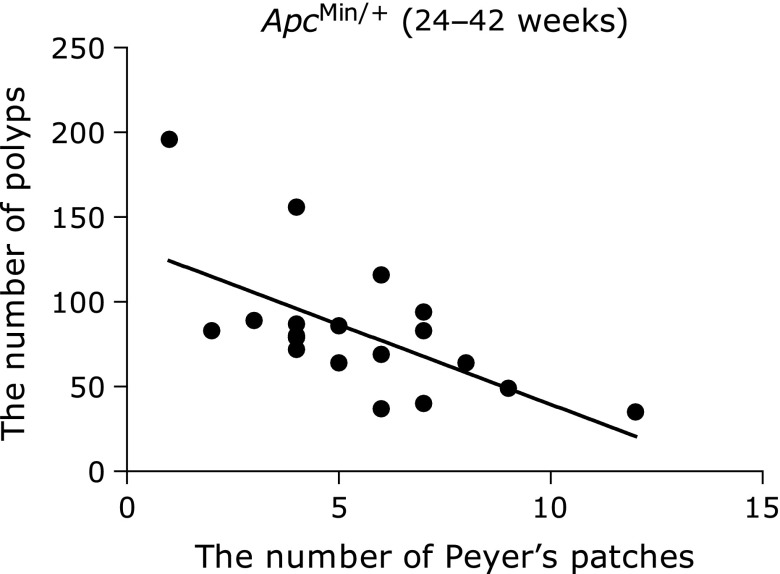
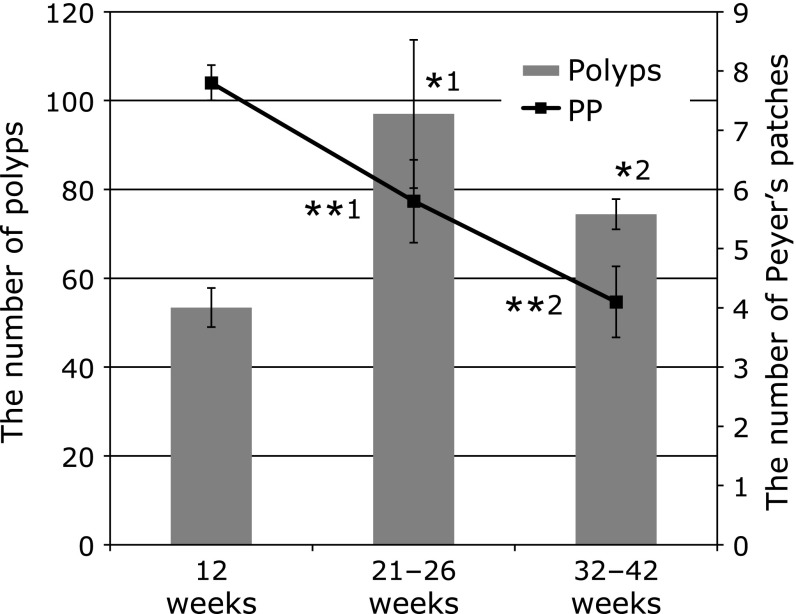
We counted the number of intestinal polyps and PP in ApcMin/+ mice >24 weeks in age. Mice that developed many intestinal polyps tended to have been fewer PP in the intestine. A statistically significant inverse relationship existed between the number of intestinal polyps and PP (r = −0.62, n = 20, p = 0.0035; Fig. 1). In addition, the amount of PP in the intestines decreased in older mice groups (Fig. 2). The quantity of PP in the intestines was significantly reduced in ApcMin/+ mice >21 weeks of age compared with 12-week-old ApcMin/+ mice. Moreover, the number of polyps significantly increased in older mice groups.
Fig. 1.
The correlation between the number of intestinal polyps and Peyer’s Patches (r = –0.62, n = 20, p = 0.0035).
Fig. 2.
The number of intestinal polyps and Peyer’s Patches according to age. The p values are *1 = 0.0385 and *2 = 0.0046 for the number of polyps. The p values are **1 = 0.0099 and **2 = 0.0004 for the number of PP (Mann–Whitney t test). n = 12 per group.
CHAX increases the number of PP in ApcMin/+ mice
It remains unclear whether or not PP suppresses polyp formation, and what factors affect the development of each condition. However, CHAX has been reported to increase the number of PP; therefore, we used this adjuvant to elucidate the effects of PP on polyp formation.
Mice were fed a diet of 0.1, 0.5, 1, or 4% CHAX to determine the optimal concentration that leads to the greatest volume of PP in WT mice. There were 7.7 ± 0.5 (n = 14) PP in 12-week-old WT mice administered 0.1% CHAX. The number significantly increased to 8.9 ± 0.5 when the mice were fed 0.5% CHAX and higher. (p = 0.0246, Mann–Whitney test) We chose the 0.1% and 1% concentrations of CHAX as optimal doses for PP formation. Furthermore, we fed 0.1% and 1% CHAX to ApcMin/+ and WT mice for 4 weeks. The number of PP significantly increased in the ApcMin/+ and WT groups (Table 1). Moreover, the number of intestinal polyps was significantly decreased in ApcMin/+ mice fed both 0.1% and 1% CHAX (Table 2). There were no significant differences between Aly−/−ApcMin/+ mice fed or not fed CHAX (Table 3). Aly−/−ApcMin/+ mice have no PP in the intestine.
Table 1.
The number of PP in ApcMin/+ mice fed CHAX
| CHAX dosage (%) | Small intestine |
|||
|---|---|---|---|---|
| Proximal | Middle | Distal | Total | |
| 0 | 2.8 ± 0.3 | 1.6 ± 0.2 | 3.4 ± 0.2 | 7.8 ± 0.3 |
| 0.1 | 3.0 ± 0.2 | 2.3 ± 0.2* | 3.9 ± 0.3 | 9.3 ± 0.3** |
| 1 | 2.9 ± 0.2 | 2.8 ± 0.3** | 3.8 ± 0.3 | 9.5 ± 0.2*** |
Values are mean ± SEM (n = 12/group). *p<0.05, **p<0.01, ***p<0.001; Mann–Whitney t test.
Table 2.
The number of intestinal polyps in 12-week-old ApcMin/+ mice
| CHAX dosage (%) | Small intestine |
Large intestine | Total | |||
|---|---|---|---|---|---|---|
| Proximal | Middle | Distal | Total | |||
| 0 | 3.9 ± 0.7 | 22.3 ± 2.2 | 27.2 ± 2.7 | 53.4 ± 4.4 | 0.5 ± 0.2 | 53.8 ± 4.3 |
| 0.1 | 2.1 ± 0.5 | 11.0 ± 1.6** | 16.2 ± 2.3* | 29.3 ± 3.8** | 0.3 ± 0.2 | 29.6 ± 3.9** |
| 1 | 3.0 ± 0.4 | 10.0 ± 1.7** | 17.9 ± 2.5* | 30.9 ± 3.3** | 0.4 ± 0.2 | 31.3 ± 3.4** |
Values are mean ± SEM (n = 12/group). *p<0.05, **p<0.01, ***p<0.001; Mann–Whitney t test.
Table 3.
The number of intestinal polyps in 12-week-old Aly−/−ApcMin/+ mice (negative control group)
| CHAX dosage (%) | Small Intestine |
Large intestine | Total | |||
|---|---|---|---|---|---|---|
| Proximal | Middle | Distal | Total | |||
| 0 | 4.3 ± 2.9 | 13.7 ± 1.6 | 11.3 ± 1.4 | 29.3 ± 2.9 | 0.2 ± 0.1 | 29.6 ± 3.0 |
| 0.1 | 2.0 ± 0.3 | 8.4 ± 1.6 | 10.6 ± 2.8 | 21.1 ± 3.7 | 0.1 ± 0.1 | 21.1 ± 3.7 |
| 1 | 3.4 ± 0.9 | 11.1 ± 3.2 | 11.3 ± 2.7 | 25.8 ± 5.9 | 0 | 25.8 ± 5.9 |
Values are mean ± SEM (n = 10/group).
Increasing the number of PP caused a decrease in IL-17 production
PP plays a central role in immunity; therefore, we examined the changes in cytokine levels in serum. We identified four cytokines, IL-1β, IL-5, IL-10, and IL-17, in which concentration levels were increased or decreased more than three-fold (Table 4). IL-1β and IL-17 levels in serum diminished when ApcMin/+ mice were fed CHAX.
Table 4.
Serum cytokine levels in WT, ApcMin/+ and Aly−/−ApcMin/+ mice fed with or without CHAX
| WT mice |
Rate of change |
ApcMin/+ mice |
Rate of change |
Aly−/−ApcMin/+ mice |
Rate of change | ||||
|---|---|---|---|---|---|---|---|---|---|
| – (without CHAX) | + (with CHAX) | – | + | – | + | ||||
| IL-1β (pg/ml) | <0.64 | <0.64 | — | 4.38 | <0.64 | 6.84 | <0.64 | <0.64 | – |
| IL-5 (pg/ml) | 10.68 | 4.37 | 2.44 | 8.58 | 7.11 | 1.21 | 22.64 | 129.94 | 5.74 |
| IL-10 (pg/ml) | 3.47 | 7.55 | 2.18 | 4.87 | 4.87 | 1 | 4.18 | 17.56 | 4.2 |
| IL-17 (pg/ml) | 3.86 | 1.05 | 3.68 | 3.24 | 0.93 | 3.48 | <0.64 | <0.64 | — |
The values are averages of extracted cytokines from double experiments of the cytokine array.
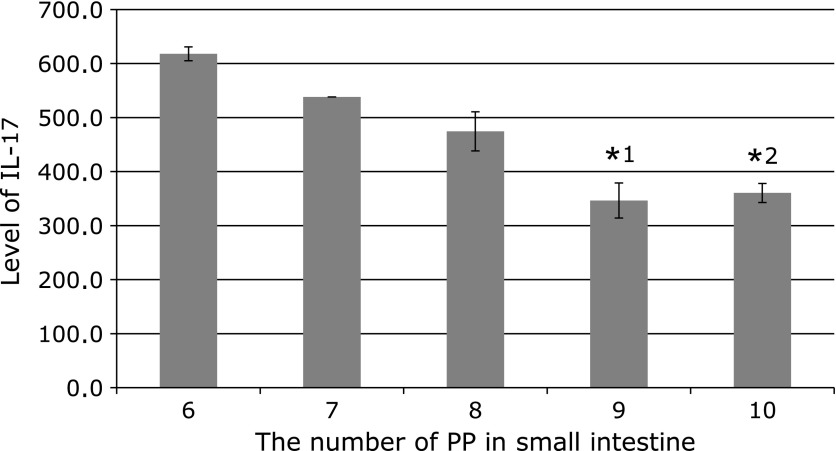
Using ELISA, we measured IL-17 concentration in the serum of ApcMin/+ mice fed or not fed CHAX based on the number of PP in the small intestine. IL-17 levels gradually decreased with increasing number of PP (Fig. 3).
Fig. 3.
The level of IL-17 in serum and the number of Peyer’s Patches in 12-week-old ApcMin/+ mice. The p values are *1 = 0.0058 and *2 = 0.0294 for IL-17 levels compared in mice which have six Peyer’s Patches (Mann–Whitney t test). n = 2, 2, 6, 8, and 5 from the left.
CHAX leads to a decrease in polyp development through the β-catenin pathway
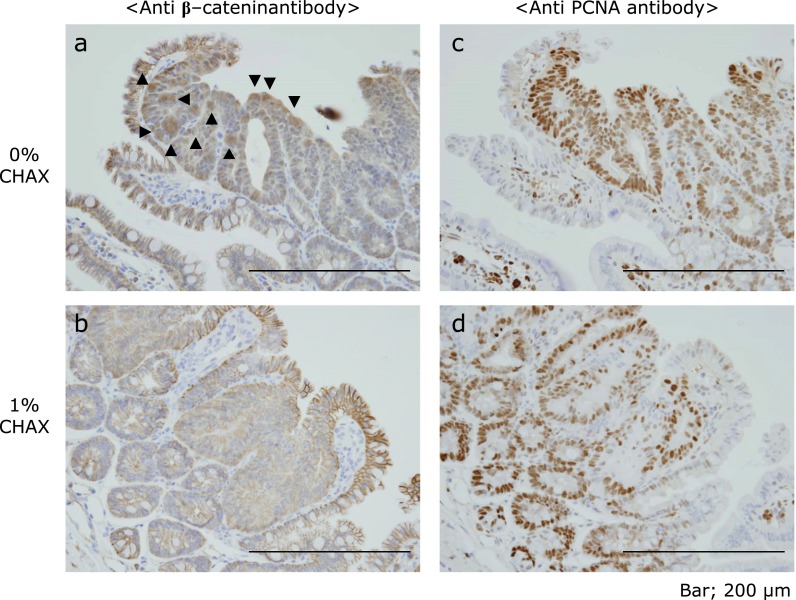
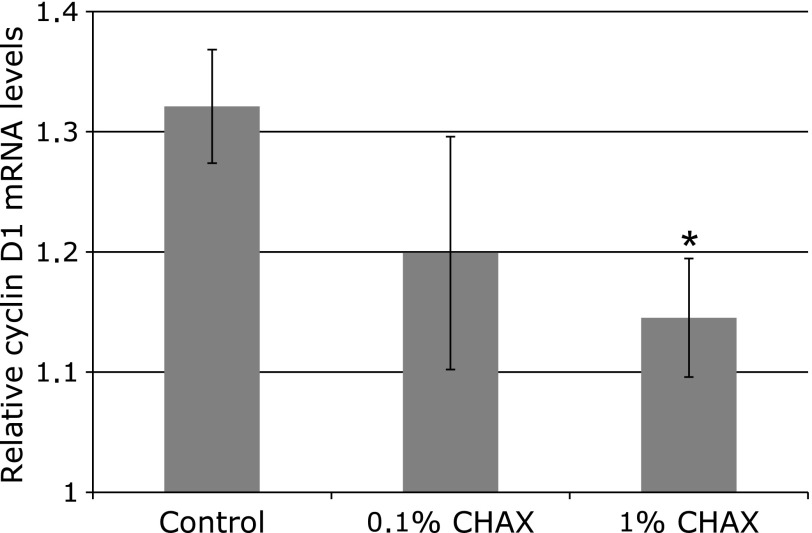
Furthermore, we examined immunohistochemical staining of paraffin-embedded intestinal polyps for β-catenin and proliferation cell nuclear antigen (PCNA). Although β-catenin is well known for accumulating in the nucleus of intestinal polyps in ApcMin/+ mice, this accrual was not observed in mice fed a 1% CHAX diet (Fig. 4a and b). In addition, the staining intensity of PCNA was weaker in mice fed 1% CHAX (Fig. 4c and d). Furthermore, we measured the messenger RNA expression level of cyclin D1, which is downstream of Wnt signaling, in intestinal polyps by quantitative real-time PCR. We found that the expression of cyclin D1 tended to decrease as CHAX concentration increased (Fig. 5).
Fig. 4.
Immunostaining of formalin-fixed paraffin-embedded polyps of the small intestine of ApcMin/+ mice fed with (b and d) or without (a and c) CHAX. ▼ shows β-catenin aggregation in the nucleus.
Fig. 5.
Quantitative real-time PCR of cyclin D1. The p value is * = 0.0426 (Mann–Whitney t test). The number of polyps used: n = 6–8, respectively. cyclin D1 mRNA levels were corrected using GAPDH as a control.
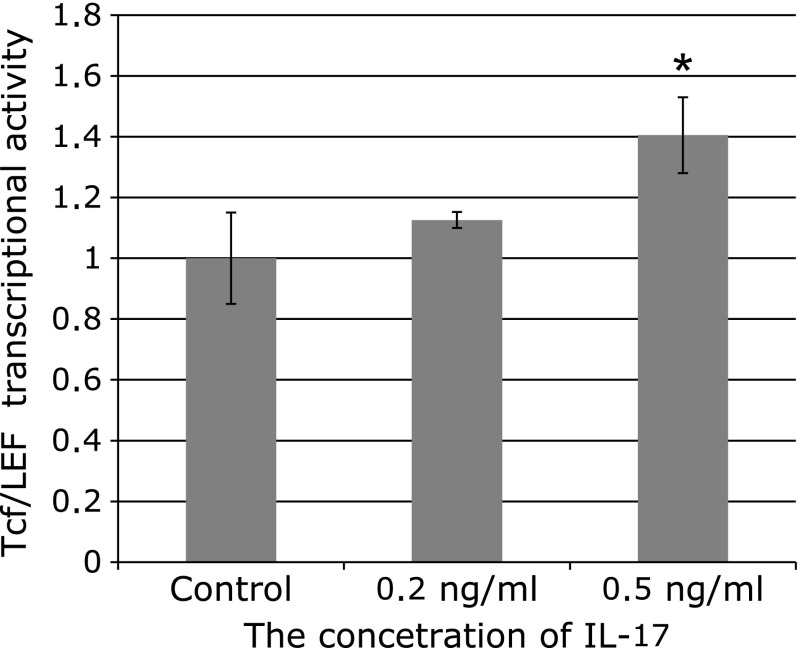
IL-17 promotes TCF/LEF transcriptional activation in vitro
We examined TCF/LEF-dependent transcriptional activity after 24-h treatment with IL-17 in Caco-2 cell transfected with pTOP-Flash or pFOP-Flash and SV40-RenillaLuc, and differentiated with 5 mM sodium butyrate (Fig. 6). Caco-2 cells are an established cell line derived from colorectal tumors with loss of APC gene function.
Fig. 6.
TCF/LEF transcriptional activity was induced by IL-17 in Caco-2 cells. The cells were stimulated with 5 mM sodium butyrate. Data are representative of at least there independent experiments. The TCF/LEF transcriptional activity was significantly increased with 0.5 ng/ml of IL-17 (*1 = p<0.001, Dunnett’s multiple comparison test).
There was no significant increase in TCF/LEF-dependent transcriptional activity with 0.2 ng/ml of IL-17, but this was observed at 0.5 ng/ml (p<0.001, Dunnett’s multiple comparison test). TCF/LEF activity was activated by IL-17 in a dose-dependent manner. The control, 0.2, and 0.5 ng/ml IL-17 values for TOP/FOP were 1 ± 0.15, 1.13 ± 0.03, and 1.40 ± 0.12, respectively.
Discussion
With continuing observation, we noticed that the number of intestinal polyps in ApcMin/+ mice varied. Usually, it is assumed that intestinal polyps appear in ApcMin/+ mice at 8 weeks of age, and we confirmed several polyps at this age. Furthermore, it is known that the number of polyps will increase with age; the life span of ApcMin/+ mouse is approximately 24 weeks because of bleeding caused anemia from intestinal polyps and other factors.(18–20) We noticed the presence of numerous PP when the mice have few polyps in the small intestine, we sought to examine the correlation between the number of polyps and PP. Previous reports have shown that the number of PP in the intestines is increased when mice are fed CHAX and/or short chain fructooligosaccharide diet.(21,22) However, there were no reports that described this change in PP levels in detail.
Our findings show that intestinal polyp formation in Aly−/−ApcMin/+ mice is lower in comparison with ApcMin/+ mice. Aly−/− mice are created by a point mutation in the tumor necrosis factor receptor associated factor (TRAF) binding domain, which is in the nuclear factor-κB (NF-κB)-inducing kinase (Nik) gene.(13) NF-κB is involved in many physiological phenomena such as acute and a chronic inflammation reactions, cell growth, and apoptosis. Furthermore, in many cases, NF-κB is activated in malignant tumors. Therefore, the decreased number of intestinal polyps in Aly−/−ApcMin/+ mice compared to ApcMin/+ mice may be because of the TRAF family, which have tumor promoting properties, not binding Nik.(23,24)
We examined changes in immunoassociated cytokines using cytokine array, because PP are critical organs in intestinal immunity and may be involved in intestinal polyp formation. In this study, we focused on IL-17, which increased the number of PP in intestine; the levels of IL-1β remained unchanged in WT mice. IL-5 and IL-10 were excluded from analysis because the changes in concentration were only observed in Aly−/−ApcMin/+ mice that do not develop PP. IL-17 is known to be produced by T helper 17 cells (Th17) cells and plays an important role in allergic responses, such as contact hypersensitivity, delayed hypersensitivity, and airway hyper-reactivity, as determined by IL-17 gene knockout mouse studies.(25) We found an inverse correlation existed between IL-17 concentration and the number of PP, suggesting a relationship between IL-17 and the number of PP. Although the reason of this phenomenon is not known in detail, Hirota et al.(26) reported that expression of IL-17 decreases when Th17 cells induce B cell formation in PP.
Short chain fatty acids, such as sodium butyrate, are mainly produced by intestinal bacteria, and are largely involved in differentiation and nutrition of intestinal epithelial cells.(27–29) Our cytokine array revealed that IL-17 levels were low (Table 4). Moreover, our results demonstrate a role for IL-17 in the enhancement of Wnt signal in the intestine. There are several reports highlighting that IL-17 is involved in intestinal polyp formation in ApcMin/+ mice or it can aggravate inflammatory bowel disease.(30–32) These findings support the hypothesis that the number of intestinal polyps is decreased due to an increase in PP in ApcMin/+ mice fed CHAX. Moreover, reduced expression of IL-17 in serum possibly causes destabilization of Wnt signaling. In the future, we propose that quantification of intestinal PP will be a useful tool for the prevention of tumorigenesis.
Acknowledgments
The authors thank K. Ogawa and Y. Kimoto (NIHON SHOKUHIN KAKO CO., LTD) for the gift of CHAX. This study was funded by a Grant-in-Aid for Challenging Exploratory Research from the Ministry of Education Culture, Sports, Science, and Technology of Japan.
Conflict of interest
No potential conflicts of interest were disclosed.
References
- 1.Center for Cancer Control and Information Services, National Cancer Center, author. Cancer Statistics in Japan-2013. http://ganjoho.jp/pro/statistics/en/backnumber/2013_en.html
- 2.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 3.Quadrilatero J, Hoffman-Goetz L. Physical activity and colon cancer. A systematic review of potential mechanisms. J Sports Med Phys Fitness. 2003;43:121–138. [PubMed] [Google Scholar]
- 4.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 5.Slack E, Balmer ML, Macpherson AJ. B cells a critical node in the microbiota-host immune system network. Immunol Rev. 2014;260:50–66. doi: 10.1111/imr.12179. [DOI] [PubMed] [Google Scholar]
- 6.Chiba Y, Shida K, Nagata S, et al. Well-controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus casei. Immunology. 2010;130:352–362. doi: 10.1111/j.1365-2567.2009.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cording S, Fleissner D, Heimesaat MM, et al. Commensal microbiota drive proliferation of conventional and Foxp3+ regulatory CD4+ T cells in mesenteric lymph nodes and Peyer’s patches. Eur J Microbiol Immunol (Bp) 2013;3:1–10. doi: 10.1556/EuJMI.3.2013.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao F, Li M, Liu Y, Gao C, Wen S, Tang L. Intestinal dysbacteriosis induces changes of T lymphocyte subpopulations in Peyer’s patches of mice and orients the immune response towards humoral immunity. Gut Pathog. 2012;4:19. doi: 10.1186/1757-4749-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebisawa M, Hase K, Takahashi D, et al. CCR6hiCD11c(int) B cells promote M-cell differentiation in Peyer’s patch. Int Immunol. 2011;23:261–269. doi: 10.1093/intimm/dxq478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- 11.Li Y, Kundu P, Seow SW, et al. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231–1238. doi: 10.1093/carcin/bgs137. [DOI] [PubMed] [Google Scholar]
- 12.Fodde R, Edelmann W, Yang K, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci U S A. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinkura R, Kitada K, Matsuda F, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa K, Takeuchi M, Nakamura N. Immunological effects of partially hydrolyzed arabinoxylan from corn husk in mice. Biosci Biotechnol Biochem. 2005;69:19–25. doi: 10.1271/bbb.69.19. [DOI] [PubMed] [Google Scholar]
- 15.Mochida Y, Taguchi K, Taniguchi S, et al. The role of P-glycoprotein in intestinal tumorigenesis: disruption of mdr1a suppresses polyp formation in ApcMin/+ mice. Carcinogenesis. 2003;24:1219–1224. doi: 10.1093/carcin/bgg073. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto K, Fujii G, Mutoh M, Yasunaga M, Tanaka H, Wada M. Suppression of intestinal polyp development in ApcMin/+ mice through inhibition of P-glycoprotein using verapamil. Eur J Cancer Prev. 2013;22:8–10. doi: 10.1097/CEJ.0b013e328353ede8. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu S, Fujii G, Takahashi M, et al. Sesamol suppresses cyclooxygenase-2 transcriptional activity in colon cancer cells and modifies intestinal polyp development in ApcMin/+ mice. J Clin Biochem Nutr. 2014;54:95–101. doi: 10.3164/jcbn.13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baltgalvis KA, Berger FG, Peña MM, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in ApcMin/+ mice. J Appl Physiol (1985) 2010;109:1155–1161. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 20.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 21.Vos AP, Knol J, Stahl B, M'Rabet L, Garssen J. Specific prebiotic oligosaccharides modulate the early phase of a murine vaccination response. Int Immunopharmacol. 2010;10:619–625. doi: 10.1016/j.intimp.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Kimoto Y, Ogawa K. The hemicellulose which induces Peyer’s Patches formation. 2008-063299 Japan Patent Kokai.
- 23.Qiao YQ, Shen J, Gu Y, et al. Gene expression of tumor necrosis factor receptor associated-factor (TRAF)-1 and TRAF-2 in inflammatory bowel disease. J Dig Dis. 2013;14:244–250. doi: 10.1111/1751-2980.12044. [DOI] [PubMed] [Google Scholar]
- 24.Nata T, Fujiya M, Ueno N, et al. MicroRNA-146b improves intestinal injury in mouse colitis by activating nuclear factor-κB and improving epithelial barrier function. J Gene Med. 2013;15:249–260. doi: 10.1002/jgm.2717. [DOI] [PubMed] [Google Scholar]
- 25.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 26.Hirota K, Turner JE, Villa M, et al. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14:994–1008. doi: 10.2174/1389200211314090006. [DOI] [PubMed] [Google Scholar]
- 28.Daroqui MC, Augenlicht LH. Transcriptional attenuation in colon carcinoma cells in response to butyrate. Cancer Prev Res (Phila) 2010;3:1292–1302. doi: 10.1158/1940-6207.CAPR-10-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmanová J, Vaculová A, Koubková Z, Hýzd'alová M, Kozubík A. Human fetal colon cells and colon cancer cells respond differently to butyrate and PUFAs. Mol Nutr Food Res. 2009;53:S102–S113. doi: 10.1002/mnfr.200800175. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Shibata M, Nishiyama H, et al. Serum levels of rapid turnover proteins are decreased and related to systemic inflammation in patients with ovarian cancer. Oncol Lett. 2014;7:373–377. doi: 10.3892/ol.2013.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chae WJ, Bothwell AL. IL-17F deficiency inhibits small intestinal tumorigenesis in ApcMin/+ mice. Biochem Biophys Res Commun. 2011;414:31–36. doi: 10.1016/j.bbrc.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Avelino MA, Wastowski IJ, Ferri RG, et al. Interleukin-17A expression in patients presenting with nasal polyposis. Braz J Otorhinolaryngol. 2013;79:616–619. doi: 10.5935/1808-8694.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]