Abstract
Fridovich identified CuZnSOD in 1969 and manganese superoxide dismutase (MnSOD) in 1973, and proposed ”the Superoxide Theory,” which postulates that superoxide (O2•−) is the origin of most reactive oxygen species (ROS) and that it undergoes a chain reaction in a cell, playing a central role in the ROS producing system. Increased oxidative stress on an organism causes damage to cells, the smallest constituent unit of an organism, which can lead to the onset of a variety of chronic diseases, such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis and other neurological diseases caused by abnormalities in biological defenses or increased intracellular reactive oxygen levels. Oxidative stress also plays a role in aging. Antioxidant systems, including non-enzyme low-molecular-weight antioxidants (such as, vitamins A, C and E, polyphenols, glutathione, and coenzyme Q10) and antioxidant enzymes, fight against oxidants in cells. Superoxide is considered to be a major factor in oxidant toxicity, and mitochondrial MnSOD enzymes constitute an essential defense against superoxide. Mitochondria are the major source of superoxide. The reaction of superoxide generated from mitochondria with nitric oxide is faster than SOD catalyzed reaction, and produces peroxynitrite. Thus, based on research conducted after Fridovich’s seminal studies, we now propose a modified superoxide theory; i.e., superoxide is the origin of reactive oxygen and nitrogen species (RONS) and, as such, causes various redox related diseases and aging.
Keywords: superoxide theory, MnSOD, mitochondria, ROS, oxidative stress diseases and aging
Introduction
Countless harmful substances contribute to environmental problems. These substances enter the food chain, destroy living environments, and even threaten the very survival of the human race. Contact between organisms and harmful substances can lead to diseases in organisms. Some harmful substances are linked to reactive oxygen generators and ultimately cause cellular damage. However, organisms possess biological defenses against these processes. Harmful substances and biological defenses battle inside cells, and these interactions can result in organisms maintaining their ”normal” status. This review focuses on biological defenses, especially antioxidant enzyme systems.
Studies have reported that reactive oxygen causes aging and a number of diseases; for example, rheumatism, hepatitis, enteritis, and carcinogenesis.(1) It is now known that many neurological diseases are caused by reactive oxygen species (ROS); for example, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS) and the like, which are caused by abnormalities in biological defenses or increased intracellular reactive oxygen levels. Increased oxidative stress on an organism causes damage to cells, the smallest constituent unit of an organism, which can lead to the onset of a variety of diseases.
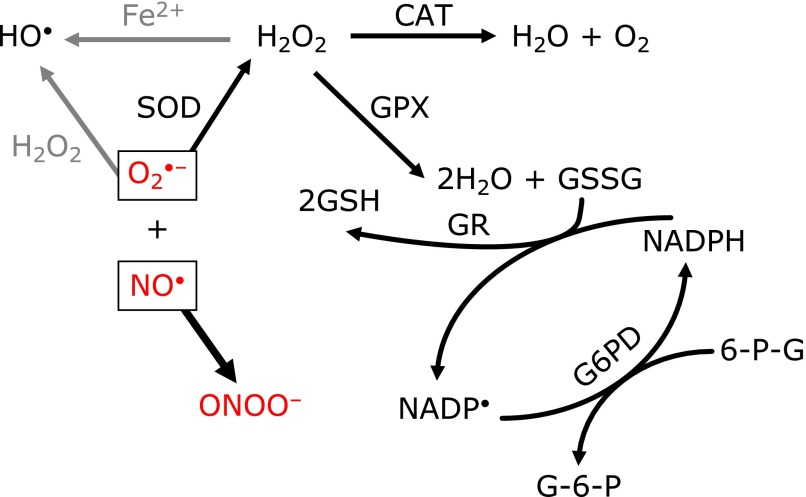
The definition of a free radical and a list of reactive oxygen species are included in Table 1.(2) Due to the emergent role of nitric oxide (NO•) in oxidative stress related diseases, reactive nitrogen cascades are sometimes included in reactive oxygen cascades. Against those oxidant reactions, biological defense systems exist in cells, including enzyme-based systems and non-enzyme-based systems, as shown in Table 2. The relationship between intracellular antioxidant systems and the functions of these systems are shown in Fig. 1.
Table 1.
Reactive oxygen species
| Free radicals: a free radical is any species capable of independent existence that contains one or more unpaired electrons. (B. Halliwell and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, 2007) | |
| Reactive Oxygen Species | |
| O2•− | superoxide |
| HO• | hydroxyl radical |
| 1O2 | singlet oxygen |
| H2O2 | hydrogen peroxide |
| ONOO− | peroxynitrite |
Table 2.
Antioxidant defense systems
| Enzymes | |
| • Mn Superoxide Dismutase (MnSOD) - Mitochondria | |
| • CuZnSOD - Cytosol | |
| • Glutathione Peroxidase - Cytosol and Mitochondria | |
| • Peroxiredoxins - Cytosol, Extracellular Space and Mitochondria | |
| Non-enzyme | |
| • Vitamins A, C, E | |
| • Glutathione | |
| • Polyphenols | |
| • Coenzyme Q10 | |
Fig. 1.
Intracellular antioxidant enzymes and their chain reactions. Superoxide (O2•−), predominantly induced from the mitochondrial electron transport chain (ETC), reacts with nitric oxide (NO•) and forms peroxynitrite (ONOO−). Peroxynitrite, a potent oxidant, then induces apoptosis or necrosis. MnSOD, which locates in mitochondria, eliminates O2•− and inhibits binding with NO•.
A New Mitochondrial Superoxide Theory
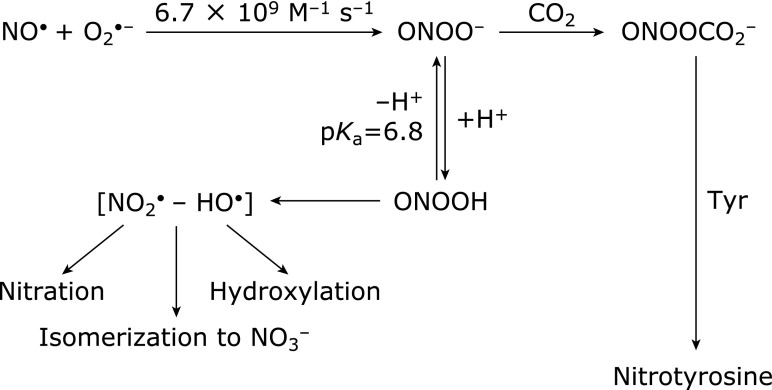
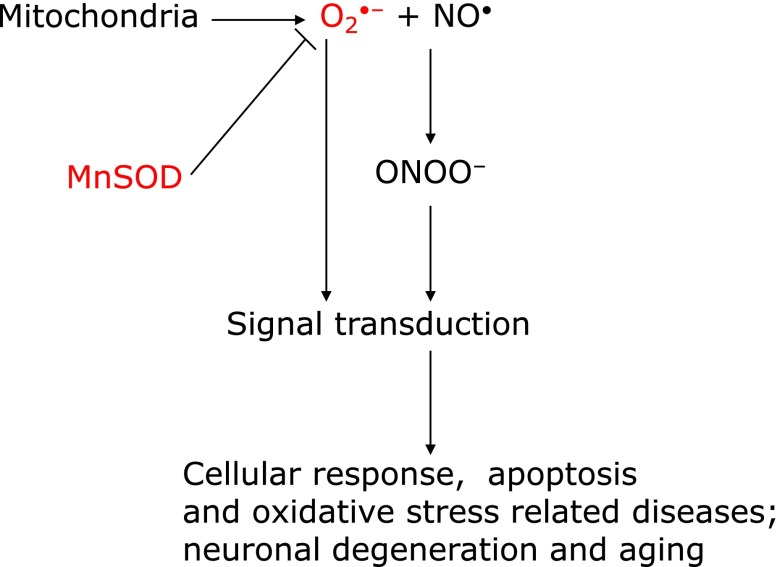
In 1969, when Fridovich discovered superoxide dismutase (CuZnSOD), an enzyme that eliminates superoxide,(3) he established that superoxide (O2•−) is an important substance that is responsible for initiating a series of ROS. The history of this discovery is shown in Table 3. Fridovich also discovered manganese superoxide dismutase (MnSOD), in 1973.(4) However, a research published approximately 10 years later showed that superoxide has low activity and poor reactivity, and concluded that the superoxide theory was invalid.(5) Rate constants for the principal radicals with ascorbic acid in ion-balanced solutions, shown in Table 4,(6) confirm that superoxide has low activity and poor reactivity. It was believed that this was also the case in bio-environments. In the same period, studies showing that nitric oxide, which plays an important role in the physiological activities of organisms, may participate in superoxide-mediated reactivity and received much attention.(7) It was eventually established that superoxide and nitric oxide readily react to form an extremely reactive substance called peroxynitrite (ONOO−) (Fig. 2).(8) In 1997, we demonstrated that cells transfected with MnSOD genes exhibited resistance to apoptotic death caused by alkaline, which is an oxidative stress condition.(9) MnSOD precursor protein has a mitochondrial targeting signal (MTS) composed of 24 amino acids that transport MnSOD protein from cytosol into the matrix of mitochondria (Fig. 3). MTS is cleaved, and then mature MnSOD protein eliminates superoxide inside the matrix of mitochondria, protecting mitochondrial DNA by inhibiting superoxide from reacting with nitric oxide (Fig. 4). These results suggest that superoxide generated from mitochondria controls subsequent oxidative reactions. As a result of this finding, we propose ”A Mitochondrial Superoxide Theory.”(10)
Table 3.
History of superoxide theory of oxygen toxicity
| • | Discovery of Superoxide dismutase (CuZnSOD); McCord JM, Fridovich I. J Biol Chem 1969; 244: 6049–6055. |
| • | Discovery of Mitochondrial Superoxide dismutase (MnSOD); Weisiger RA, Fridovich I. J Biol Chem 1973; 248: 4793–4796. |
| • | Superoxide (O2•−) is not a strong oxidant; Sawyer DT, Valentine JS. Acc Chem Res 1981; 14: 393–400. |
| • | Endothelium-derived relaxing factor (EDRF) is nitric oxide (NO); Palmer RMJ, Ferrige AG, Moncada S. Nature 1987; 327: 524–526. |
| • | Peroxynitrite (ONOO−) is a potent oxidant and nitrating agent; Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA, Proc Natl Acad U S A 1990; 87: 1620–1624. |
| • | MnSOD protects against cell death: Superoxide induction from mitochondria relates apoptosis; Majima HJ, Oberley TD, Furukawa K, Mattson MP, Yen H-C, Szweda LI, St. Clair D. J Biol Chem 1998; 273: 8217–8224. |
| • | Nobel prize laureates: Murat F, Ignarro L, Furchgott R. 1998. |
Table 4.
Second-order rate constants for the reaction of the free radicals with ascorbate
| Free Radicals | kobs (M−1s−1) |
|---|---|
| HO• | 1.1 × 1010 |
| RO• (tert-butoxy radical) | 1.6 × 109 |
| ROO• (alkylperoxyl radical) | 1–2 × 106 |
| GS• (glutathionyl radical) | 6 × 108 |
| O2•− | 1 × 105 |
Fig. 2.
Formation of peroxynitrite (ONOO−) and its further reactions. Nitrates and/or hydroxylates of proteins, lipids and DNAs, by formation of NO2• and HO• (adapted from Beckman, et al.(8)).
Fig. 3.
MnSOD has a mitochondria targeting signal (MTS). The sequence consists of an alternating pattern (amphipathic helix) composed of 24 amino acids, which includes three positively charged peptides (arginine [R]).
Fig. 4.
A Mitochondrial Superoxide Theory. The binding reaction of superoxide (O2•−) and nitric oxide (NO•) forms peroxynitrite (ONOO−) (amendment to Motoori, et al.(71)).
The Role of Superoxide Dismutase Enzymes
Enzymes scavenging superoxide are referred to as SODs (EC 1. 15. 1. 1). Three types of SODs have been identified in mammals: CuZnSOD (SOD1), MnSOD (SOD2) and ECSOD (SOD3, extracellular SOD).(3,4,11,12) The oxidation-reduction active centers of these SODs are zinc and copper for CuZnSOD and ECSOD, and manganese for MnSOD. CuZnSOD is homodimeric and present in cytoplasm. The genes of CuZnSOD are present in chromosome 21 in humans and in chromosome 16 in mice. CuZnSOD is localized in cytosol, but recent studies have shown that it is also localized inside mitochondria, in intermembrane space,(13–16) where superoxide anions are released from Complex III. SOD1 is linked to amyotrophic lateral sclerosis (ALS),(17–20) aging(21) and Alzheimer’s disease.(22) The enzyme MnSOD is located in mitochondria, and it is homotetrameric. Genes of this enzyme are present in chromosome 6 in humans and in chromosome 8 in mice. The enzyme has a mitochondrial localizing signal, comprised of 24 amino acids that target the protein to mitochondria. Upon reaching its destination, the signal part is cleaved and the mature protein becomes localized inside the mitochondria.(9,10)
ECSOD is homotetrameric and a glycosylated CuZnSOD. Genes of ECSOD are present in chromosome 4 in humans and in chromosome 5 in mice. ECSOD is found predominantly in the extracellular matrix of tissues and situated to prevent cell and tissue damage initiated by extracellularly produced ROS. ECSOD may protect against pulmonary fibrosis and other extracellular superoxide mediated diseases.(12,23,24)
Mitochondrial dysfunction has been linked to aging and a wide range of degenerative and metabolic diseases, including cancer.(25) In cells, superoxide is produced from oxygen molecules by xanthine oxidase, NADPH oxidase and mitochondrial electron transfer systems. Superoxide produced in mitochondria is generated by electrons leaking from the electron transfer system, which is located in the inner membrane of mitochondria. These electrons are then captured by molecular oxygen and become superoxide.(26–29) It is estimated that an adult at rest utilizes 3.5 ml O2/kg/minute or 352.8 L/day (assuming 70 kg body mass) or 14.7 mol/day.(30) If 1% makes superoxide, a human produces 0.147 mol/day or 53.66 moles/year or about 1.72 kg/year of superoxide. During physical exertion, this would increase up to 10-fold, assuming that the 1% still applied. Therefore, superoxide generated from mitochondria in normal cells is present in large quantities in cells. SODs eliminate these superoxides. The importance of these SODs can be seen from the results of experiments using genetic knockout mice.(31,32) Cutler reported on the correlation between SOD activity and survival time using 2 rodents and 12 primate species of maximum lifespan potentials (MLSPs) ranging from 3.5 to 95 years.(33) Recently, Page et al.(34) reported no correlation between antioxidant enzyme activities and longevity among 14 mammalian and avian species with MLSPs ranging from 3 years to over 100 years. However, they also found that MnSOD and catalase positively correlated with MLSP only for brain tissue. Although the Mitochondrial Free Radical Theory of Aging (MFRTA) remains unproven due to various complex data,(35) it seems that MnSOD could play an important role in aging. In other words, a control of ROS generated from mitochondria could be an important factor for the aging process. These reports, then, suggest that superoxide has an important effect in cells, which in turn implies that ”the superoxide theory” is still valid. The regulation of reactive oxygen has implications for many types of degenarative conditions, including aging.
Mitochondria-localized Manganese Superoxide Dismutase (MnSOD)
While CuZnSOD is present in the cytoplasm and forms a dimer, MnSOD forms a tetramer (homotetrameric), as described previously. The gene encoding MnSOD is located on chromosome 6 in humans and chromosome 8 in mice.(36) The cDNA of the MnSOD gene consists of 666 bps, the first 72 bps of which correspond to 24 amino acids MTS for translocation of the precursor protein into the mitochondrion (Fig. 3).(37) MTS is followed by the start signal codon (AUG; methionine). This mitochondrial translocation signal contains many basic amino acids and thus is a cation as a whole. Because the mitochondrial outer membrane is negatively charged, the speculation was that it electrically attracts MTS. Subsequent studies identified TOM and TIM in the mitochondrial outer and inner membranes, which were found to attract proteins into the mitochondrion.(38–40) To attract proteins into the mitochondrion, these molecules have a stable primary structure via the binding of a chaperone protein to the amino acid chain.(38–40) An MnSOD molecule attracted into the mitochondrial matrix is cleaved at the MTS portion and assumes a three-dimensional structure, which then forms a tetramer with manganese at the active center to form the mature and active form of the molecule.(38–40)
Several studies conducted in the 1970s demonstrated a leakage of electrons from some proteins present in the mitochondrial inner membrane, particularly complexes I and III, and the resulting production of superoxide.(28,29,41) However, the exact percentage of all intracellularly generated active oxygen species accounted for by mitochondria-derived superoxide remains unclear. The intracellular systems that produce superoxide include peroxidases (non-specific),(42) xanthine oxidase,(43,44) nitric oxide synthase (NOS),(45–50) aldehyde oxidase,(51,52) NADPH oxidase,(53) fumarate reductase,(54) heme proteins(55) and the mitochondrial electron transport system.(56) We have demonstrated that the mitochondrion is the most abundant source of superoxide of all these systems.(56) Generation of active oxygen (O2•−) from the mitochondrial electron transport system causes oxidative stress to the cell and subsequently induces oxidative-related diseases.(26,57,58) Although other antioxiant enzymes play important roles in mitochondria, e.g., peroxiredoxin (Prx)(59,60) and glutathione peroxidase (GPx),(61,62) as mentioned above, MnSOD, an enzyme localized in the mitochondrion, and scavenging superoxide, has several important roles.(4) The following findings of MnSOD suggest the critical role of MnSOD in the survival of aerobic life: (1) Escherichia coli and yeasts lacking the MnSOD gene are highly sensitive to oxidative stress.(63–65) (2) MnSOD gene knockout mice can only survive 10–18 days after birth, with pathological findings of cardiomyopathy, fatty liver, skeletal muscle acidosis and degeneration of neurons in the central nervous system due to mitochondrial disorder, suggesting a critical role of the enzyme.(31,32) Superoxide can directly oxidize [4Fe–4S] of aconitase, etc., to form hydrogen peroxide (H2O2) with subsequent release of Fe2+. The cluster is also in complex I, and so aconitase and complex I are inactivated in SOD2 KO mice, which is important for superoxide toxicity.(66,67) (3) Cells transfected with MnSOD cDNAs are resistant to paraquat,(68) tumor necrosis factor,(69,70) doxorubicin,(69) mitomycin C,(69) irradiation,(69,71–76) alkaline treatment,(9) hypoxic condition,(77) ischemic reperfusion,(78) smoking toxicity(79) and radiation carcinogenesis.(80) (4) Human MnSOD gene transgenic mice show reduced severity of hyperbaric oxygen-induced pulmonary damage(81) and adriamycin-induced myocardial damage.(82) Free radicals generated from mitochondria could play roles in any kind of cell death; i.e., apoptosis, necrosis and autophagy.(83,84)
How SOD Works to Remove Oxidative Stress in Mitochondria
Several hypotheses describe the important role MnSOD plays as an antioxidant.(76) Hydroxyl radicals are produced from the Fenton reaction (1) or Haber-Weiss reaction. Thus, they are formed H2O2 and superoxide (O2•−).
The Fenton reaction is defined as:
| Fe2+ + H2O2 → Fe3+ + HO• + HO− | (1) |
The Haber-Weiss reaction is initiated by
| Fe3+ + O2•− → Fe2+ + O2 | (2) |
The overall reaction is:
| O2•− + H2O2 → HO• + HO− + O2 | (3) |
Cells with more MnSOD should generate more H2O2 due to more substrate being available for reactions, and more HO• could be produced by the Fenton and Harber-Weiss reactions. However, the absence of superoxide prevents the first step of the Haber-Weiss reaction and thus HO• formation is reduced. This is consistent with the general observation that the level of ROS (HO•), subsequent lipid peroxidation and apoptosis are decreased by MnSOD overexpression. Thus, the Fenton reaction alone does not correspond with these results and does not explain the observed reduced amounts of ROS (mostly HO•) by MnSOD transfection. The excess production of H2O2 by MnSOD could be quickly detoxified by GPx by reducing it to water.(85) This reaction could be accompanied by glutathione, of which the level for most cells is ~5 mM, an excess amount for the reaction.(85)
Superoxide radicals can react with NO• to form ONOO− with a diffusion-controlled rate, because NO• has an unpaired electron.(86) ONOO− is a potent biological oxidant that has recently been implicated in diverse forms of free radical-induced tissue injury.(8,87) The reaction of ONOO− with membrane lipids induces a phospholipid membrane peroxidation product even without iron being present.(88) Various aldehydes are generated as final products when lipid hydroperoxides break down. Among them, HNE is a highly toxic nine-carbon α,β-unsaturated aldehyde that can be generated by the peroxidation of ω-6 unsaturated fatty acids, such as arachidonic acid and linoleic acid.(89,90) Peroxynitrous acid (protonated forms of ONOO−:ONOOH) subsequently produces HO• and nitrogen dioxide radicals (NO2•), resulting in oxidation and nitration, respectively.(8) Thus, MnSOD inhibits the formation of ONOO− by decreasing superoxide levels that prevent the peroxidant effects produced by ONOO− (Fig. 2 and 4).
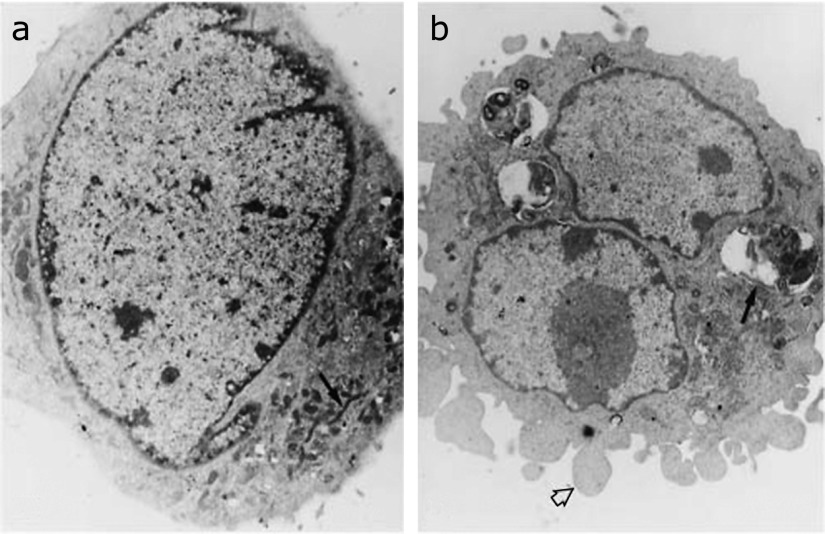
Free radicals generated from mitochondria could play a role in many kinds of cell death; i.e., apoptosis, necrosis, autophagy.(83,84) In our published paper that reported the relationship between ROS generated from mitochondria and apoptosis,(9) we showed an electron microscopic picture of mitophagy impacted by an alkaline condition, whereas MnSOD transfected cells seemed normal, as shown in Fig. 5. These findings indicate that MnSOD is essential for maintenance of life and cellular resistance to oxidative stress in the presence of oxygen, and suggest that superoxide generated from mitochondria plays an important role in oxidative stress and its related diseases, including aging.
Fig. 5.
Ultrastructural analysis of cells cultured at pH 8.3 for 6 h. Electron microscopy of (a) SOD transfected cells that appear normal with normal mitochondria (arrow) and (b) NEO cells show accumulation of lysosomes with membrane debris observed internally (arrow). The cell surface demonstrates prominent membrane blebs (arrowhead). Mitochondria show focal swelling and loss of cristae. Focal condensation of chromosomal material is present. This research was originally published in J Biol Chem., Majima HJ, Oberley TD, Furukawa K, Mattson MP, Yen H-C, Szweda LI, St Clair DK: Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem 1998; 273: 8217–8224. Reprint from ref. 9 with permission.
Conclusion
Mitochondrial ETC generates superoxide under physiological conditions, and oxidative stress increases ROS production. Mitochondria are the major source of intracellular superoxide production, and damage of mtDNA appears to damage mitochondrial DNA encoded proteins in ETC, causing more superoxide to be produced. Reducing excess amounts of mitochondria-generated superoxide seems important to protecting against oxidative stress related diseases. The reactivity of superoxide is relatively low, as shown in Table 4. However, when elevated levels of NO• are present, nitric oxide binds to mitochondrial ETC-generated superoxide. Subsequently, ONOO− is formed with the rate constant close to that of the reaction between hydroxyl radical (HO•) and ascorbic acid (Table 4, Fig. 2). Then, ONOO− produces hydroxyl radicals and nitrogen dioxide, and oxidizes and nitrates DNAs, lipids and proteins, etc., and induces apoptosis, autophagy, mitophagy and necrosis (Fig. 2 and 5). MnSOD exists in mitochondria (Fig. 3) to block the binding of mitochondrial ETC-generated superoxide with nitric oxide (Fig. 4). This theory, called ”A Mitochondrial Superoxide Theory” (Fig. 4), could explain the initiation of numerous chronic diseases, such as aging and carcinogenesis.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (No. 22592093 to H.J.M.) and the Strategic Promotion Program for Basic Nuclear Research (to H.J.M.) of the Ministry of Education, Culture, Sports, Science and Technology of Japan; and the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN) (to H.J.M.) by the Ministry of Agriculture, Forestry and Fisheries of Japan.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Halliwell B, Gutterridge JMC. Reactive species and disease: fact, fiction or filibuster? In: Halliwell B, Gutterridge JMC, editors. Free Radicals in Biology and Medicine (4th ed.) Oxford: Oxford University Press; 2007. pp. 488–613. [Google Scholar]
- 2.Halliwell B, Gutterridge JMC. Oxygen is a toxic gas—an introduction to oxygen toxicity and reactive species. In: Halliwell B, Gutterridge JMC, editors. Free Radicals in Biology and Medicine (3rd ed.) Oxford: Oxford University Press; 2007. pp. 19–21. [Google Scholar]
- 3.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 4.Weisiger RA, Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 5.Sawyer DT, Valentine JS. How super is superoxide? Acc Chem Res. 1981;14:393–400. [Google Scholar]
- 6.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 7.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 8.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majima HJ, Oberley TD, Furukawa K, et al. Prevention of mitochondrial injury by manganese superoxide dismutase reveals a primary mechanism for alkaline-induced cell death. J Biol Chem. 1998;273:8217–8224. doi: 10.1074/jbc.273.14.8217. [DOI] [PubMed] [Google Scholar]
- 10.Majima HJ, Indo HP, Suenaga S, et al. Mitochondria as Source of Free Radicals. In: Naito Y, Suematsu M, Yoshikawa T, editors. Free Radical Biology in Digestive Diseases, Front Gastrointest Res (Vol. 29) Basel: Karger; 2011. pp. 12–22. [Google Scholar]
- 11.von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol. 2000;150:1027–1036. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 14.Kawamata H, Manfredi G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid Redox Signal. 2010;13:1375–1384. doi: 10.1089/ars.2010.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki Y, Ali M, Fischer M, Riemer J. Human copper chaperone for superoxide dismutase 1 mediates its own oxidation-dependent import into mitochondria. Nat Commun. 2013;4:2430. doi: 10.1038/ncomms3430. [DOI] [PubMed] [Google Scholar]
- 16.Dröse S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 17.Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998;1366:211–223. doi: 10.1016/s0005-2728(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 18.Okado-Matsumoto A, Fridovich I. Amyotrophic lateral sclerosis: a proposed mechanism. Proc Natl Acad Sci U S A. 2002;99:9010–9014. doi: 10.1073/pnas.132260399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan W, Naniche N, Bogush A, Pedrini S, Trotti D, Pasinelli P. Small peptides against the mutant SOD1/Bcl-2 toxic mitochondrial complex restore mitochondrial function and cell viability in mutant SOD1-mediated ALS. J Neurosci. 2013;33:11588–11598. doi: 10.1523/JNEUROSCI.5385-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solsona C, Kahn TB, Badilla CL, et al. Altered thiol chemistry in human Amyotrophic Lateral Sclerosis-linked mutants of superoxide dismutase 1. J Biol Chem. 2014;289:26722–26732. doi: 10.1074/jbc.M114.565333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostrominova TY. Advanced age-related denervation and fiber-type grouping in skeletal muscle of SOD1 knockout mice. Free Radic Biol Med. 2010;49:1582–1593. doi: 10.1016/j.freeradbiomed.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Oladzad Abbasabadi A, Javanian A, Nikkhah M, Meratan AA, Ghiasi P, Nemat-Gorgani M. Disruption of mitochondrial membrane integrity induced by amyloid aggregates arising from variants of SOD1. Int J Biol Macromol. 2013;61:212–217. doi: 10.1016/j.ijbiomac.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Gao F, Kinnula VL, Myllärniemi M, Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid Redox Signal. 2008;10:343–354. doi: 10.1089/ars.2007.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47:344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace DC. Mitochondrial DNA in aging and disease. Sci Am. 1997;277:40–47. doi: 10.1038/scientificamerican0897-40. [DOI] [PubMed] [Google Scholar]
- 27.Beyer RE. An analysis of the role of coenzyme Q in free radical generation and as an antioxidant. Biochem Cell Biol. 1992;70:390–403. doi: 10.1139/o92-061. [DOI] [PubMed] [Google Scholar]
- 28.Takeshige K, Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J. 1970;180:129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Huang TT, Carlson EJ, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 32.Lebovitz RM, Zhang H, Vogel H, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolmasoff JM, Ono T, Cutler RG. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page MM, Richardson J, Wiens BE, et al. Antioxidant enzyme activities are not broadly correlated with longevity in 14 vertebrate endotherm species. Age (Dordr) 2010;32:255–270. doi: 10.1007/s11357-010-9131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanz A, Stefanatos RK. The mitochondrial free radical theory of aging: a critical view. Curr Aging Sci. 2008;1:10–21. doi: 10.2174/1874609810801010010. [DOI] [PubMed] [Google Scholar]
- 36.Church SL, Grant JW, Meese EU, Trent JM. Sublocalization of the gene encoding manganese superoxide dismutase (MnSOD/SOD2) to 6q25 by fluorescence in situ hydridization and somatic cell hybrid mapping. Genomics. 1992;14:823–825. doi: 10.1016/s0888-7543(05)80202-2. [DOI] [PubMed] [Google Scholar]
- 37.Ho YS, Crapo JD. Isolation and characterization of complementary DNAs encoding human manganese-containing superoxide dismutase. FEBS Lett. 1988;229:256–260. doi: 10.1016/0014-5793(88)81136-0. [DOI] [PubMed] [Google Scholar]
- 38.Mihara T, Onuma T. Cytoplasmic chaperons in precursor targeting to mitochondria: the role of MSF and hsp 70. Trends Cell Biol. 1996;6:104–108. doi: 10.1016/0962-8924(96)81000-2. [DOI] [PubMed] [Google Scholar]
- 39.Bohnert M, Pfanner N, van der Laan M. A dynamic machinery for import of mitochondrial precursor proteins. FEBS Lett. 2007;581:2802–2810. doi: 10.1016/j.febslet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138:628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boveris A, Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975;1:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 42.Smith AM, Morrison WL, Milham PJ. Oxidation of indole-3-acetic acid by peroxidase: involvement of reduced peroxidase and compound III with superoxide as a product. Biochemistry. 1982;21:4414–4419. doi: 10.1021/bi00261a034. [DOI] [PubMed] [Google Scholar]
- 43.Dockrell HM, Playfair JH. Killing of plasmodium yoelii by enzyme-induced products of the oxidative burst. Infect Immun. 1984;43:451–456. doi: 10.1128/iai.43.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas MJ, Mehl KS, Pryor WA. The role of superoxide in xanthine oxidase-induced autooxidation of linoleic acid. J Biol Chem. 1982;257:8343–8347. [PubMed] [Google Scholar]
- 45.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaesemeyer WH, Ogonowski AA, Jin L, Caldwell RB, Caldwell RW. Endothelial nitric oxide synthase is a site of superoxide synthesis in endothelial cells treated with glyceryl trinitrate. Br J Pharmacol. 2000;131:1019–1023. doi: 10.1038/sj.bjp.0703665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pou S, Keaton L, Surichamorn W, Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 48.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 49.Xia Y, Roman LJ, Masters BS, Zweier JL. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J Biol Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- 50.Vásquez-Vivar J, Kalyanaraman B, Martásek P, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarepour M, Simon K, Wilch M, et al. Identification of superoxide production by Arabidopsis thaliana aldehyde oxidases AAO1 and AAO3. Plant Mol Biol. 2012;80:659–671. doi: 10.1007/s11103-012-9975-1. [DOI] [PubMed] [Google Scholar]
- 52.Kundu TK, Velayutham M, Zweier JL. Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry. 2012;51:2930–2939. doi: 10.1021/bi3000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura Y, Ohtaki S, Makino R, Tanaka T, Ishimura Y. Superoxide anoin is the initial product in the hydrogen peroxide formation catalyzed by NADPH oxidase in porcine thyroid plasma membrane. J Biol Chem. 1989;264:4759–4761. [PubMed] [Google Scholar]
- 54.Imalay JA. A metabolic enzyme that rapidly produces superoxide, fumarate reductase of Escherichia coli. J Biol Chem. 1995;270:19767–19777. [PubMed] [Google Scholar]
- 55.Dershwitz M, Novak RF. Genaration of superoxide via the interaction of nitrofuration with oxyhemoglobin. J Biol Chem. 1982;257:75–79. [PubMed] [Google Scholar]
- 56.Indo HP, Davidson M, Yen H-C, et al. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2000;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 57.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 58.Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6:69–81. doi: 10.1023/a:1009676112184. [DOI] [PubMed] [Google Scholar]
- 59.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Cao Z, Lindsay JG, Isaacs NW. Mitochondrial peroxiredoxins. Subcell Biochem. 2007;44:295–315. doi: 10.1007/978-1-4020-6051-9_14. [DOI] [PubMed] [Google Scholar]
- 61.Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Margis R, Dunand C, Teixeira FK, Margis-Pinheiro M. Glutathione peroxidase family - an evolutionary overview. FEBS J. 2008;275:3959–3970. doi: 10.1111/j.1742-4658.2008.06542.x. [DOI] [PubMed] [Google Scholar]
- 63.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farr SB, D'Ari R, Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986;83:8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Loon AP, Pesold-Hurt B, Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986;83:3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- 67.Liang LP, Patel M. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson’s disease. J Neurochem. 2004;90:1076–1084. doi: 10.1111/j.1471-4159.2004.02567.x. [DOI] [PubMed] [Google Scholar]
- 68.St Clair DK, Oberley TD, Ho YS. Overproduction of human Mn-Superoxide dismutase modulates paraquat-mediated toxicity in mammalian cells. FEBS Lett. 1991;293:199–203. doi: 10.1016/0014-5793(91)81186-c. [DOI] [PubMed] [Google Scholar]
- 69.Hirose K, Longo DL, Oppenheim JJ, Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993;7:361–368. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- 70.Wong GH, Elwell JH, Oberley LW, Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 71.Motoori S, Majima HJ, Ebara M, et al. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res. 2001;61:5382–5388. [PubMed] [Google Scholar]
- 72.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich R, Mikkeisan RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 73.Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 1998;24:586–593. doi: 10.1016/s0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 74.Epperly MW, Gretton JE, Sikora CA, et al. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 75.Majima HJ, Indo HP, Tomita K, et al. Intracellular oxidative stress caused by ionizing radiation. In: Singh K, editor. Oxidative Stress, Disease and Cancer. London: Imperial College Press; 2006. pp. 61–83. [Google Scholar]
- 76.Indo HP, Inanami O, Koumura T, et al. Roles of mitochondria-generated reactive oxygen species on X-ray-induced apoptosis in a human hepatocellular carcinoma cell line, HLE. Free Radic Res. 2012;46:1029–1043. doi: 10.3109/10715762.2012.698012. [DOI] [PubMed] [Google Scholar]
- 77.Kiningham KK, Oberley TD, Lin S, Mattingly CA, St Clair DK. Overexpression of manganese superoxide dismutase protects against mitochondrial-initiated poly(ADP-ribose) polymerase-mediated cell death. FASEB J. 1999;13:1601–1610. doi: 10.1096/fasebj.13.12.1601. [DOI] [PubMed] [Google Scholar]
- 78.Hirai F, Motoori S, Kakinuma S, et al. Mitochondrial signal lacking manganese superoxide dismutase failed to prevent cell death by reoxygenation following hypoxia in a human pancreatic cancer cell line, KP4. Antioxid Redox Signal. 2004;6:523–535. doi: 10.1089/152308604773934288. [DOI] [PubMed] [Google Scholar]
- 79.St Clair DK, Jordan JA, Wan S, Gairola CG. Protective role of manganese superoxide dismutase against cigarette smoke-induced cytotoxicity. J Toxicol Environ Health. 1994;43:239–249. doi: 10.1080/15287399409531918. [DOI] [PubMed] [Google Scholar]
- 80.St Clair DK, Wan XS, Oberley TD, Muse KE, St Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinog. 1992;6:238–242. doi: 10.1002/mc.2940060404. [DOI] [PubMed] [Google Scholar]
- 81.Wispé JR, Warner BB, Clark JC, et al. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992;267:23937–23941. [PubMed] [Google Scholar]
- 82.Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98:1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majima HJ, Indo HP, Tomita K, et al. Bio-assessment of risk in long-term manned space exploration—cell death factors in space radiation and/or microgravity: a review. Biol Sci Space. 2009;23:43–53. [Google Scholar]
- 84.Majima HJ, Indo HP, Suenaga S, Matsui H, Yen HC, Ozawa T. Mitochondria as possible pharmaceutical targets for the effects of vitamin E and its homologues in oxidative stress-related diseases. Curr Pharm Des. 2011;17:2190–2195. doi: 10.2174/138161211796957490. [DOI] [PubMed] [Google Scholar]
- 85.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 86.Blough NV, Zafiriou OC. Reaction of superoxide with nitric oxide to form peroxynitrite in alkaline aqueous solution. Inorg Chem. 1985;24:3502–3504. [Google Scholar]
- 87.Keller JN, Kindy MS, Holtsberg FW, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 89.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde, and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 90.Pryor WA, Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic Biol Med. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]