Abstract
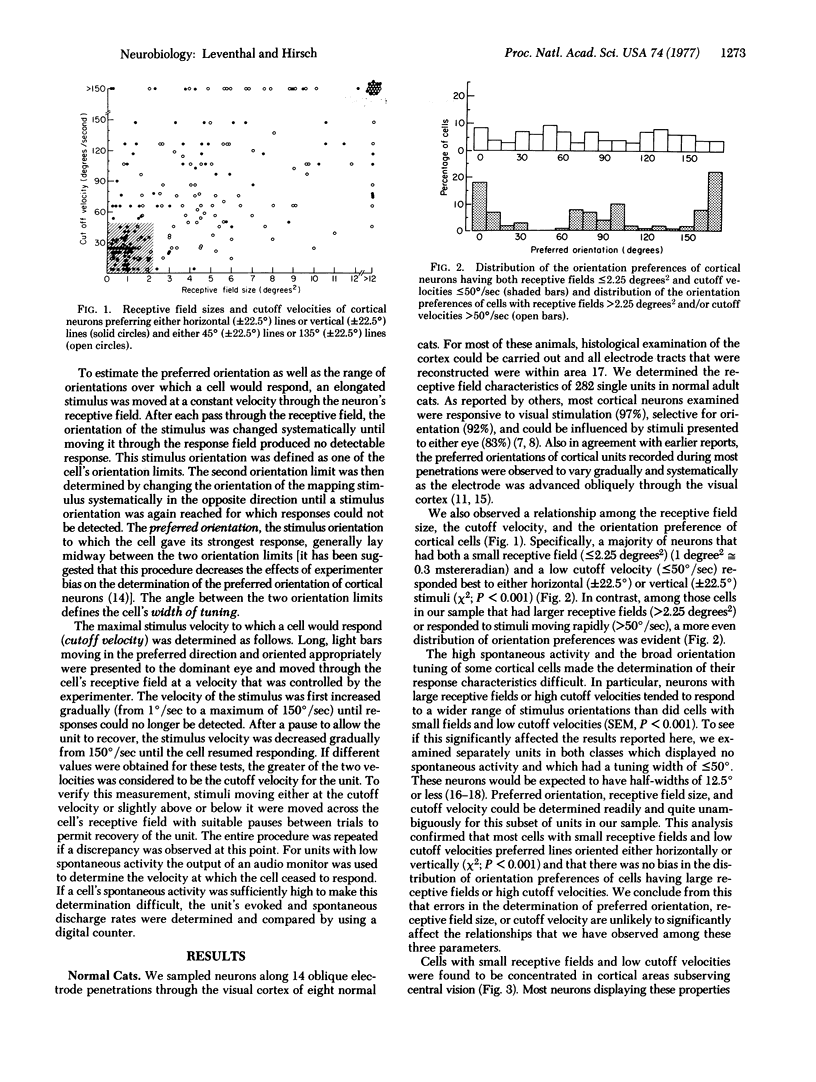
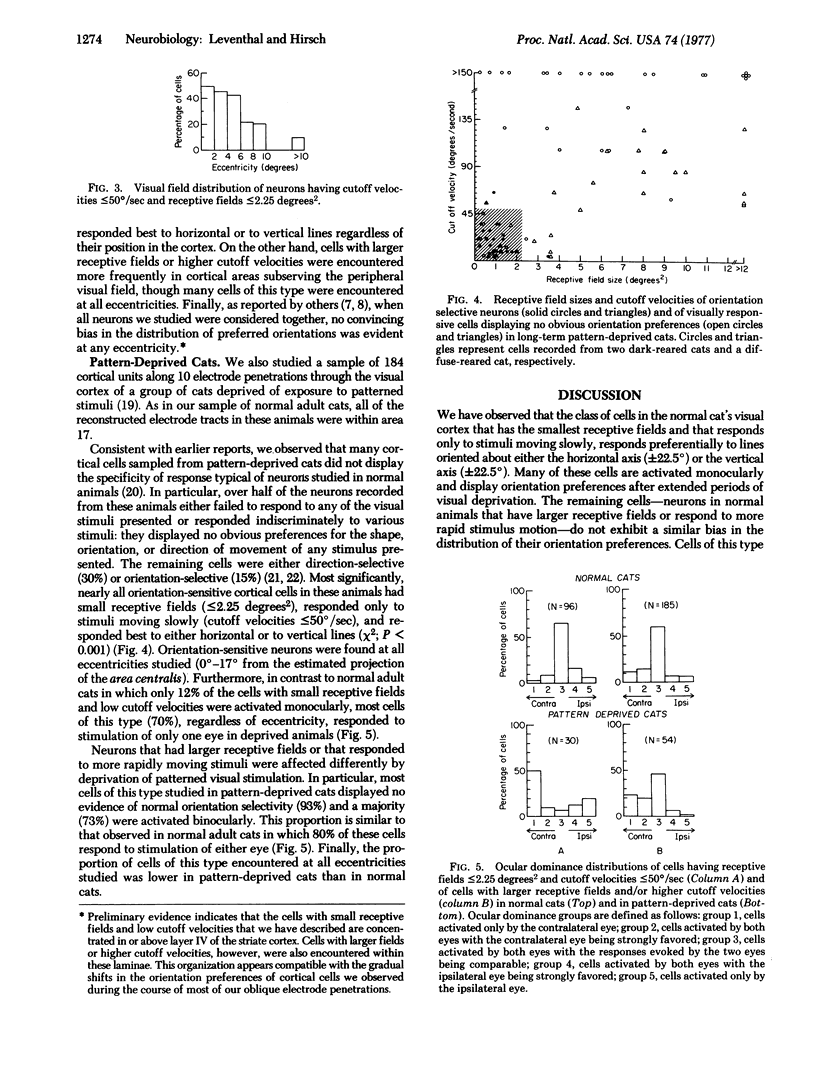
The class of neurons within the visual cortex of normal adult cats that has the smallest receptive fields (less than or equal to 2.25 degrees2) and that responds only to low rates of stimulus motion (less than or equal to 50 degrees / sec) responds preferentially to lines oriented about either the horizontal axis (+/-22.5 degrees) or the vertical axis (+/-22.5 degrees). In animals reared without exposure to patterned visual stimulation, many of these cells display orientation preferences but are activated monocularly. In contrast, in normal animals, neurons that have larger receptive fields or that respond to higher rates of stimulus motion do not exhibit a similar bias in the distribution of their orientation preferences. Cells of this type, studied in animals reared without exposure to patterned visual stimuli, are activated binocularly but do not display orientation preferences.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. I. The precision of the topography. Exp Brain Res. 1975 Dec 22;24(2):159–179. doi: 10.1007/BF00234061. [DOI] [PubMed] [Google Scholar]
- Appelle S. Perception and discrimination as a function of stimulus orientation: the "oblique effect" in man and animals. Psychol Bull. 1972 Oct;78(4):266–278. doi: 10.1037/h0033117. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley M. A., Kitterle F., Watkins D. W. Grating visibility as a function of orientation and retinal eccentricity. Vision Res. 1975 Feb;15(2):239–244. doi: 10.1016/0042-6989(75)90213-8. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Antidromic responses of orthodromically identified ganglion cells in monkey retina. J Physiol. 1969 Oct;204(2):407–419. doi: 10.1113/jphysiol.1969.sp008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968 Dec;199(3):533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Shape and arrangement of columns in cat's striate cortex. J Physiol. 1963 Mar;165:559–568. doi: 10.1113/jphysiol.1963.sp007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Tupper R. M., Dreher B. Orientation specificity and response variability of cells in the striate cortex. Vision Res. 1973 Sep;13(9):1771–1779. doi: 10.1016/0042-6989(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Sherman S. M. Effects of early binocular deprivation on visual input to cat superior colliculus. J Neurophysiol. 1975 Sep;38(5):1049–1059. doi: 10.1152/jn.1975.38.5.1049. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Sherman S. M. Effects of early monocular deprivation on visual input to cat superior colliculus. J Neurophysiol. 1974 Nov;37(6):1276–1286. doi: 10.1152/jn.1974.37.6.1276. [DOI] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Leehey S. C., Moskowitz-Cook A., Brill S., Held R. Orientational anisotropy in infant vision. Science. 1975 Nov 28;190(4217):900–902. doi: 10.1126/science.1188370. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Cortical effect of early selective exposure to diagonal lines. Science. 1975 Nov 28;190(4217):902–904. doi: 10.1126/science.1188371. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol. 1967 Feb;188(3):285–307. doi: 10.1113/jphysiol.1967.sp008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATURANA H. R., FRENK S. DIRECTIONAL MOVEMENT AND HORIZONTAL EDGE DETECTORS IN THE PIGEON RETINA. Science. 1963 Nov 15;142(3594):977–979. doi: 10.1126/science.142.3594.977. [DOI] [PubMed] [Google Scholar]
- Mansfield R. J. Neural basis of orientation perception in primate vision. Science. 1974 Dec 20;186(4169):1133–1135. doi: 10.1126/science.186.4169.1133. [DOI] [PubMed] [Google Scholar]
- Oyster C. W. The analysis of image motion by the rabbit retina. J Physiol. 1968 Dec;199(3):613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J., Olson C., Barlow H. B. Kitten visual cortex: short-term, stimulus-induced changes in connectivity. Science. 1973 Jun 15;180(4091):1202–1203. doi: 10.1126/science.180.4091.1202. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. An analysis of orientation selectivity in the cat's visual cortex. Exp Brain Res. 1974 Apr 30;20(1):1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- Sherk H., Stryker M. P. Quantitative study of cortical orientation selectivity in visually inexperienced kitten. J Neurophysiol. 1976 Jan;39(1):63–70. doi: 10.1152/jn.1976.39.1.63. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Hoffmann K. P., Stone J. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. J Neurophysiol. 1972 Jul;35(4):532–541. doi: 10.1152/jn.1972.35.4.532. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Stone J. Physiological normality of the retinal in visually deprived cats. Brain Res. 1973 Sep 28;60(1):224–230. doi: 10.1016/0006-8993(73)90861-5. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Wilson J. R., Guillery R. W. Evidence that binocular competition affects the postnatal development of Y-cells in the cat's lateral geniculate nucleus. Brain Res. 1975 Dec 19;100(2):441–444. doi: 10.1016/0006-8993(75)90498-9. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Sherk H. Modification of cortical orientation selectivity in the cat by restricted visual experience: a reexamination. Science. 1975 Nov 28;190(4217):904–906. doi: 10.1126/science.1188372. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Sherman S. M. Receptive-field characteristics of neurons in cat striate cortex: Changes with visual field eccentricity. J Neurophysiol. 1976 May;39(3):512–533. doi: 10.1152/jn.1976.39.3.512. [DOI] [PubMed] [Google Scholar]



