Abstract
This study investigated the effect and mechanism of pre-germinated brown rice (PGBR) prevented hyperglycemia in C57BL/6J mice fed high-fat-diet (HFD). Normal six-week-old mice were randomly divided into three groups. Group 1 was fed standard-regular-diet (SRD) and group 2 was fed HFD for 16 weeks. In group 3, the mice were fed a HFD with its carbohydrate replaced with PGBR for 16 weeks. Comparing the SRD and HFD groups, we found the HFD group had higher blood pressure, higher concentrations of blood glucose and HbA1c. The HFD group had less protein expression of insulin receptor (IR), insulin receptor substrate-1 (IRS-1), phosphatidylinositol-3-kinase (PI3K), glucose transporter-4 (GLUT-4) and glucokinase (GCK) and greater expression of glucogen synthase kinase (GSK) in skeletal muscle. The HFD group also had less expression of IR, serine/threonine kinase PI3K-linked protein kinase B (Akt/PKB), AMP-activated protein kinase (AMPK), GCK and peroxisome proliferator-activated receptor γ (PPARγ) in liver. In the HFD + PGBR group, the PGBR could reverse the disorders of blood pressure, blood glucose, HbA1c and increase insulin concentration. PGBR increased the IR, IRS-1, PI3K, Akt, GLUT-1 and GLUT-4 proteins, and ameliorated AMPK, GCK, GSK and PPARγ proteins. Together, PGBR prevented HFD-induced hyperglycemia through improving insulin levels, insulin receptor, glucose transporters and enhancing glucose metabolism.
Keywords: pre-germinated brown rice, hyperglycemia, insulin, glucose metabolism
Introduction
Type II diabetes mellitus (DM) increases a person’s risk of having myocardial and cerebral infarctions in highly industrialized countries.(1) This disease has genetic and environmental causes, with diet being an important environmental factor. A high-carbohydrate diet increases postprandial levels of blood glucose and insulin, and long-term consumption of diets high in carbohydrates leads to insulin resistance.(2) Insulin resistance increases risk for DM and coronary artery disease.(3,4) The recommend beneficial controlling blood glucose concentration is that supplies suitable energy intake and well-balanced daily diet.(5–7)
Food digestibility directly affects blood glucose and insulin levels.(8) White rice is an important staple carbohydrate food, but its glycemic index (GI) makes it inappropriate for frequent consumption by patients with diabetes.(9) A new type of rice, pre-germinated brown rice (PGBR), has become available in Japan and Taiwan. PGBR, which created by soaking brown rice kernels in water to slightly germinate, is considered more healthful than white rice because it is richer in dietary fiber.(10) PGBR comprised endosperm, aleurone layer, bran layer and germ were considered to have physical shape that delayed digestion and absorption of carbohydrate.(11) Moreover, PGBR contained various functional compounds such as γ-aminobutyric acid (GABA) and γ-oryzanol, which improved levels of blood glucose, lipids and peroxidation.(10–13)
Many reports had been proved that PGBR could ameliorate DM or hyperlipidemia,(10–16) however, not much research presented the biochemical mechanisms of PGBR in improving DM or hyperlipidemia. The aim of this study is to investigate the effect and action mechanism of long-term consumption of PGBR in the prevention of high fat diet (HFD)-induced hyperglycemia. We examined the mechanism on the secretion of insulin and the protein levels of insulin receptor, glucose transporters, and the enzymes involved in the metabolism of glucose.
Experimental Methods
Animals
Mice (C57BL/6J strain) were obtained by the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan) and housed under constant temperature and illumination (light between 7:30 and 19:30). Water and standard regular diet (SRD, purchased from National Laboratory Animal Breeding and Research Center, the crude fat and crude protein in SRD are soybean oil and soybean protein) were made available ad libitum. After an acclimatization period, the six-week-old mice were randomly divided into three groups. Group 1 (n = 8) was fed the SRD and group 2 (n = 8) was fed high-fat diet (HFD) for 16 weeks. The HFD was made from SRD with the addition of lard oil and cholesterol for increasing the calories and metabolic syndrome. And the whey protein was added in HFD enhancing the protein levels for the cause of similar with SRD. HFD contained 60% energy from fat, 21.4% energy from carbohydrates and 18.7% energy from protein. In group 3 (n = 8), we replaced the source of carbohydrates in HFD, which was, with PGBR (Asia RICE Biotech, Inc, Taipei, Taiwan) and fed it to the mice for 16 weeks. The levels of dietary fibers of all diets had no significant different. The nutrient compositions of diets we used in this experiment are shown in Table 1. For all groups, body weight was measured weekly, and oral glucose tolerance test (OGTT) was recorded every four weeks. At the end of the study, we collected blood for biochemical assay and excised the liver and skeletal muscle from all mice and stored these tissues in protein-lysis buffer solution at −80°C until analysis. This study was approved by the Animal Care and Use Committee of Meiho University.
Table 1.
Nutrient compositions of experimental diets
| Ingredient | SRD | HFD | HFD + PGBR |
|---|---|---|---|
| Energy (kcal/100 g) | 358 | 505 | 493.9 |
| Carbohydrates (g/100 g) | 58 | 36.6 | 37.6 |
| Crude fat (g/100 g) | 6 | 32.6 | 32.7 |
| Crude protein (g/100 g) | 18 | 16.3 | 12.3 |
| Moisture (g/100 g) | 10.5 | 8.8 | 10.8 |
| Dietary fiber (g/100 g) | 6 | 4.1 | 5.8 |
| Cholesterol (g/100 g) | — | 0.5 | 0.5 |
| Calcium (mg/100 g) | 1,000 | 680 | 115.2 |
| Iron (mg/100 g) | 100 | 68.1 | 0.6 |
| Magnesium (mg/100 g) | 250 | 170.3 | 43.5 |
| Zinc (mg/100 g) | 60 | 40.9 | 3.1 |
| Vitamin E (mg/100 g) | 15 | 10.2 | 2.1 |
| Vitamin B1 (mg/100 g) | 3 | 2.1 | 0.4 |
| Vitamin B2 (mg/100 g) | 2 | 1.4 | 0.1 |
| GABA (mg/100 g) | — | — | 9.1 |
| γ-oryzanol (µg/100 g) | — | — | 2,031 |
SRD, standard regular diet; HFD, high fat diet; HFD + PGBR, high fat diet with pre-germinated brown rice.
Measurement of blood pressure and heart rate
For all groups, blood pressure and heart rate were monitored by mice tail cuff blood pressure system: Softron BP 98-A (Softron Co., LTD, Tokyo, Japan).
Measurement of OGTT
The mice of all groups were given glucose (2 g/kg) after fasting 12 h in the last week. Blood samples were collected prior to glucose administration (0 min) and then at 30, 60 and 120 min after glucose loading. Blood glucose was measured using a glucose test strip (Glucotide, Bayer, Indianapolis, Indiana). The incremental area under the curve (IAUC) for glucose concentration was calculated according to the method described by Wolever and Jenkins.(17)
Measurement of serum biochemical parameters
Blood samples were collected to measure glucose, glycated hemoglobin (HbA1c) which was performed using a HITACHI Clinical Analyzer 7070 and insulin levels, which were performed using an ELISA kit (Mercodia, Uppsala, Sweden).
Western blot analysis of skeletal muscle tissue
Following previously described procedures,(18) the homogenized tissues were centrifuged at 12,500 × g for 30 min and the supernatants were stored at –70°C until further analysis. Aliquots of tissue homogenates were used for protein assay (Bio-Rad protein assay reagent (Bio-Rad Laboratories Taiwan Ltd, Taipei, Taiwan) and Western blot analysis. The skeletal muscle homogenates were probed for insulin receptor (IR), insulin receptor substrate-1 (IRS-1), phosphatidylinositol-3-kinase (PI3K), Akt, glucose transporter-1 (GLUT-1), GLUT-4, AMP-activated protein kinase (AMPK), glucogen synthase kinase (GSK), glucose 6-phosphatase (G6Pase), glucokinase (GCK), peroxisome proliferator-activated receptor γ (PPARγ) and phosphoenolpyruvate carboxykinase (PEPCK) antibodies (Santa Cruz Biotechnology, Paso Robles, CA; 1:500 dilution) and IgG conjugated antibody (Santa Cruz Biotechnology; 1:10000 dilution). The relative expression of those proteins in each tissue was quantified by densitometric scanning of the western blots using Image-pro plus software (Media Cybernetics, Rockville, MD).
Western blot analysis of liver tissue
The homogenized liver tissues were analyzed by IR, IRS-1, PI3K, Akt, AMPK, GSK, G6Pase, GCK, PPARγ and PEPCK antibodies (Santa Cruz Biotechnology, Santa Cruz; 1:500 dilution) and IgG conjugated antibody (Santa Cruz Biotechnology, Santa Cruz; 1:10000 dilution). The relative expression of those proteins in each tissue was quantified by densitometric scanning of the western blots using Image-pro plus software (Media Cybernetics, MD) as previously described.(18)
Statistical analysis of data
Results are expressed as mean ± SE. Statistical differences were determined by independent and paired Student’s t test in unpaired samples. If a significant difference was found, Tukey’s or Scheffe’s test was used for further analysis. A p value <0.05 was considered significant in all experiments. Analysis of data and plotting of figures was performed using SigmaStat: ver. 2.03 and SigmaPlot: ver. 8.0 (Systat Software, Point Richmond, CA).
Results
Effect of PGBR in body weight and weight gain
After 16 weeks, SRD group weight gained approximately 50.4%, and HFD group weight gained approximately 116.8%. The HFD group gained significantly more weight in total than the SRD group. In the HFD + PGBR group, weight was gained but more gradually (68.2%). Compared with the HFD group, weight gain was inhibited in the HFD + PGBR group (Table 2). Comparing the food intake per day, all groups had no significant difference in SRD group: 22.6 ± 8.4 g, in HFD group: 25.6 ± 7.6 g and in HFD + PGBR group: 26.2 ± 7.8 g, respectively.
Table 2.
Effects of experimental diets on body weight in mice every 4 weeks
| Feed time (weeks) | Body weight (g) |
||
|---|---|---|---|
| SRD (n = 8) | HFD (n = 8) | HFD + PGBR (n = 8) | |
| 0 | 16.3 ± 0.7 | 17.9 ± 0.5 | 17.2 ± 0.3 |
| 4 | 21.4 ± 1.1 | 24.8 ± 0.9# | 21.4 ± 0.8* |
| 8 | 22.1 ± 1.3 | 29.8 ± 1.9# | 23.8 ± 1.2* |
| 12 | 24.7 ± 1.3 | 34.7 ± 1.7# | 28.5 ± 2.1* |
| 16 | 24.5 ± 1.1 | 38.8 ± 1.7# | 28.9 ± 3.8* |
SRD, standard regular diet; HFD, high fat diet; HFD + PGBR, high fat diet with pre-germinated brown rice. #p<0.05 vs SRD; *p<0.05 vs HFD.
Effect of PGBR in blood pressure and heart rate
The systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean blood pressure (MBP) of HFD group were higher significantly in mice eating the HFD, compared those fed the standard regular diet. In the HFD + PGBR group, the SBP, DBP and MBP were similar with SRD group (Table 3).
Table 3.
The effects of PGBR in blood pressure and heart rate of mice fed HFD
| Measurement | SRD (n = 8) | HFD (n = 8) | HFD + PGBR (n = 8) |
|---|---|---|---|
| SBP (mmHg) | 102.4 ± 8.6 | 114.1 ± 3.8# | 90.3 ± 13.5* |
| DBP (mmHg) | 70.2 ± 9.5 | 83.8 ± 12.1# | 62.9 ± 12.9* |
| MBP (mmHg) | 80.8 ± 8.5 | 93.8 ± 8.5# | 72.1 ± 12.9* |
| HR | 576.1 ± 68.7 | 632.3 ± 34.8 | 587.1 ± 58.9 |
SRD, standard regular diet; HFD, high fat diet; HFD + PGBR, high fat diet with pre-germinated brown rice; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; HR, heart rate. #p<0.05 vs SRD; *p<0.05 vs HFD.
Effect of PGBR in OGTT
After glucose administration (oral, 2 g/kg) 0, 30, 60 and 120 min, the HFD group had a higher blood glucose levels than the SRD group. In the HFD + PGBR group, the blood glucose was recovered, suggesting that PGBR exerted a significant hypoglycemic effect in hyperglycemic mice (Table 4).
Table 4.
The effect of PGBR in OGTT and IAUC of mice fed HFD
| Measurement | SRD (n = 8) | HFD (n = 8) | HFD + PGBR (n = 8) |
|---|---|---|---|
| OGTT (mg/dl) | |||
| 0 (min) | 86 ± 11.2 | 197.3 ± 46.9# | 108 ± 5.3* |
| 30 (min) | 389 ± 47.1 | 442.3 ± 92.4 | 403 ± 96.9 |
| 60 (min) | 301 ± 42.9 | 334.5 ± 46.1 | 334 ± 77.6 |
| 120 (min) | 197 ± 28.5 | 281 ± 32.9# | 171 ± 29.1* |
| IAUC (mg*min/dl) | 32,427 ± 1,922 | 39,713 ± 3,339# | 32,820 ± 4,212* |
SRD, standard regular diet; HFD, high fat diet; HFD + PGBR, high fat diet with pre-germinated brown rice; IAUC, the incremental area under curve of the blood glucose. #p<0.05 vs SRD; *p<0.05 vs HFD.
Effect of PGBR in biochemical parameters
The HFD group had a 2.3-fold higher blood glucose level and 1.1-fold higher HbA1c level than the SRD group, though no obvious change in insulin levels was found. In the HFD + PGBR group, blood glucose and HbA1c levels were found to be obviously ameliorated. PGBR significantly increased the insulin levels.
The proteins expressions of glucose metabolism in skeletal muscle
Western blot analysis of the skeletal muscle isolated from HFD group revealed lower levels of IR (2.6-fold), IRS-1 (2.7-fold), PI3K (1.3-fold), GLUT-4 (1.2-fold) and GCK (1.7-fold) proteins, and a higher level of GSK (1.8-fold) protein, compared with the SRD group. PGBR was found to improve the expressions of those proteins. In the HFD + PGBR group, there were significant increases in IR (2.4-fold), IRS-1 (2.4-fold), PI3K (1.2-fold), Akt (1.4-fold), GLUT-1 (1.7-fold), GLUT-4 (1.3-fold) (Fig. 1), GCK (2.4-fold) and PPARγ (1.4-fold) protein levels. GSK (1.4-fold) protein level was recovered in skeletal muscle (Fig. 2). There were not obvious changes in the protein expressions of AMPK, G6Pase and PEPCK in any of the groups (data not shown).
Fig. 1.
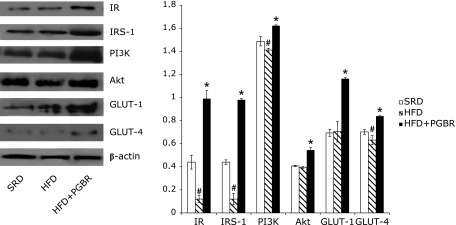
Effects of PGBR on IR, IRS-1, PI3K, Akt, GLUT-1, GLUT-4 protein expressions in skeletal muscle of high-fat diet (HFD) fed mice. Mice were fed a standard regular diet (SRD), HFD and HFD with PGBR for 16 weeks. Each value represents the mean ± SE. (n = 8). #p<0.05 vs SRD; *p<0.05 vs HFD.
Fig. 2.
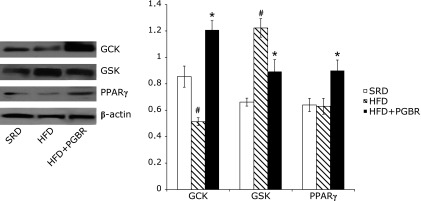
Effects of PGBR on GCK, GSK, PPARγ protein expressions in skeletal muscle of high-fat diet (HFD) fed mice. Mice were fed a standard regular diet (SRD), HFD and HFD with PGBR for 16 weeks. Each value represents the mean ± SE. (n = 8). #p<0.05 vs SRD; *p<0.05 vs HFD.
The protein expressions of glucose metabolic enzymes in liver
Fig. 3 and 4 show the expressions of IR, IRS-1, PI3K, Akt, AMPK, GCK, GSK and PPARγ proteins in the mice. Compared with SRD group, the protein expressions of the following were decreased in the HFD group: IR (10%), Akt (13%), AMPK (11%), GCK (10%) and PPARγ (10%), though expression of GSK showed no significant change. In the HFD + PGBR group, PGBR significantly increased the protein expressions of IR (30%), IRS-1 (33%), PI3K (20%), Akt (14%), AMPK (15%) (Fig. 3), GCK (7%) and PPARγ (24%), and decreased GSK (27%) (Fig. 4). There were no obvious changes in the protein expressions of G6Pase and PEPCK in all groups (data not shown).
Fig. 3.
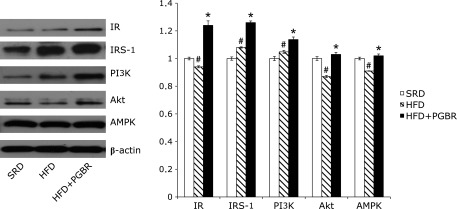
Effects of PGBR on IR, IRS-1, PI3K, Akt, AMPK protein expressions in liver of high-fat diet (HFD) fed mice. Mice were fed a standard regular diet (SRD), HFD and HFD with PGBR for 16 weeks. Each value represents the mean ± SE. (n = 8). #p<0.05 vs SRD; *p<0.05 vs HFD.
Fig. 4.
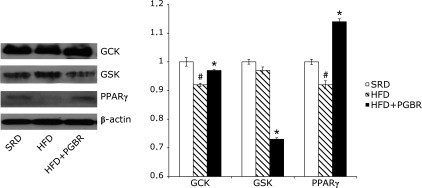
Effects of PGBR on GCK, GSK, PPARγ protein expressions in liver of high-fat diet (HFD) fed mice. Mice were fed a standard regular diet (SRD), HFD and HFD with PGBR for 16 weeks. Each value represents the mean ± SE. (n = 8). #p<0.05 vs SRD; *p<0.05 vs HFD.
Discussion
In developed countries, the cure and prevention of type II diabetes mellitus (T2DM) have become important concerns. The development of T2DM has been related with diets high in fat and sugar. Therefore, good dietary habits are helpful for decreasing the pathogenesis and mortality of T2DM.(14) PGBR, which is a new type of rice comprised of endosperm, aleurone layer, bran layer and germ, is thought to have physical shape that delays digestion and absorption of carbohydrate.(11) The present shows that consuming PGBR as a staple carbohydrate markedly decreased fasting blood glucose and HbA1c and enhanced insulin levels in hyperglycemic mice.
Based on the findings of our OGTT, which reflects current blood glucose status and insulin reaction, PGBR can improve HFD-induced glucose tolerance. Therefore, the use of PGBR as a main source of carbohydrate can efficaciously ameliorate hyperglycemia. Table 1 compares the nutrient compositions of the diets used in this experiment. The SRD had a lower proportion of calories, crude fat, and cholesterol and higher proportion of carbohydrate. HFD and HFD + PGBR had the similar ratio of calories, carbohydrate, crude fat, protein and cholesterol. Only HFD + PGBR had γ-aminobutyric acid (GABA) and γ-oryzanol. Although, the dietary fiber of SRD and HFD + PGBR was slightly higher than that of HFD, we do not believe that this slight increase in higher dietary fiber had an obvious effect in the regulation of weight, blood glucose and blood pressure. GABA and γ-oryzanol which are markedly enhanced in PGBR,(10,13,15) regulates blood pressure, affects nervous system as neurotransmitter, and potentiates insulin secretion from the pancreases.(16,19,20) The current study also found GABA and γ-oryzanol to be higher in PGBR (Table 1). The mice that ate HFD + PGBR in our study had less weight gain and lower blood glucose and blood pressure (Table 2–5), suggesting that the GABA and γ-oryzanol of PGBR can help regulate HFD-induced disorders. To examine the hypoglycemic effects of PGBR extracts will be our next challenges.
Table 5.
The effects of PGBR in blood glucose, HbA1c and insulin levels of mice fed HFD
| Measurement | SRD (n = 8) | HFD (n = 8) | HFD + PGBR (n = 8) |
|---|---|---|---|
| Blood glucose (mg/dl) | 91 ± 9.5 | 202.7 ± 42.7# | 108.3 ± 6.1* |
| HbA1c (%) | 3.9 ± 0.2 | 4.5 ± 0.1# | 4.2 ± 0.1* |
| Insulin (ng/ml) | 0.043 ± 0.005 | 0.056 ± 0.002 | 0.115 ± 0.03#,* |
SRD, standard regular diet; HFD, high fat diet; HFD + PGBR, high fat diet with pre-germinated brown rice. #p<0.05 vs SRD; *p<0.05 vs HFD.
A significant metabolic abnormality in DM is the diminished ability of insulin-sensitive tissues to take up and metabolize glucose.(21) Skeletal muscle is the primary site of insulin-stimulated glucose disposal and has been suggested to be the major tissue responsible for postprandial hyperglycemia in insulin-resistant subjects.(22) It is likely that the impaired insulin action associated with DM alters the expression or function of proteins which are components of the insulin receptor (IR) signing pathway and the glucose transport system.(23,24) Insulin action is limited through hormone binding to cell surface insulin receptor, which triggers a cascade of intracellular phosphorylation. Insulin binding to the extracellular subunit stimulates the tyrosine kinase, and the kinase induces the phosphorylation of nonreceptor proteins, including insulin receptor substrate (IRS). IRS stimulates phosphatidyl-inositol 3-kinase (PI 3-kinase), and serine/threonine kinase PI3K-linked protein kinase B (Akt/PKB) activity,(25–27) and then activates the glucose transporter (GLUT) to uptake glucose into cell.(28) GLUT-1 is the basal glucose transporter isoform for glucose transporter, and GLUT-4, an insulin-sensitive isoform, is only expressed in insulin-responsive cells.(29–31) Although the insulin levels of HFD group had no significant different with SRD group, the protein expressions of IR, IRS-1, PI3K, Akt and GLUT-4 were reduced by hyperglycemia in the HFD group. We suggested that the course of insulin resistance in this animal model was not induced yet. However, PGBR significantly increased insulin levels, and the expressions of IR, IRS-1, PI3K, Akt, GLUT-1 and GLUT-4 in the group whose HFD had been modified by the replacement with of the original carbohydrate with PGBR (Fig. 1 and 3). Based on our results, PGBR was able to ameliorate hyperglycemia-induced insulin related proteins reducing and increase the glucose uptake into the cells thereby regulating blood glucose levels.
T2DM is a metabolic disease characterized by chronic hyperglycemia caused by abnormal glucose use in skeletal muscle(32) or increased hepatic glucose production.(33,34) AMP-activated protein kinase (AMPK), glucokinase (GCK), peroxisome proliferator-activated receptor γ (PPARγ) and glycogen synthase kinase (GSK) are related to the metabolism of glucose. AMPK is reduced in the skeletal muscle of obese and type 2 diabetic humans and rodents, and it may be a potential target for the treatment of obesity induced insulin resistance. Pharmacological activation of AMPK promotes glucose uptake and insulin sensitivity.(35) Glucokinase (GCK) is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate, an important preparer of glucose for glycolysis and glycogen synthesis.(36) Peroxisome proliferator-activated receptor γ (PPARγ) has been identified as a key regulator of adipocyte differentiation and lipid metabolism, and its role in regulating glucose homeostasis has been established.(37) An increase in the expression of PPAR-γ increases the ability of insulin to mediate tissue glucose uptake and maintain glucose homeostasis.(38) Glycogen synthase kinase (GSK) is initially characterized as a critical enzyme involved in glycogen biosynthesis. Enhanced GSK activation decreases glycogen production, and its inhibitors have also shown promise in the treatment of T2DM.(39,40) In our results, HFD-induced hyperglycemia reduced the protein expressions of AMPK, GCK and PPARγ and increased GSK. PGBR ameliorated the protein expressions of AMPK, GCK, PPARγ and GSK (Fig. 2, 3 and 4). We suggest that PGBR improved HFD-induced hyperglycemia by increasing AMPK and PPARγ expressions to enhance insulin-mediated glucose uptake and glucose homeostasis. At the same time, PGBR increased GCK and inhibited GSK to enhance glycolysis and glycogen synthesis.
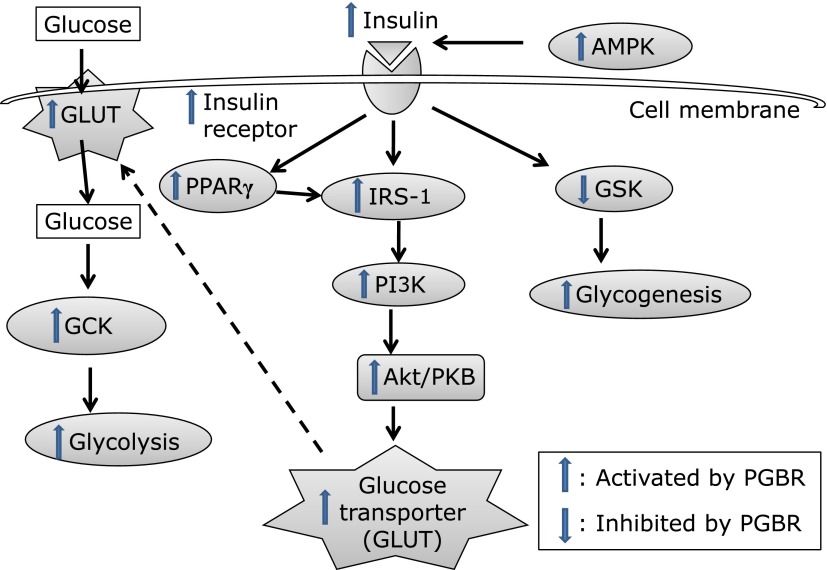
In conclusion, PGBR lowers blood glucose and blood pressure and improves glucose tolerance in mice with HFD-induced hyperglycemia. Moreover, PGBR recovered the protein expressions of IR, IRS-1, PI3K, Akt, GLUT-1, GLUT-4, AMPK, GCK, PPARγ and GSK. Based on our results, PGBR ameliorated hyperglycemia by increasing insulin secretion, insulin receptor levels, enhancing glucose to transport into cell and glucose metabolism. PGBR was able to increase insulin secretion, insulin receptor expression, glucose uptake, and glycolysis, glycogenesis (Fig. 5). Taken together, these findings suggest that PGBR might be used to help control HFD-induced metabolic syndrome, especially in those who often use rice as a staple food.
Fig. 5.
Hypothetical mechanisms of PGBR in HFD-induced hyperglycemia. The action mechanisms of PGBR ameliorating hyperglycemia occurred through increases in insulin secretion, insulin receptor levels and enhancement of glucose to transport into cell and glucose metabolisms. The protein levels of IR, IRS-1, PI3K, Akt, GLUT-1, GLUT-4, AMPK, GCK, PPARγ and GSK in skeletal muscle and liver were recovered by PGBR. Taken together, PGBR was able to increase insulin secretion, insulin receptor sensitivity, glucose uptake, and glycolysis, glycogenesis.
Acknowledgments
This work was supported by grants 100-FI-DFN-IAC-R-007 to Dr. Kuo-Ping Shen and Dr. Hui-Li Lin from Meiho University, Pingtung, Taiwan.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lin YC, Yang CC, Chen YJ, Peng WC, Li CY, Hwu CM. Utilization of statins and aspirin among patients with diabetes and hyperlipidemia: Taiwan, 1998–2006. J Chin Med Assoc. 2012;75:567–572. doi: 10.1016/j.jcma.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Ginter E, Simko V. Global prevalence and future of diabetes mellitus. Adv Exp Med Biol. 2012;771:35–41. doi: 10.1007/978-1-4614-5441-0_5. [DOI] [PubMed] [Google Scholar]
- 3.Hong JH, Cha YS, Rhee SJ. Effects of the cellcultured Acanthopanax senticosus extract on antioxidative defense system and membrane fluidity in the liver of type 2 diabetes mouse. J Clin Biochem Nutr. 2009;45:101–109. doi: 10.3164/jcbn.08-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou CH, Tsai WC, Wang MC, et al. Effects of deranged glucose homeostasis on peripheral arterial stiffness index in patients with pre-diabetes mellitus. Int Heart J. 2013;54:27–32. doi: 10.1536/ihj.54.27. [DOI] [PubMed] [Google Scholar]
- 5.Bohdjalian A, Aviv R, Prager G, et al. Gastric stimulation in the digestive period modifies length and contractility of the inter-digestive period in obese non-diabetic and diabetic subjects. Obes Surg. 2012;22:1465–1472. doi: 10.1007/s11695-012-0703-3. [DOI] [PubMed] [Google Scholar]
- 6.Siew ED, Ikizler TA. Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial. 2010;23:378–382. doi: 10.1111/j.1525-139X.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- 7.Faulkner MS, Chao WH, Kamath SK, et al. Total homocysteine, diet, and lipid profiles in type 1 and type 2 diabetic and nondiabetic adolescents. J Cardiovasc Nurs. 2006;21:47–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Wanapat M, Pilajun R, Rowlinson P. Effect of carbohydrate source and cottonseed meal level in the concentrate: IV. Feed intake, rumen fermentation and milk production in milking cows. Trop Anim Health Prod. 2013;45:447–453. doi: 10.1007/s11250-012-0238-6. [DOI] [PubMed] [Google Scholar]
- 9.Komindr S, Ingsriswang S, Lerdvuthisopon N, Boontawee A. Effect of long-term intake of Asian food with different glycemic indices on diabetic control and protein conservation in type 2 diabetic patients. J Med Assoc Thai. 2001;84:85–97. [PubMed] [Google Scholar]
- 10.Hsu TF, Kise M, Wang MF, et al. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J Nutr Sci Vitaminol. 2008;54:163–168. doi: 10.3177/jnsv.54.163. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Mizukuchi A, Kise M, et al. Postprandial blood glucose and insulin responses to pre-germinated brown rice in healthy subjects. J Med Invest. 2005;52:159–164. doi: 10.2152/jmi.52.159. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara H, Seki T, Ariga T. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2004;68:444–447. doi: 10.1271/bbb.68.444. [DOI] [PubMed] [Google Scholar]
- 13.Roohinejad S, Omidizadeh A, Mirhosseini H, et al. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J Sci Food Agric. 2010;90:245–251. doi: 10.1002/jsfa.3803. [DOI] [PubMed] [Google Scholar]
- 14.Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev. 2013;71:433–440. doi: 10.1111/nure.12038. [DOI] [PubMed] [Google Scholar]
- 15.Imam MU, Ismail M, Ithnin H, Tubesha Z, Omar AR. Effects of germinated brown rice and its bioactive compounds on the expression of the peroxisome proliferator-activated receptor gamma gene. Nutrients. 2013;5:468–477. doi: 10.3390/nu5020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imam MU, Azmi NH, Bhanger MI, Ismail N, Ismail M. Antidiabetic properties of germinated brown rice: a systematic review. Evid Based Complement Alternat Med. 2012;2012:816501. doi: 10.1155/2012/816501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–172. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 18.Lin HL, Shen KP, Chang WT, et al. Eugenosedin-A prevents high fat diet increased adhesion molecules through inhibition of MAPKs and p65 mediated NF-κB pathway in rat model. J Pharm Pharmacol. 2013;65:300–309. doi: 10.1111/j.2042-7158.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 19.Taneera J, Jin Z, Jin Y, et al. γ-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia. 2012;55:1985–1994. doi: 10.1007/s00125-012-2548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendt A, Birnir B, Buschard K, et al. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53:1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- 21.Satoh H, Audrey Nguyen MT, Kudoh A, Watanabe T. Yacon diet (Smallanthus sonchifolius, Asteraceae) improves hepatic insulin resistance via reducing Trb3 expression in Zucker fa/fa rats. Nutr Diabetes. 2013;3:e70. doi: 10.1038/nutd.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPherron AC, Guo T, Bond ND, Gavrilova O. Increasing muscle mass to improve metabolism. Adipocyte. 2013;2:92–98. doi: 10.4161/adip.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N, Chai W, Zhao L, Tao L, Cao W, Liu Z. Losartan increases muscle insulin delivery and rescues insulin’s metabolic action during lipid infusion via microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304:E538–E545. doi: 10.1152/ajpendo.00537.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukahara T, Haniu H, Matsuda Y. Effect of alkyl glycerophosphate on the activation of peroxisome proliferator-activated receptor gamma and glucose uptake in C2C12 cells. Biochem Biophys Res Commun. 2013;433:281–285. doi: 10.1016/j.bbrc.2013.02.101. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, Hakuno F, Northcott P, Pessin JE, Rozakis Adcock M. Nexilin, a cardiomyopathy-associated F-actin binding protein, binds and regulates IRS1 signaling in skeletal muscle cells. PLoS One. 2013;8:e55634. doi: 10.1371/journal.pone.0055634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WY, Lee JJ, Kim IS, Kim Y, Park JS, Myung CS. 7-O-methylaromadendrin stimulates glucose uptake and improves insulin resistance in vitro. Biol Pharm Bull. 2010;33:1494–1499. doi: 10.1248/bpb.33.1494. [DOI] [PubMed] [Google Scholar]
- 27.Chappell DS, Patel NA, Jiang K, et al. Functional involvement of protein kinase C-betaII and its substrate, myristoylated alanine-rich C-kinase substrate (MARCKS), in insulin-stimulated glucose transport in L6 rat skeletal muscle cells. Diabetologia. 2009;52:901–911. doi: 10.1007/s00125-009-1298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma CJ, Nie AF, Zhang ZJ, et al. Genipin stimulates glucose transport in C2C12 myotubes via an IRS-1 and calcium-dependent mechanism. J Endocrinol. 2013;216:353–362. doi: 10.1530/JOE-11-0473. [DOI] [PubMed] [Google Scholar]
- 29.Osorio-Fuentealba C, Contreras-Ferrat AE, Altamirano F, et al. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes. 2013;62:1519–1526. doi: 10.2337/db12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong KW, Hsu A, Tan BK. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7:e32718. doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temofonte N, Sajan MP, Nimal S, et al. Combined thiazolidinedione-metformin treatment synergistically improves insulin signalling to insulin receptor substrate-1-dependent phosphatidylinositol 3-kinase, atypical protein kinase C and protein kinase B/Akt in human diabetic muscle. Diabetologia. 2009;52:60–64. doi: 10.1007/s00125-008-1180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oak S, Tran C, Castillo MO, Thamotharan S, Thamotharan M, Devaskar SU. Peroxisome proliferator-activated receptor-gamma agonist improves skeletal muscle insulin signaling in the pregestational intrauterine growth-restricted rat offspring. Am J Physiol Endocrinol Metab. 2009;297:E514–E524. doi: 10.1152/ajpendo.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Y, Ho G, Li Y, et al. Beneficial metabolic effects of CB1R anti-sense oligonucleotide treatment in diet-induced obese AKR/J mice. PLoS One. 2012;7:e42134. doi: 10.1371/journal.pone.0042134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol. 2012;142:65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Viollet B, Andreelli F. AMP-activated protein kinase and metabolic control. Handb Exp Pharmacol. 2011;203:303–330. doi: 10.1007/978-3-642-17214-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shukla K, Dikshit P, Shukla R, Gambhir JK. The aqueous extract of Withania coagulans fruit partially reverses nicotinamide/streptozotocin-induced diabetes mellitus in rats. J Med Food. 2012;15:718–725. doi: 10.1089/jmf.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui AM, Cui X, Wu R, et al. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-γ. Crit Care Med. 2006;34:1874–1882. doi: 10.1097/01.CCM.0000221921.71300.BF. [DOI] [PubMed] [Google Scholar]
- 38.Park SH, Ko SK, Chung SH. Euonymus alatus prevents the hyperglycemia and hyperlipidemia induced by high-fat diet in ICR mice. J Ethnopharmacol. 2005;102:326–335. doi: 10.1016/j.jep.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 39.Zhu T, Zhao R, Zhang L, Bernier M, Liu J. Pyrrolidine dithiocarbamate enhances hepatic glycogen synthesis and reduces FoxO1-mediated gene transcription in type 2 diabetic rats. Am J Physiol Endocrinol Metab. 2012;302:E409–E416. doi: 10.1152/ajpendo.00453.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav HN, Singh M, Sharma PL. Involvement of GSK-3β in attenuation of the cardioprotective effect of ischemic preconditioning in diabetic rat heart. Mol Cell Biochem. 2010;343:75–81. doi: 10.1007/s11010-010-0500-z. [DOI] [PubMed] [Google Scholar]