Abstract
Hyaluronan (HA) has been increasingly used as a dietary supplement to improve the skin. However, the effect of ingested HA may depend on its molecular weight (MW) because its physiological activities in the body vary with its MW. In this study, we examined the effects of ingested HA with varying MW on the skin. In this randomized, double blind, placebo controlled study, 61 subjects with dry skin received oral HA (120 mg/day), of MWs 800 k and 300 k or placebo, for 6 weeks. The skin moisture contents of the first two groups increased more than those of the placebo group during the ingestion period. In addition, group HA 300 k exhibited significant improvements in skin moisture content 2 weeks after ingestion ended compared with the placebo group. A questionnaire survey about subjective facial aging symptoms showed that the HA treated groups exhibited significantly improved the skin condition compared with the placebo treated group. Furthermore, dermatologists objectively evaluated the clinical symptoms of the facial and whole body skin, showing that no adverse events were related to daily ingestion of HA. This study shows that both of ingesting HAs (MWs 800 k and 300 k) improved the skin condition by increasing the moisture content.
Keywords: hyaluronan, molecular weight, ingestion, skin moisture content, dry skin
Introduction
The skin is the largest organ of the body, and it acts as a barrier protecting against exogenous factors and preventing water loss from the body. Diseases such as atopic dermatitis, which is characterized by skin dryness and intense itching, are associated with reductions in this barrier function.(1,2) In addition to marked decreases in its barrier function, early symptoms of dry skin are also caused by age-related declines in intracellular lipids and natural moisturizing factors such as free amino acids and specific salts in the stratum corneum.(3,4) Other factors that cause dry skin include extrinsic stimuli such as a sudden change in the weather and contact with chemical agents such as soap, detergents and cosmetics.(5–8) Dry skin reduces a person’s quality of life not only because of itching but also the discomfort associated with tight and dry skin. Moreover, dry skin also contributes to the formation of fine wrinkles. Therefore, many people use cosmetics and dietary supplements to moisturize their skin and to improve their skin condition.
Hyaluronan (HA) is an anionic linear glycosaminoglycan comprising repeated polymeric disaccharides of d-glucuronic acid and N-acetyl-d-glucosamine, which are linked via alternating β-1,4 and β-1,3 glycosidic bonds. The molecular weight (MW) of HA is over 107, and thus it is the largest macromolecular polymer in the body.(9) HA has high viscosity because it can incorporate a large volume of water,(10) and it forms stable tertiary structures in aqueous solution.(11)
HAs are found in various tissues and organs in the body, but more than 50% of the total body HA is present in the skin,(12,13) where it plays a major role in maintaining extracellular spaces, preserving tissue hydration, and facilitating the transport of ion solutes and nutrients to cells in the upper layer because of its water-retaining capacity.(14) Therefore, HA is important for maintaining healthy skin.
Recently, HA has been used in cosmetics and foods with the aim of improving skin condition. The topical application of all HAs with MWs from 50 k to 200 k moisturizes the skin.(15) Furthermore, HA is used increasingly as a dietary supplement as well as a food additive. However, the effects of HA ingestion on the skin have been rarely studied outside Japan. Thus, we evaluated the clinical efficacy of HA ingestion in subjects with dry skin. The physiological activities of HA differ according to its MW,(16–19) and thus we also studied the effects obtained using HA with MWs of 800 k and 300 k.
Materials and Methods
Supplements and dosage
The two types of HA, Hyaluronsan HA-F and Hyabest® (S) LF-P, used in this study were manufactured by Kewpie Corporation (Tokyo, Japan) and had a 95% purity according to HPLC and the mean MWs were approximately 800 k and 300 k, respectively. Microcrystalline cellulose and hydrogenated maltose were obtained from Asahi Kasei Chemicals Corporation (Tokyo, Japan) and Mitsubishi Shoji Foodtech Co., Ltd (Tokyo, Japan), respectively. Each HA (60 mg) was mixed with 210 mg of hydrogenated maltose; the placebo comprised 270 mg of cellulose. They were administered as capsules. There were no differences in the appearance and taste of HA and placebo capsules. The capsules were manufactured by Aliment Industry Co., Ltd (Yamanashi, Japan).
Subjects
The subjects were healthy Japanese female volunteers aged 35 to 60 years who were conscious of dry and sagging skin or wrinkles around the outer canthus. This clinical trial was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board. Informed consent was obtained in writing from each subject prior to enrolment in the study. Two hundred candidates were recruited from healthy female volunteers, and after eliminating volunteers using the exclusion standards (Table 1), 66 subjects were selected primarily on the basis of low moisture content in the stratum corneum of their right cheek and declining skin viscoelasticity. The selected subjects were assigned randomly to three groups, i.e., placebo, HA 800 k, and HA 300 k treated groups in which the moisture value of the stratum corneum, skin viscoelasticity, or age were not significantly different. After grouping, a double-blind, placebo-controlled study was conducted. Immediately before the trial began, three and two subjects left groups HA 800 k group and HA 300 k, respectively, thus the final number of trial subjects was 61 (Table 2).
Table 1.
Exclusion criteria
| 1. | Currently taking oral or topical medication which may affect the test result. |
| 2. | Pregnant, possibly pregnant, or lactating. |
| 3. | Currently taking excessive alcohol. |
| 4. | Likely to have a skin allergy (with symptoms of atopic dermatitis). |
| 5. | Likely to be acutely allergic to the test material. |
| 6. | Participating in another clinical test. |
| 7. | Judged to be inappropriate by the test managing doctor. |
| 8. | Continuously taking hyaluronan as a medicine or a dietary supplement. |
| 9. | Usual symptoms of diarrhea or stomach ache. |
Table 2.
Baseline characteristics of the study subjects
| Placebo | HA 800 k | HA 300 k | Total | |
|---|---|---|---|---|
| Number of subjects | 22 | 19 | 20 | 61 |
| Mean age (Age Range) | 42.73 ± 0.92 (35–60) | 43.68 ± 1.23 (35–60) | 43.90 ± 1.06 (35–60) | 43.41 ± 0.61 |
| Sex | female | |||
| Skin moisture (stratum corneum) | 19.50 ± 1.85 | 19.31 ± 1.78 | 21.5 ± 1.61 | 20.10 ± 0.96 |
| Skin viscoelasticity | ||||
| Max. amplitude | 0.32 ± 0.01 | 0.35 ± 0.01 | 0.32 ± 0.01 | 0.33 ± 0.01 |
| Min. amplitude | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.00 |
| Return rate | 73.84 ± 1.76 | 73.86 ± 1.21 | 72.74 ± 1.50 | 73.49 ± 0.88 |
Clinical trial design
The clinical study was conducted by Souken Corporation (Tokyo, Japan), which accepted a randomized, double-blind, placebo-controlled trial. Microcrystalline cellulose was used as the placebo, Hyaluronsan HA-F in the HA 800 k group, and Hyabest® (S) LF-P in the HA 300 k group. They were consumed by the designated subjects as two capsules (total weight 540 mg containing 120 mg HA in the treatment groups) each day after breakfast for 6 consecutive weeks. Each subject was asked to visit a hospital for a skin condition assessment by a dermatologist prior to treatment; after 2, 4, and 6 weeks of treatment; and 2 weeks after the treatment ended. Several measurements and skin examinations were performed by the dermatologist after the subjects washed their face followed by a 25 min rest in a waiting room with mild environmental conditions (room temperature (R.T.): 22 ± 2°C, relative humidity: 50 ± 15%) and another 5 min rest in an environmentally controlled room (R.T.: 21 ± 2°C, relative humidity: 50 ± 10%), thereby maintaining homogeneous environmental and measurement conditions as much as possible.
Clinical trial register
This study is registered at the Japan Medical Association Center for Clinical Trials (JMACCT) with the clinical trial number JMA-IIA00178.
Skin moisture
Skin moisture was measured in the stratum corneum at three points along a line between the right earlobe root and lip end using the Corneometer CM825 (Courage + Khazaka; Electronic Gmbh, Cologne, Germany). The maximum values were treated as the final moisture value at the time when the subject was screened, prior to ingestion, after 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. A Corneometer measures the variation in the electrostatic capacity, which depends on the moisture content in the stratum corneum, and thus the measurements used arbitrary units (a.u.) of the electrostatic capacity. The skin moisture in the stratum corneum was compared among groups over time, as well as between the HA treated and placebo treated groups.
Skin viscoelasticity
Skin viscoelasticity was measured in the right cheek using the Cutometer® MPA580 (Courage + Khazaka; Electronic Gmbh) at the time when the subject was screened, prior to ingestion, after 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. The Cutometer measures the dynamic characteristics of the skin height, which is lifted by low pressure in the probe sensors that adhere to the skin, and the value obtained reflects the state of the dermis layer. The maximum measured variation (length extended by suction force) indicates the skin hardness, whereas the minimum measured variation (minimum length after suction release) indicates the skin shrinkage ability and the return rate of overall skin elasticity. Skin viscoelasticity was compared among groups over time, as well as between the HA treated and placebo treated groups.
Questionnaire survey
A questionnaire survey was completed by the subjects to assess the luster, suppleness, and wrinkles of the facial skin using the five-point evaluation scale (Table 3) at the following times: prior to ingestion, after 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. The mean variation between the initial and treated values was compared among groups. In addition, the differences in the answers and the ratios of subjects among groups were tested by comparing the evaluations using three scoring levels, i.e., combined scores of 1, 2, and 3 only, and combined scores of 4 and 5, at each time point.
Table 3.
Methods used to evaluate the skin conditions in the questionnaire
| Scores | Evaluation items |
||
|---|---|---|---|
| Skin luster | Skin suppleness | Wrinkles | |
| 1 | sufficient | sufficient | no care |
| 2 | moderate | moderate | little care |
| 3 | normal | normal | normal |
| 4 | insufficient | insufficient | some care |
| 5 | no luster | no supple | much care |
Skin examinations by dermatologists
Objective evaluations were performed by dermatologists who were approved by a specialized medical doctors association. They assessed the clinical symptoms for the facial and whole-body skin of subjects using a four-point scale, i.e., 0: no symptoms, 1: slight degree, 2: medium degree, and 3: severe degree, in which the assessments were performed prior to ingestion, after 3 and 6 weeks ingestion, and 2 weeks after ingestion ended. Evaluation criteria were as follows. The facial skin: cosmetic rash, dry skin, rough skin, poor luster of skin, blushing, skin redness, and a poor sense of makeup; the whole-body skin: itching, dry skin, blushing, sore skin, desquamation, papules, phlyctenula, swelling, and general symptoms.
Statistical analysis
All values obtained are expressed as the mean ± SE. Paired t tests were used to compare the skin moisture in the stratum corneum and the skin viscoelasticity relative to the initial values. Dunnett’s test was used to compare the moisture variation in the stratum corneum between the placebo and HA treated groups. Steel Dwass test was used to compare the variations relative to the initial values obtained in the questionnaire and the dermatologist examinations among groups. All statistical analyses were performed using Statcel 2 of 4 Steps Excel Statistics second edition (the publisher OMS Ltd., Saitama, Japan). A p value of <0.05 was considered statistically significant.
Results
Skin moisture
Table 4 shows the transitions in the skin moisture content in the stratum corneum during the test period. The skin moisture content did not change in the placebo group. In contrast, the skin moisture contents in the HA 800 k and 300 k groups increased at 3 and 6 weeks ingestion compared with prior to its ingestion (p<0.001). In addition, the skin moisture content in HA 300 k group was significantly higher at 2 weeks after the end of ingestion than prior to its ingestion (p<0.001).
Table 4.
Changes in the skin moisture content with time following ingestion of hyaluronans and the placebo
| Skin Moisture (a.u.) | ||||
|---|---|---|---|---|
| Prior to ingestion | 3 weeks of ingestion | 6 weeks of ingestion | 2 weeks after ingestion ended | |
| Placebo | 21.18 ± 2.41 | 27.88 ± 2.97 | 26.03 ± 2.03 | 24.16 ± 2.23 |
| HA 800 k | 19.87 ± 2.63 | 28.66 ± 2.42*** | 29.95 ± 2.32*** | 26.09 ± 2.66 |
| HA 300 k | 21.2 ± 1.79 | 29.10 ± 1.64*** | 30.10 ± 2.43*** | 33.9 ± 2.59*** |
*p<0.05, **p<0.01, and ***p<0.001 vs prior to ingestion according to the paired t test. Means ± SE. HA, hyaluronan.
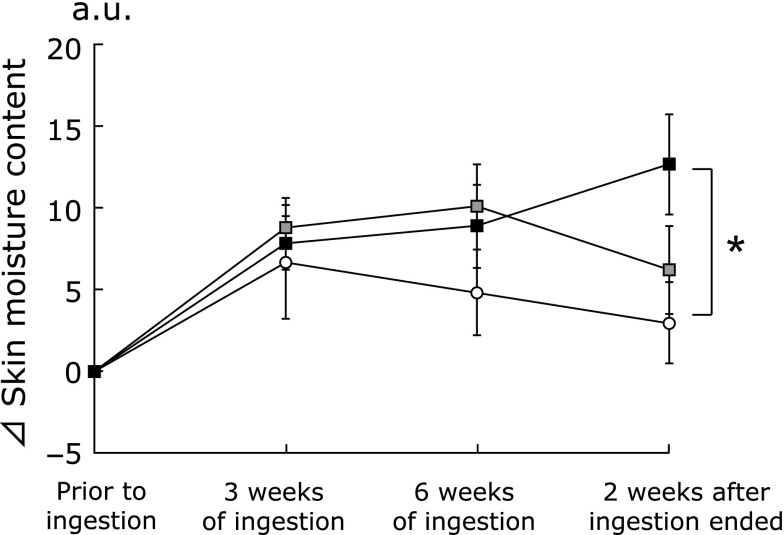
Fig. 1 shows the changes in the skin moisture content in the stratum corneum of HA and placebo treated subjects during the test period. The skin moisture content in the HA 300 k group was significantly higher than that in the placebo group 2 weeks after consumption ended (p<0.05).
Fig. 1.
Changes in the skin moisture with time after ingestion of hyaluronans or the placebo. The skin moisture contents of the right cheek were measured at three points prior to ingestion, 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. Variations in the skin moisture content relative to the baseline condition (⊿):  indicates the placebo group;
indicates the placebo group;  indicates the HA 800 k group;
indicates the HA 800 k group;  indicates the HA 300 k group. Dunnett’s test was used to compare the three groups. Data are presented as means ± SE. *p<0.05.
indicates the HA 300 k group. Dunnett’s test was used to compare the three groups. Data are presented as means ± SE. *p<0.05.
Skin viscoelasticity
Table 5 shows the transitions in the maximum amplitude value, minimum amplitude value, and return rate, which are indicators of skin viscoelasticity. None of the values differed significantly between the placebo and HA treated groups at the end of the treatment. However, the maximum and minimum amplitude values were significantly lower after 3 weeks consumption than those before consumption, and the return rate after 3 weeks consumption was significantly higher than that before consumption in all groups (p<0.05, p<0.01 and p<0.001, respectively). However, there were no significant differences after 6 weeks of consumption or before consumption in each group.
Table 5.
Changes in skin viscoelasticity following ingestion of hyaluronans and the placebo
| Skin viscoelasticity | Prior to ingestion | 3 weeks of ingestion | 6 weeks of ingestion | 2 weeks after ingestion ended | |
|---|---|---|---|---|---|
| Max. amplitude (10−1 mm) | Placebo | 3.02 ± 0.01 | 2.33 ± 0.01* | 3.00 ± 0.01 | 3.19 ± 0.01 |
| HA 800 k | 3.15 ± 0.01 | 2.51 ± 0.02*** | 2.88 ± 0.02 | 3.21 ± 0.02 | |
| HA 300 k | 3.23 ± 0.01 | 2.52 ± 0.01*** | 3.08 ± 0.02 | 3.45 ± 0.01 | |
| Min. amplitude (10−1 mm) | Placebo | 0.71 ± 0.01 | 0.24 ± 0.00** | 0.58 ± 0.00* | 0.66 ± 0.00 |
| HA 800 k | 0.72 ± 0.00 | 0.24 ± 0.00*** | 0.66 ± 0.00 | 0.70 ± 0.01 | |
| HA 300 k | 0.81 ± 0.00 | 0.23 ± 0.00*** | 0.68 ± 0.00** | 0.74 ± 0.00 | |
| Return rate (%) | Placebo | 76.44 ± 1.57 | 89.75 ± 1.32*** | 80.12 ± 1.51* | 79.00 ± 1.53 |
| HA 800 k | 76.37 ± 1.81 | 89.94 ± 0.94*** | 76.45 ± 1.66 | 77.05 ± 2.36 | |
| HA 300 k | 74.11 ± 1.57 | 90.46 ± 1.10*** | 77.08 ± 1.66 | 77.70 ± 1.72 |
*p<0.05, **p<0.01, and ***p<0.001 vs prior to ingestion according to a paired t test. Means ± SE. HA, hyaluronan.
Fig. 2 shows the change in the skin viscoelasticity indicators, i.e., the maximum amplitude value, minimum amplitude value, and the return rate. The maximum amplitude value and return rate did not differ significantly between the HA treated groups and placebo treated group. However, the minimum amplitude value in the HA 300 k group tended to be lower than that in the placebo group after 3 weeks of ingestion (p<0.10).
Fig. 2.
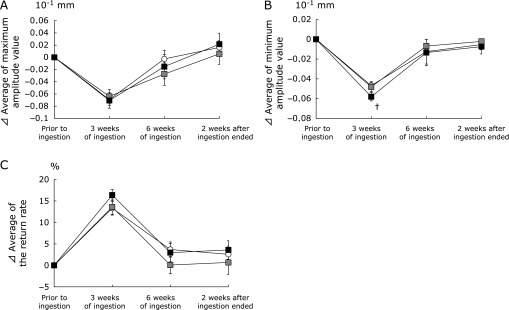
Changes in the skin viscoelasticity with time following ingestion of hyaluronans or the placebo. The skin viscoelasticity in the right cheek was measured prior to ingestion, 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. The maximum amplitude value, minimum amplitude value, and the return rate were determined. Variations in skin viscoelasticity relative to the baseline condition (⊿): (A) maximum amplitude value, (B) minimum amplitude value, (C) the return rate.  indicates the placebo group;
indicates the placebo group;  indicates the HA 800 k group;
indicates the HA 800 k group;  indicates the HA 300 k group. Dunnett’s test was used to compare the three groups. Data are presented as means ± SE. †p<0.10.
indicates the HA 300 k group. Dunnett’s test was used to compare the three groups. Data are presented as means ± SE. †p<0.10.
Questionnaire survey to determine subjective skin symptoms
The effects of HA on the subjective symptoms of facial skin aging, i.e., luster, suppleness, and wrinkles, were evaluated using a questionnaire. The luster and suppleness were improved significantly in the HA treated groups than in the placebo-treated group (Fig. 3, 4, 5, 6).
Fig. 3.
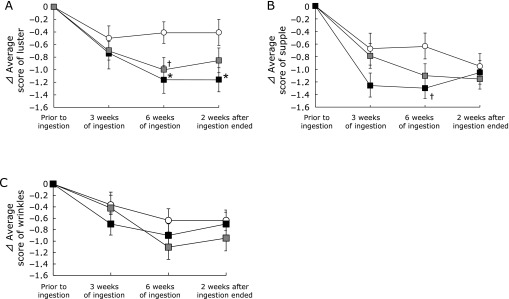
Changes in the subjective skin condition with time following ingestion of hyaluronans and placebo. The subjective skin conditions, i.e., luster, suppleness, and wrinkles, were evaluated using a questionnaire prior to ingestion, 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. Differences in each questionnaire result relative to the baseline condition (⊿): (A) luster, (B) suppleness, and (C) wrinkles.  indicates the placebo group;
indicates the placebo group;  indicates the HA 800 k group;
indicates the HA 800 k group;  indicates the HA 300 k group. Dunnett’s test was used to compare the three groups. Data are present as means ± SE. †p<0.10 and *p<0.05.
indicates the HA 300 k group. Dunnett’s test was used to compare the three groups. Data are present as means ± SE. †p<0.10 and *p<0.05.
Fig. 4.
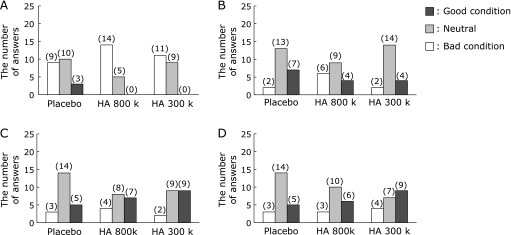
Changes in the skin luster score with time after the ingestion of hyaluronans and placebo. A questionnaire was completed to evaluate the subjective skin luster prior to ingestion, 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. The number of answers with each score are indicated in the histogram: (A) prior to ingestion, (B) 3 weeks of ingestion, (C) 6 weeks of ingestion, and (D) 2 weeks after ingestion ended.
Fig. 5.
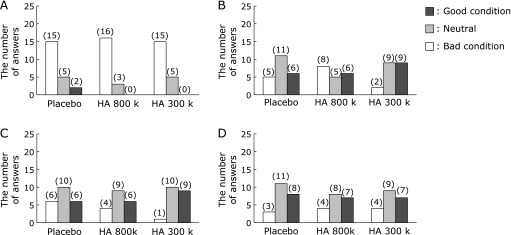
Changes in the skin suppleness score with time after the ingestion of hyaluronans and the placebo. A questionnaire was completed to evaluate the subjective skin suppleness prior to ingestion, 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. The number of answers with each score is indicated in the histogram: (A) prior to ingestion, (B) 3 weeks of ingestion, (C) 6 weeks of ingestion, and (D) 2 weeks after ingestion ended.
Fig. 6.
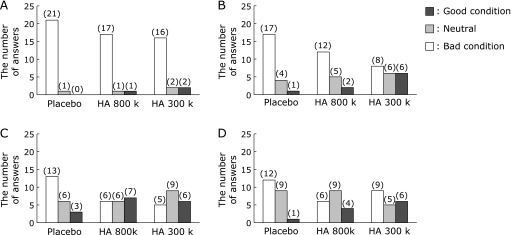
Changes in the skin wrinkles with time score after the ingestion of hyaluronans and the placebo. A questionnaire was completed to evaluate the subjective skin wrinkles prior to ingestion, 3 and 6 weeks of ingestion, and 2 weeks after ingestion ended. The number of answers for each score is indicated in the histogram: (A) prior to ingestion, (B) 3 weeks of ingestion, (C) 6 weeks of ingestion, and (D) 2 weeks after ingestion ended.
As shown in Fig. 3A, the mean skin luster scores after 6 weeks of consumption tended to improve and improved significantly in the HA 800 k and 300 k treated groups than in the placebo-treated group (p<0.10 and p<0.05, respectively). Furthermore, the significant improvement in skin luster was maintained in the HA 300 k group 2 weeks after consumption ended (p<0.05). Approximately half the subjects gave assessments of a good skin luster condition (scores of 1 and 2) after 6 weeks of consumption compared with that before consumption in groups HA 800 k and 300 k (7/16 and 9/20, respectively; Fig. 4). Good skin luster condition responses (scores of 1 and 2) were maintained 2 weeks after consumption ended in approximately half of the subjects in the HA 300 k group.
The mean skin suppleness scores after 6 weeks of consumption tended to improve in the HA 300 k group compared with those of the placebo group (p<0.10; Fig. 3B). Approximately half of the subjects had good scores for skin suppleness after 3 and 6 weeks of consumption compared with those before consumption in the HA 300 k group (9/20; Fig. 5).
The HA treated groups exhibited decreases in the subjective symptoms of wrinkles compared with the placebo treated group, but the differences were not significant (Fig. 3C). The numbers of improved skin wrinkle condition ratings (score of 1 and 2) after 6 weeks consumption compared with that before consumption were higher in the HA treated groups than in the placebo treated group (7/19 and 6/20, respectively; Fig. 6). Moreover, a high proportion of subjects reported an improved wrinkle condition (scores of 1 and 2) 2 weeks after consumption ended in the HA 300 k group.
Skin examination by dermatologists
The clinical symptoms of the facial and whole-body skin in the subjects treated using the placebo or each HA were evaluated periodically by dermatologists (data not shown). In terms of facial skin symptoms, no abnormal symptoms were observed before consumption in all groups; however, slight dry skin, rough skin, and poor skin luster were observed at 3 weeks in only one subject in the placebo group. No further changes in facial skin symptoms were observed in the HA or placebo treated groups.
In terms of the whole body skin symptoms, slight dry skin was observed before consumption in only one subject from the HA 300 k group. No further changes in whole-body skin symptoms were observed in the HA or placebo treated groups.
Discussion
This study confirmed that daily ingestion of 120 mg of HA (MWs: 800 k and 300 k) for 6 weeks increased the skin moisture content and improved the facial aging symptoms such as luster and suppleness, in subjects with dry skin in a randomized, double blind, placebo controlled study.
The HA treated groups exhibited significant increased skin moisture contents after ingestion compared with that before ingestion and they remained higher than those of the placebo treated group (Table 4). In addition, we confirmed that the HA 300 k group had significantly increased skin moisture contents ended compared with the placebo 2 weeks after ingestion (Fig. 1). To understand why the skin moisture content only increased significantly in the HA 300 k group, it is necessary to examine the differences in absorption of HA by the body and its effects on skin cells. In a previous randomized, double blind, placebo controlled study, healthy subjects received collagen hydrolysate once daily for 8 weeks, it improved the skin elasticity after 4 weeks of ingestion and 2 weeks after ingestion ended compared with when the subjects received a placebo.(20) However, the ingestion of collagen hydrolysate did not increase the skin moisture content significantly compared with the placebo but only maintained a high skin moisture content.(20) In another randomized, double blind, placebo controlled study where healthy subjects received glucosylceramide once daily for 12 weeks, it tended to reduce the loss of the skin moisture content compared with the placebo but did not increase the skin moisture content significantly compared with placebo.(21) These effects suggest that a more prolonged ingestion period may be necessary to confirm any effects on the skin moisture content by ingestion of functional food.
In the present study, the variation in the minimum measured value in the HA 300 k group tended to be lower than that in the placebo group, indicating that the skin viscoelasticity was reduced (Fig. 2). In addition, each group exhibited significant reductions in the maximum amplitude value and the minimum amplitude value, but the return rate increased significantly (Table 5). Each skin viscoelasticity measure was decreased slightly or increased in all groups, but they returned to the initial values subsequently. Thus, it was not possible to confirm the effects of HA ingestion on skin viscoelasticity. HA in the dermis affects the skin viscoelasticity, but fiber components such as elastin and collagen are mainly related to skin viscoelasticity. Elastin fibers are present between collagen fibers in the dermis, and they confer skin viscoelasticity by forming a network from the basement membrane up to the skin tissue.(22) Thus, the significant increase in skin viscoelasticity due to the continued ingestion of collagen hydrolysate is related to the protection of collagen fibers and the prevention of abnormal accumulations of elastin fibers in the dermis.(20) In the present study, HA ingestion did not improve the skin viscoelasticity, probably because the ingested HA had no direct effects on fiber components.
In addition, the questionnaire results confirmed that the HA treated groups exhibited significantly improved facial aging symptoms such as luster and suppleness compared with the placebo group (Fig. 3). Loss of luster and suppleness, and increased wrinkles in facial skin are signs of aging, which are induced by time and external stimuli such as ultraviolet irradiation, and they along with skin dryness affect the appearance of the skin. Dehydration of the skin by excoriation can lead to the loss of luster and suppleness and the formation of fine wrinkles around the outer canthus. Thus, the improved skin symptoms as a result of continuous ingestion of HA lead to skin moisturizing.
In a previous oral administration test using radioactively labeled HA (MW: 1,000 k) in rats, approximately 90% of the ingested HA was absorbed into the body and almost 3% of the total ingested HA was transferred to the skin.(23) It was confirmed that the ingestion of HA not only with a MW of 1,000 k but also with 10 k was transferred to the skin.(24) Therefore, we considered that both of the HA types used in the present study were transferred to the skin after ingestion. The ingested HA is believed to be absorbed via the intestinal route. The intestinal permeability of low-MW HA has been confirmed in cultured monolayers of human intestinal Caco-2 cells.(25) The low-MW HA is absorbed primarily through the Caco-2 cell monolayer via the paracellular pathway, which increases inversely in proportion to the molecular size of HA.(25) In addition, ingested HA is reportedly absorbed intact as well as in the form of its decomposed metabolites. Orally administered HA was shown to be decomposed into low-MW molecules by intestinal bacteria in mice.(26) Bacteroides stercoris HJ-15, which potently degrades glycosaminoglycan,(27) and bacteria such as Staphylococcus aureus and Clostridium perfringens,(28,29) which produce hyaluronidases, are found in the human intestine. Thus, it is possible that ingested HA is also degraded by intestinal bacteria in humans. It is not clear what the configurations the HAs used in this study adopted when they were absorbed after ingestion, but it is considered that these HAs were absorbed as intact HA and depolymerized HA. HA oligosaccharides (MW: 1–2 k) increase HA production in human fibroblasts, probably by displacing endogenous HA from receptors.(30) In addition, high-MW HA (MW: 110 k) promotes cell proliferation during the production of human fibroblast-populated collagen lattices.(31) The amount of HA in the skin is one of the main factors that determines the skin moisture content.(32) Furthermore, we considered that increases in cell numbers elevate the amount of HA synthesis in the skin, thereby suppressing skin water loss by filling the gaps between skin cells. Thus, depending on its MW, HA has different effects on fibroblasts. Different HA-binding proteins such as cluster of differentiation 44 and receptor for HA-mediated mobility are present in the cell membrane, which are involved in intracellular signal transduction by interacting with HA.(33,34) The pharmacokinetics of ingested HA should be determined in order to elucidate the precise mechanism underlying its effect on the skin.
The examinations by dermatologists confirmed that there were no adverse effects on the skin. Other studies have also reported the safety of long-term HA ingestion. Randomized, double-blind, placebo-controlled studies have reported no abnormal hematological or medical findings in human subjects who received 240 mg/day and 200 mg/day of HA (Hyabest® (J): MW 9 × 105, Kewpie Co., Tokyo) for 12 weeks and 12 months, respectively.(35,36) Therefore, the safety of HA as a food material has been confirmed by long-term ingestion.
The present study suggests that the ingestion of HA increased the skin moisture content resulting moisturized skin, which improved facial aging symptoms such as luster, suppleness, and wrinkles. In addition, there were no differences in the effects of ingesting HA of various MWs, which we confirmed by using HAs with MWs of 800 k and 300 k as they had the same moisturizing and skin improvement effects. However, the physiological activities of HA differ according to its MWs.(16–19) Therefore, it will be necessary to further examine the effects of ingesting HA of various MWs, in addition to those tested in this study.
Conclusion
This study showed that the ingestion of HA (MW: 800 k and 300 k) by subjects with dry skin increased their skin moisture content and improved skin aging symptoms such as the luster and suppleness of facial skin. Furthermore, we confirmed that HA is safe for use as a food because there were no adverse effects on the skin according to the examinations by dermatologists. In conclusion, it is expected that HA can be used as a functional food for improving the skin condition as it moisturizes dry skin internally.
Abbreviations
- a.u.
arubitary unit
- HA
hyaluronan
- MW
molecular weight
- R.T.
room temperature
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lodén M, Olsson H, Axéll T, Linde YW. Friction, capacitance and transepidermal water loss (TEWL) in dry atopic and normal skin. Br J Dermatol. 1992;126:137–141. doi: 10.1111/j.1365-2133.1992.tb07810.x. [DOI] [PubMed] [Google Scholar]
- 2.Thune P. Evaluation of the hydration and the water-holding capacity in atopic skin and so-called dry skin. Acta Derm Venereol Suppl (Stockh) 1989;144:133–135. doi: 10.2340/00015555144133135. [DOI] [PubMed] [Google Scholar]
- 3.Rogers J, Harding C, Mayo A, Banks J, Rawlings A. Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res. 1996;288:765–770. doi: 10.1007/BF02505294. [DOI] [PubMed] [Google Scholar]
- 4.Nordstrom KM, McGinley J, Leyden KJ, Kligman AM. Sebaceous lipids in xerosis of the skin. J Cutan Aging Cosmet Dermatol. 1988;89:29–33. [Google Scholar]
- 5.Sunwoo Y, Chou C, Takeshita J, Murakami M, Tochihara Y. Physiological and subjective responses to low relative humidity. J Physiol Anthropol. 2006;25:7–14. doi: 10.2114/jpa2.25.7. [DOI] [PubMed] [Google Scholar]
- 6.Sunwoo Y, Chou C, Takeshita J, Murakami M, Tochihara Y. Physiological and subjective responses to low relative humidity in young and elderly men. J Physiol Anthropol. 2006;25:229–238. doi: 10.2114/jpa2.25.229. [DOI] [PubMed] [Google Scholar]
- 7.Tupker RA, Bunte EE, Fidler V, Wiechers JW, Coenraads PJ. Irritancy ranking of anionic detergents using one-time occlusive, repeated occlusive and repeated open tests. Contact Dermatitis. 1999;40:316–322. doi: 10.1111/j.1600-0536.1999.tb06082.x. [DOI] [PubMed] [Google Scholar]
- 8.Hashizume H. Skin aging and dry skin. J Dermatol. 2004;31:603–609. doi: 10.1111/j.1346-8138.2004.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 9.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 10.Turino GM, Cantor JO. Hyaluronan in respiratory injury and repair. Am J Respir Crit Care Med. 2003;167:1169–1175. doi: 10.1164/rccm.200205-449PP. [DOI] [PubMed] [Google Scholar]
- 11.Scott JE, Heatley F. Hyaluronan forms specific stable tertiary structures in aqueous solution: a 13C NMR study. Proc Natl Acad Sci U S A. 1999;96:4850–4855. doi: 10.1073/pnas.96.9.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed RK, Lilja K, Laurent TC. Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand. 1988;134:405–411. doi: 10.1111/j.1748-1716.1988.tb08508.x. [DOI] [PubMed] [Google Scholar]
- 13.Laurent UB, Dahl LB, Reed RK. Catabolism of hyaluronan in rabbit skin takes place locally, in lymph nodes and liver. Exp Physio. 1991;76:695–703. doi: 10.1113/expphysiol.1991.sp003536. [DOI] [PubMed] [Google Scholar]
- 14.Manuskiatti W, Maibach HI. Hyaluronic acid and skin: wound healing and aging. Int J Dermatol. 1996;35:539–544. doi: 10.1111/j.1365-4362.1996.tb03650.x. [DOI] [PubMed] [Google Scholar]
- 15.Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990–1000. [PubMed] [Google Scholar]
- 16.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Ito T, Tawada A, et al. A. Effect of hyaluronan oligosaccharides on the expression of heat shock protein 72. J Biol Chem. 2002;277:17308–17314. doi: 10.1074/jbc.M112371200. [DOI] [PubMed] [Google Scholar]
- 18.Hodge-Dufour J, Noble PW, Horton MR, et al. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–2500. [PubMed] [Google Scholar]
- 19.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 20.Proksch E, Segger D, Degwert J, Schunck M, Zague V, Oesser S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47–55. doi: 10.1159/000351376. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama T, Fukaya Y, Nakano Y, et al. Dietary konjac extract improves skin conditions. Pharmacometrics. 2008;75:1–6. [Google Scholar]
- 22.Kim BS, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18:2–9. doi: 10.1007/s003450050002. [DOI] [PubMed] [Google Scholar]
- 23.Balogh L, Polyak A, Mathe D, et al. Absorption, uptake and tissue affinity of high-molecular-weight hyaluronan after oral administration in rats and dogs. J Agric Food Chem. 2008;56:10582–10593. doi: 10.1021/jf8017029. [DOI] [PubMed] [Google Scholar]
- 24.Laznicek M, Laznickova A, Cozikova D, Velebny V. Preclinical pharmacokinetics of radiolabelled hyaluronan. Pharmacol Rep. 2012;64:428–437. doi: 10.1016/s1734-1140(12)70784-3. [DOI] [PubMed] [Google Scholar]
- 25.Hisada N, Satsu H, Mori A, et al. Low-molecular-weight hyaluronan permeates through human intestinal Caco-2 cell monolayers via the paracellular pathway. Biosci Biotechnol Biochem. 2008;72:1111–1114. doi: 10.1271/bbb.70748. [DOI] [PubMed] [Google Scholar]
- 26.Lee B, Lee JH, Lee HS, et al. Glycosaminoglycan degradation-inhibitory lactic acid bacteria ameliorate 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. J Microbiol Biotechnol. 2009;19:616–621. doi: 10.4014/jmb.0808.479. [DOI] [PubMed] [Google Scholar]
- 27.Ahn MY, Shin KH, Kim DH, et al. Characterization of a Bacteroides species from human intestine that degrades glycosaminoglycans. Can J Microbiol. 1998;44:423–429. doi: 10.1139/cjm-44-5-423. [DOI] [PubMed] [Google Scholar]
- 28.Jones RC, Deck J, Edmondson RD, Hart ME. Relative quantitative comparisons of the extracellular protein profiles of Staphylococcus aureus UAMS-1 and its sarA, agr, and sarA agr regulatory mutants using one-dimensional polyacrylamide gel electrophoresis and nanocapillary liquid chromatography coupled with tandem mass spectrometry. J Bacteriol. 2008;190:5265–5278. doi: 10.1128/JB.00383-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu T, Ohtani K, Hirakawa H, et al. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lüke HJ, Prehm P. Synthesis and shedding of hyaluronan from plasma membranes of human fibroblasts and metastatic and non-metastatic melanoma cells. Biochem J. 1999;343(Pt 1):71–75. [PMC free article] [PubMed] [Google Scholar]
- 31.Greco RM, Iocono JA, Ehrlich HP. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J Cell Physiol. 1998;177:465–473. doi: 10.1002/(SICI)1097-4652(199812)177:3<465::AID-JCP9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Oh JH, Kim YK, Jung JY, et al. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci. 2011;62:192–201. doi: 10.1016/j.jdermsci.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J Biol Chem. 2002;277:41046–41059. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- 34.Papakonstantinou E, Klagas I, Karakiulakis G, et al. Steroids and β2 agonists regulate hyaluronan metabolism in asthmatic airway smooth muscle cells. Am J Respir Cell Mol Biol. 2012;47:759–767. doi: 10.1165/rcmb.2012-0101OC. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Iwaso H. An effectiveness study of hyaluronic acid [Hyabest® (J)] in the treatment of osteoarthritis of the knee on the patients in the United States. J New Rem & Clin. 2009;58:249–256. [Google Scholar]
- 36.Tashiro T, Seino S, Sato T, Matsuoka R, Masuda Y, Fukui N.Oral administration of polymer hyaluronic acid alleviates symptoms of knee osteoarthritis: a double-blind, placebo-controlled study over a 12-month period ScientificWorldJournal 2012. DOI: 10.1100/2012/167928 [DOI] [PMC free article] [PubMed] [Google Scholar]