Abstract
Background
In the last stage of the C. elegans equivalent of epiboly, an open pocket in the epidermis is closed by marginal epidermal P/pocket cells that express and require VAB-1/Eph and PLX-2/plexin receptors for migration toward and alignment with contralateral partners at the ventral midline. Cellular mechanisms affected by these signaling proteins remain unknown.
Results
A cellular bridge comprising four neuron cell bodies that spans the open pocket serves as a substratum for migration of contra-lateral P cell pair P9/10 to the midline which can facilitate similar migration of neighboring P cells. This bridge is formed by a stereotypical rearrangement of five sister pairs of PLX-2 and VAB-1 expressing cells, of which three pairs serve as a scaffold for bridge assembly and two pairs form the bridge. Bridge formation requires VAB-1 kinase-dependent extension of presumptive bridge cell protrusions toward the ventral midline. An unassembled mutant bridge obstructs but does not block P cell progression toward the midline, however, cell type-specific rescue experiments show that VAB-1 or a nearly complete cytoplasmic deletion of VAB-1 expressed by scaffold and bridge cells or by P9/10 can facilitate P cell progression to the midline. MAB-20/semaphorin and VAB-1 also exhibit complex redundancies to regulate adhesion and prevent gaps between sister bridge and scaffold forming cells that would otherwise completely block P cell migration.
Conclusions
The Eph receptor functions to mediate cell extensions required for bridge formation, independently facilitates P cell migration to the ventral midline, and acts redundantly with PLX-2/plexin to prevent gaps between sister plexin band cells that normally serve as a substratum for P9/10 cell migration.
Keywords: semaphorin, Eph receptor, plexin, ventral enclosure, cell migration, morphogenesis
INTRODUCTION
Body wall closure defects are a leading cause of human birth defects, yet little is known about the cellular and molecular mechanisms involved[1]. The most studied example of body wall closure is dorsal closure in the Drosophila embryo. Dorsal closure occurs by spreading of epidermis from the ventral side to the dorsal midline to enclose the embryo and is largely mediated by DPP/TGF-beta signaling between epidermal cells lining the margins of the advancing epidermis and the underlying amnioserosa [2].
Although there is evidence for the involvement of BMP/TGF-beta signaling in mammalian body wall closure and wound healing [3], it is clear that additional signaling mechanisms, including ephrin signaling [4, 5], are involved in these processes. In spite of the known involvement of specific signaling molecules in mammalian body wall closure, the precise cellular and molecular mechanisms mediated by these molecules are largely unknown.
The C. elegans embryo provides a genetically tractable alternative model to analyze body wall closure and resembles Drosophila dorsal closure in several respects [3]. Epidermal enclosure in C. elegans involves extension, stretching, and migration of a large patch of dorsal epidermoblasts around both sides of the embryo to converge and form a seal at the ventral midline. In the final stage of this process, a ventral opening or pocket in the epidermis, lined with marginal epidermoblasts called P/pocket cells, is closed at the ventral midline (Figures 1, 2A and S1). We are studying the underlying cellular and molecular mechanisms that elicit and guide the migration of the P cells to the ventral midline during pocket closure and that regulate the midline alignment of contralateral P cell partners.
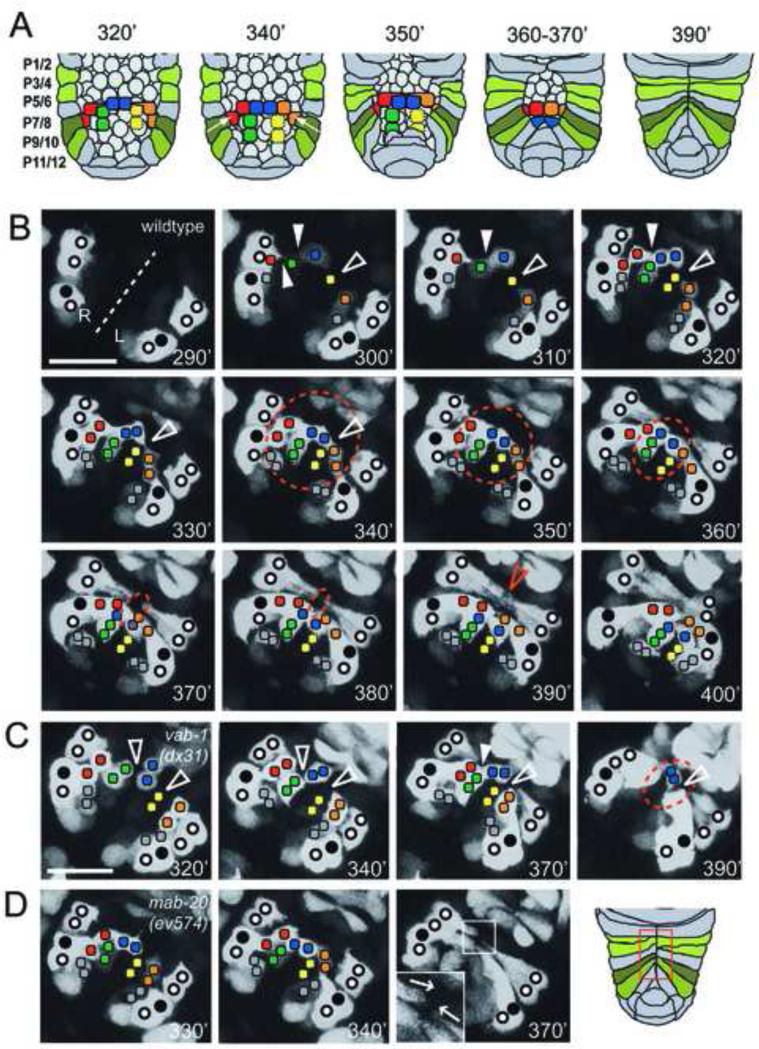
Figure 1. Pocket bridge formation and pocket closure in the wild type and in mab-20 and vab-1 mutants.
(A) Schematic of epidermal pocket closure in minutes post first cleavage (PFC) of the zygote. Ten plexin band cells span the open pocket. Cell births and movements were followed using Pplx-2::gfp. Plexin band cells of the same color are sisters. Rearrangement of plexin band cells during pocket closure are as photographed in panel B. (B) Ventral view of the pocket region of a wildtype embryo with anterior at top right. The ventral midline (dashed line) and relative positions of expressing identified P cells (circles, closed circles are P9/10) and plexin expressing cells on the surface of the open ventral pocket (colored squares) are indicated. Developmental time at 20°C is indicated in each frame. The approximate size of the open pocket (dashed circle) reflects the advance of P cell extension toward the midline. At 290’ PFC, Pplx-2::gfp is expressed in the P cells. At 300’-310’ PFC, blast cells on the surface of the open pocket express Pplx-2::gfp. At 320’-350’ PFC expressing blast cells divide and sister cells remain attached to form a contiguous band of plexin expressing cells (plexin band), which spans the open pocket (when sporadically expressing yellow cells of Figure S1 are included). The midline distal pairs (red and orange squares) form the pocket bridge by migrating toward the midline over intervening expressing scaffold cells (green, yellow and blue squares). This rearrangement requires protrusions from the presumptive bridge cells that extend over scaffold cells toward the ventral midline in two stages (330’ open arrowhead) (see summary in Figure 2). P9/10 cell migration then occurs along the forming and mature pocket bridge. At 390’ PFC, P cell migration stops when opposing leading edges meet at the midline. At 400’ PFC, contralateral marginal cells align and suture and the embryo begins to elongate and turn. (C) Plexin band cells form a nearly contiguous band of cells by 340’ PFC in vab-1(dx31) null as well as in kinase dead embryos (not shown). The non-sister cell gaps (left open arrowhead at 320’) are usually closed later by unknown mechanisms that might involve small filopodia-like projections (solid arrowhead at 370’). (D) Skewed P cell leading edges (see 370’ inset) were detected in 2 of 5 mab-20(ev574) mutant embryos examined. Scale bar in B for panels B-D is 10 μm.
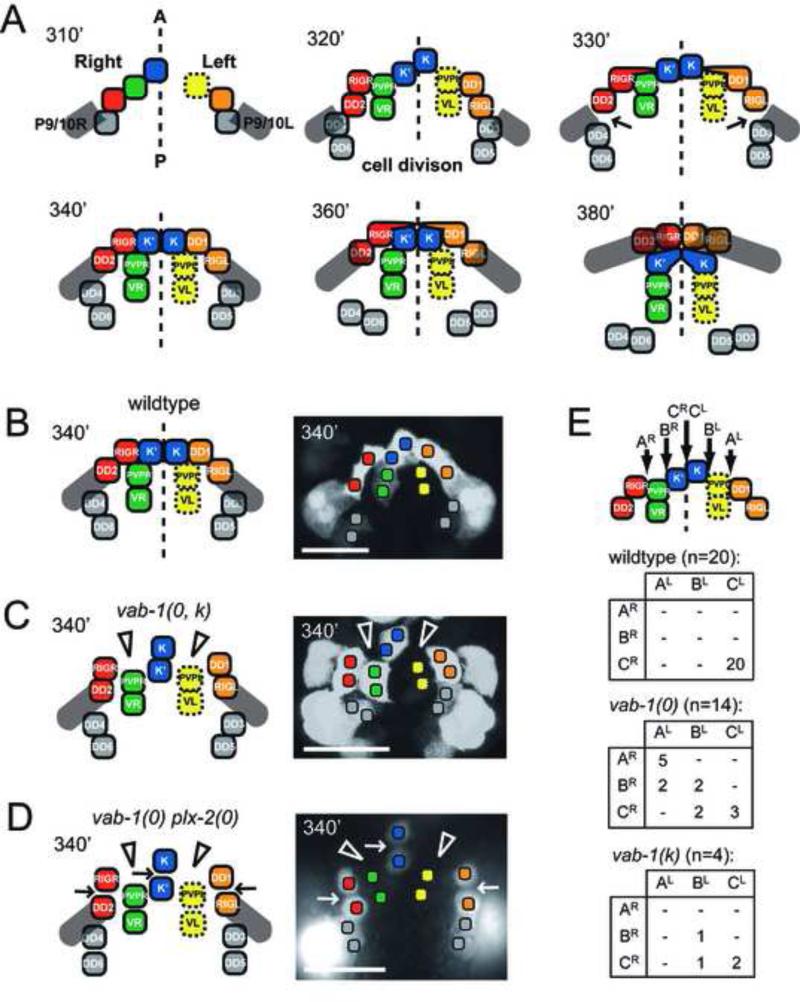
Figure 2. Schematic of pocket bridge formation in the wild type and mutants.
Relative stages of pocket bridge formation from plexin band cells are shown in minutes PFC using the Pplx-2::gfp reporter. (A) Summarizes cell identities, bridge assembly and P9/10 cell migration as derived from Figure 1 and described in the Results. (B,C) vab-1 null and kinase dead mutants (0, k), pocket bridge cells (red and orange) fail to form or maintain the directed protrusions (open arrowheads) required for their movement over the scaffold cells (green, blue and yellow cells) to the midline. Pocket bridge formation at this time remains in an obstructed state. (B,D) The plexin band is more severely disrupted in the vab-1 plx-2 double null. Gaps are present between sister (arrows) and non-sister (open arrowheads) plexin band cells. (E) P9/10 cells stop progression toward the midline at positions A, B, or C right (R) or left (L). The number of embryos with a particular position of blockage is shown as the intersection of a specific row (right side block) with a specific column (left side block). Scale bars are 10 μm.
Although conventional TGF-beta signaling is not obviously required for pocket closure in C. elegans, the axon guidance and cell association signaling pathways involving semaphorin-2a/MAB-20 [6], its PLX-2/plexin receptor [7], the Eph receptor VAB-1, and the ephrins (EFN-1 to EFN-4) are required for several aspects of this process [6, 8-11]. Previously, George et al. (1998) suggested that the ephrin receptor VAB-1 expressed in neuroblasts within the open ventral pocket could facilitate ventral enclosure [8]. These results and the involvement of ephrin signaling in mammalian body wall closure prompted us to examine the cellular and molecular functions that are mediated by these signaling proteins in C. elegans pocket closure.
To further our understanding of the role of Eph and semaphorin signaling during ventral enclosure, we examined the expression patterns of VAB-1, the only known Eph receptor in C. elegans [8], and PLX-2, one of two known Sema-2a/MAB-20 receptors [7, 12]. We discovered a complex pattern of cell births, divisions and adhesions mediating wildtype pocket closure and identified the cell specific functions of VAB-1 and MAB-20 signaling in both pocket closure and P cell alignment. We also uncovered the cause of the synergistic/synthetic pocket closure defects in double mutant embryos of semaphorin and Eph receptor signaling. These results clarify the roles that phylogenetically conserved guidance cue receptors play during epidermal enclosure and define, at the level of individual cell types and molecular interactions between them, how epithelial cell migrations are controlled in a living embryo.
RESULTS
Expression pattern of vab-1 and plx-2 and formation of the wildtype pocket bridge
Since vab-1 and mab-20/semaphorin-2A mutations affect aspects of pocket closure (see below), we decided to characterize the expression patterns of vab-1 and plx-2 during pocket closurein detail. We developed a variety of reporters for vab-1 and plx-2 expression (Table S1, see Experimental Procedures). At the onset of pocket closure, the plx-2 reporters express in right and left side analogues of QV5, and P cells P3/4, P5/6, P9/10, P11/12 (Figures 1-3 and S1), whereas, vab-1 reporters express in right and left side analogues of V3 (sporadically), V4, QV5, and P9/10 (Figure 4). Both reporters also express in bridge and scaffold cells (see below), which together comprise a band of PLX-2 and VAB-1 expressing cells that cross the open pocket (brackets in Figures 3 and 4), referred to below as the plexin band. Expression in all of these cells continues throughout pocket closure and beyond. Among the P cells, expression is most prominent in P9/10 right (R) and left (L) for all reporters.
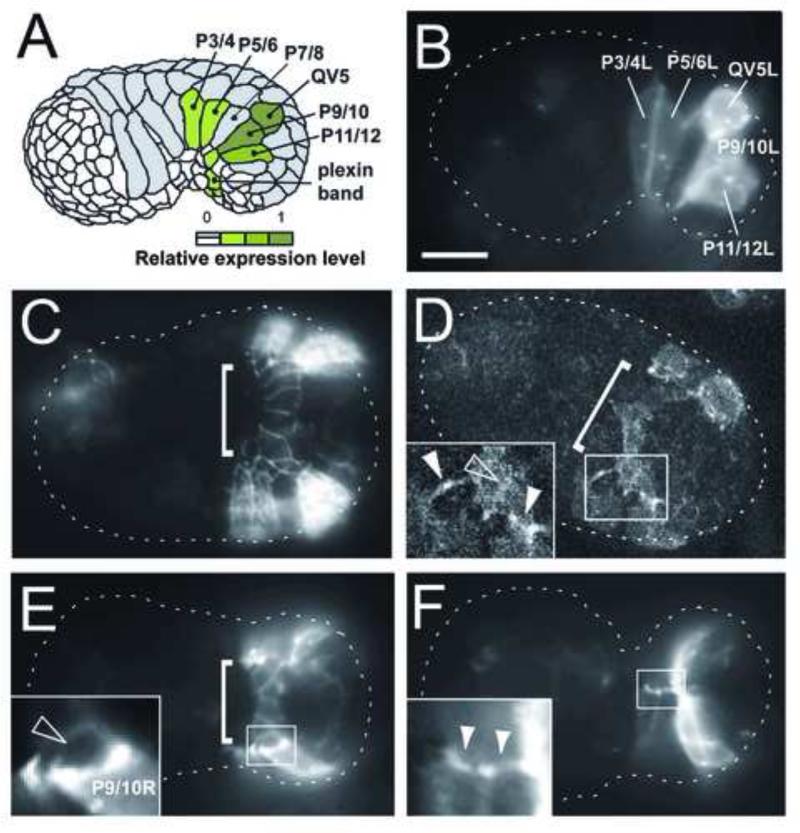
Figure 3. PLX-2 is expressed in the plexin band and P cells undergoing pocket closure.
(A-F) Fluorescence is from Pplx-2::PLX-2::GFP translational reporter. Anterior is to the left. (A) Summary of plx-2 reporter expressing (green) cells. (B) Lateral view at the beginning of pocket closure. PLX-2::GFP is expressed in a subset of P cells at the margin of the open pocket. (C) Ventral view at the onset of pocket closure showing PLX-2::GFP-expressing plexin band cells (bracket). (D) Ventral view showing a confocal section of the ventral pocket. PLX-2::GFP is enriched at the leading end and filopodial-like tips of migrating P cells (solid arrowheads). Before the onset of pocket closure, P5/6 tips contact the plexin band (left arrowhead, inset) as do extensions from P9/10 (right arrowhead, inset). (E) Ventral view showing the assembling bridge (bracket) and PLX-2::GFP-enriched P cell tips (also see inset). (F) Ventral view showing PLX-2::GFP-enriched P cell tips (inset) as they meet at the ventral midline. Scale bar is 10 μm.
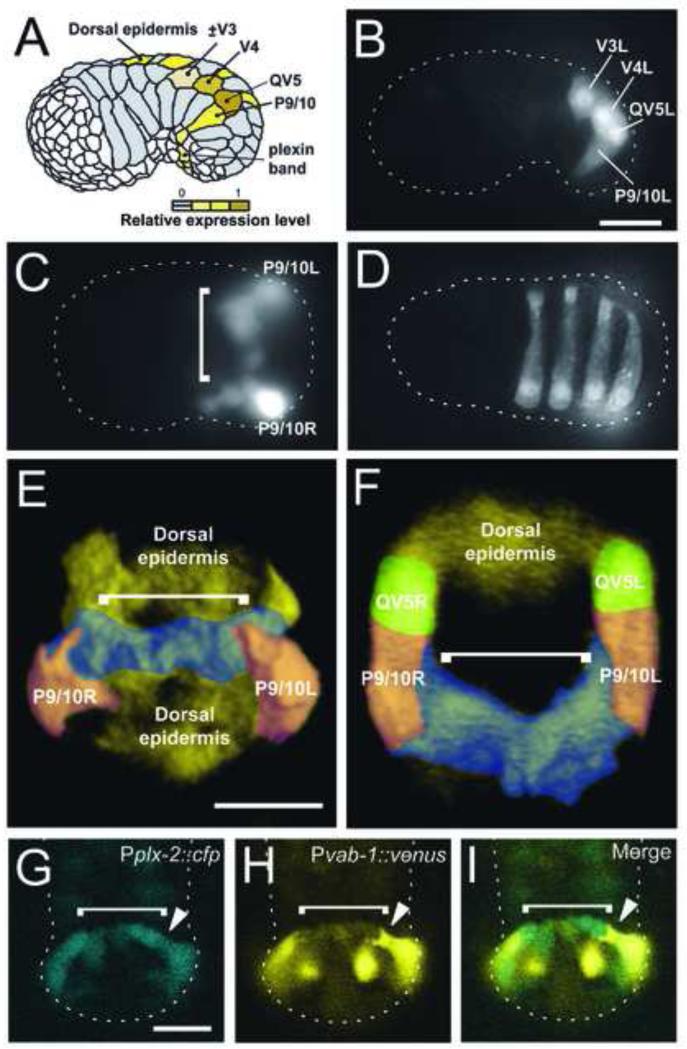
Figure 4. vab-1 and plx-2 are co-expressed in P9/10 and the plexin band.
(A-F) Fluorescence is from Pvab-1::venus(yfp) transcriptional reporter. Anterior is left unless stated otherwise. (A) Summary of vab-1 reporter expressing (yellow) cells. (B) Lateral view. vab-1 is expressed in V3, V4, and co-expressed with plx-2 in QV5 and P9/10. (C) Ventral view. vab-1 is expressed in the plexin band of single cell width on the ventral surface of the open pocket (bracket) prior to P cell migration. (D) Dorsal view. vab-1 is expressed in alternating, left side posterior epidermoblasts overlying the enclosing pocket. (E,F) Reconstructed 3-dimensional ventral view (E) and cross-sectional view (F) from a stack of confocal images of vab-1 expression during early pocket closure. False color was added to provide contrast. Differentially colored cell types are named except the plexin band (blue), indicated by a bracket. (G-I) Anterior is up. Confocal images of the Pplx-2::cfp reporter showing the pocket bridge (bracket) in (G) in direct contact with the migratory leading end of P9/10 (closed arrowhead) of Pvab-1::venus in (H). Merged image in (I) shows plx-2 and vab-1 reporter expression overlaps in P9/10 (closed arrowhead) and the pocket bridge (bracket) with P9/10 migrating on the pocket bridge. Scale bars are 10 μm.
We used time lapse analysis of a plx-2::gfp reporter to characterize cell divisions, movements and associations involved in pocket closure (Figures 1-3 and S1). The results presented below derive from time lapse analysis of 20 wildtype, 14 vab-1(dx31) null, 4 vab-1(e2) kinase dead, and 5 mab-20(ev574) mutant embryos and from visual inspection of dozens of embryos of each genotype by epifluorescence and DIC microscopy, which confirm the time lapse findings.
At the onset of pocket closure, the plx-2 reporter expresses in six blast cells (and sporadically in a 7th – see Figure S1C) on the surface of the open ventral pocket, five of which, after dividing, form a band of ten PLX-2/plexin expressing plexin band cells (Figures 1-3 and S1C in which sister cells are denoted by the same color) that extends across the midline spanning the open pocket. Time lapse analyses show that the plexin band cells are of two functional types. One type comprises 3 sister cell pairs -one roughly D/V-oriented midline proximal sister pair (born just right of the presumptive midline) whose border, following a slight rearrangement, comes to lay on the ventral midline (blue cells in Figures 1 and 2), plus flanking A/P-oriented sister pairs on each side (yellow and green cells in Figures 1 and 2). These 6 cells rearrange slightly so that they span across the ventral midline and form a scaffold for movement of the other set of 4 cells (midline-distal red and orange sister pairs) toward the midline, where they form a single cell wide and deep transverse queue of plx-2 and vab-1 expressing cells on the surface of the open pocket. These 4 cells form a bridge that serves as a substratum for the migration of contralateral P9/10 cells to the midline.
Bridge Formation
Bridge assembly occurs with near left/right mirror-image symmetry. Each midline distal pair of bridge-forming cells on each side (red cell RIGR on the right and orange cell DD1 on the left in Figures 1 and 2A) must migrate over the scaffold cells to meet at the ventral midline. This occurs in phases. First, the anterior sister of each pair of presumptive bridge cells on each side extends a narrow protrusion over the anterior surface of the anterior sister of the adjacent scaffold sister cell pair (see Figure 1B, 320’ and 330’ arrowheads, and Figure 2A, 330’). This protrusion attaches to the border between scaffold cells (Figure 2A, red to blue and orange to blue cell borders) then retracts, pulling the attached presumptive sister bridge cells toward the site of attachment (Figure 2A 330’-340’). This is repeated using the midline proximal scaffold cell as a substratum for protrusion and the border between midline straddling blue cells for attachment. Retraction then pulls the contralateral sister bridge cell pairs to the midline and aligns them along the D/V body wall axis to form the transverse pocket bridge (Figure 2A, 360’-380’), which spans the open ventral pocket from left to right margins. Coordinate with these movements is a general squeezing together of the two sides of the embryo.
P cell migration over the pocket bridge to the ventral midline
As the pocket bridge is assembling, the P9/10 cells begin to migrate over the presumptive bridge cells toward the ventral midline (Figure 1B). A translational reporter for PLX-2 is localized at the leading edge of these and other P cells during this time (Figure 3 D-F). Other P cells may initially contact the bridge cells (Figure 3D inset), but lose contact when the leading edge of each P9/10 cell begins to migrate across the bridge while spanning its entire width (Figure 4I, closed arrowhead). When the P cells reach the midline, they stop, align with their contralateral partners and seal the midline to complete pocket closure. The embryo then begins to turn, marking the onset of circumferential constriction of epidermis and embryo elongation.
Bridge formation and P cell progression to the midline in vab-1 mutants
Mutants of vab-1 and mab-20 are known to affect ventral pocket closure [6, 8]. Many vab-1 null mutant [vab-1(0)] embryos fail to close the open ventral pocket before embryo elongation begins [8, 13]. The residual hole in the mutant epidermis provides a conduit for the extrusion of internal blast cells from the embryo thereby causing lethality (Figure S2A). This closure defect identifies a role for VAB-1 in regulating the ability of the P cells to migrate to the ventral midline before embryo elongation occurs.
While approximately 40% of vab-1(0) mutant embryos die by expelling inner embryonic cells through an open pocket [Figures 5C, S2 and Table S2, see also [8]] or in principle through a weakened midline that breaks open (see below), the remaining embryos successfully complete ventral pocket closure and survive. Among these, only mild misalignments of contralateral P cells occur at the ventral midline (Figure S3 and Table S2).
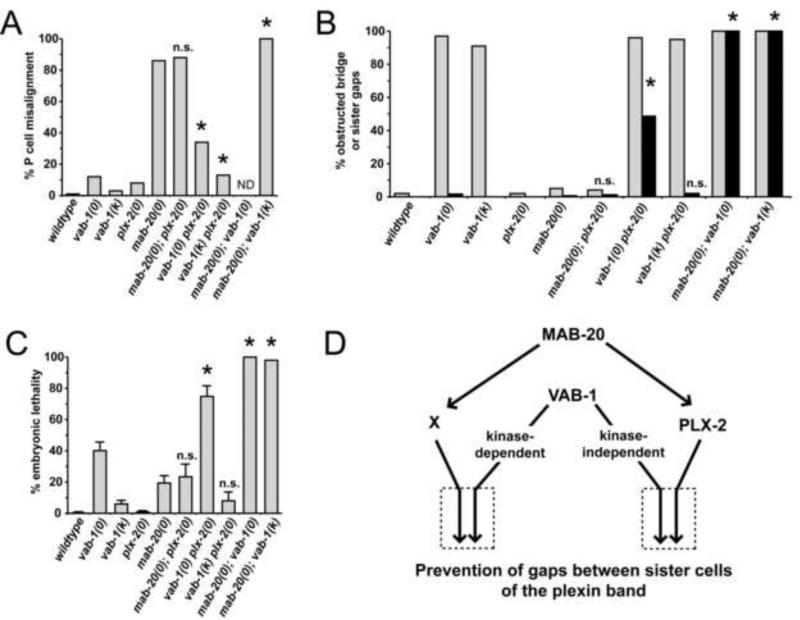
Figure 5. Analysis of P cell ventral midline alignment, pocket bridge formation, and embryonic lethality in vab-1, mab-20 and plx-2 mutants.
Data are from transgenic lines in Table S3. (A-C) Genetic analyses utilized null mutants vab-1(dx31), mab-20(ev574), plx-2(ev773), and kinase-dead mutant (k) vab-1(e2). Strain mIn1[mIs14 dpy-10(e128)] (Edgley and Riddle, 2001), was used to balance the complete and nearly complete embryonic lethality of the mab-20; vab-1(0) and mab-20; vab-1(k) double mutants, respectively. (A) The percentage of L1s with P cell ventral midline alignment defects was scored (N ranges from 121 to 173). The complete embryonic lethality of the mab-20; vab-1(0) precluded midline analysis (ND). (B) The percentage of embryos with an obstructed bridge (gray bars) or gaps between sister plexin band cells (black bars) present at 340’ PFC are shown for wildtype and mutant animals (indicated on the abscissa). Because of mab-20; vab-1 double mutant lethality, only 53 and 45 animals were scored from mab-20; vab-1(0, k) balanced lines, respectively. Essentially all vab-1 null and vab-1 plx-2 double null embryos also had gaps between non-sister plexin band cells. (C) Embryonic lethality was scored as described in Experimental Procedures. Error bars represent standard error of the mean (SEM). Data presented without error bars represent a single sample of size n (see Table S2). Significant interactions (comparing double to single mutants) are shown with an asterisk. For (A, B) a two-sided Fisher's exact test was used and for (C) an unpaired t-test (two-tailed P < 0.05) was used to determine significance of interactions. (D) Genetic model of semaphorin and Eph signaling during pocket closure. The penetrance of single and double mutant pocket closure defects indicate that MAB-20 signals through two mechanisms – PLX-2-dependent and -independent mechanisms (the latter mediated by hypothetical receptor X). Double mutant data suggest that the kinase-independent function of VAB-1 functions redundantly (boxed arrows to the right) with PLX-2, whereas, the kinase-dependent function of VAB-1 functions redundantly (boxed arrows to the left) with unknown (X) to prevent the non-sister gaps and consequent embryonic lethality.
Eph receptors like VAB-1 can have both kinase-dependent and kinase-independent activities[14]. George et al. (1998) previously found that P cell midline alignment and embryonic lethal defects are relatively rare in the kinase dead [vab-1(e2)] mutant embryos compared to vab-1(0) embryos (see also Figures 5A,C and Table S2), suggesting that a kinase independent function of VAB-1 is largely sufficient for these processes.
To further examine the roles of Eph receptor and semaphorin signaling in pocket closure, we followed dozens of carefully staged embryos of vab-1 null and kinase dead mutants and also analyzed null (n=14) and kinase dead (n=4) embryos by time lapse photomicroscopy. The spatiotemporal pattern of plx-2 expression revealed that the pocket bridge and scaffold progenitors, their subsequent divisions, and adhesion between sister cells appeared unaffected in all vab-1 null and kinase dead embryos examined (Figures 1C, 2C and S2). Reported gastrulation defects in vab-1(0) embryos [8] did not produce obvious disorganization of plexin band cells in any embryos we examined, possibly because embryos can correct or bypass these defects. However, we did find that none of the vab-1(0) or vab-1(k) mutant embryos was able to form or maintain the narrow cellular bridge cell protrusions that normally extend over the anterior surface of the scaffold cells to pull the presumptive bridge cells to the midline. These protrusions were also never observed in the staged mutant embryos.
Although the absence of these protrusions is the likely cause of ventral pocket defects in vab-1 null embryos, 97% of these also have gaps between non-sister plexin band cells beyond 340’ post-first cleavage (PFC) of the zygote (Figures 1C 340’, 2C, and S2) when these gaps are usually closed in wildtype embryos (Figures 1B 340’ and 2B). These gaps could, in principle, also be causal for embryonic lethality, however, in most null and kinase dead embryos these gaps are closed with a delay (i.e., after 340’ PFC), possibly by small filopodia-like protrusions sometimes seen emanating from bridge and scaffold cells (Figure S2, 360’ closed arrowhead) or by constriction of the entire midline region. This suggests that these gaps delay rather than block pocket closure.
As a consequence of the protrusion defects or gaps between non-sister cells, the presumptive pocket bridge cells are impeded in their migration over the scaffold cells to reach the midline. The entire group of un-rearranged cells caused by this defect is referred to hereafter as the obstructed bridge even though in many embryos it appears to assemble with a delay just in advance of P9/10 cell migration (see below).
An obstructed pocket bridge variably hindered progression of P9/10 cells toward the midline (Figure 2C,E). In some vab-1 mutant embryos, P9/10 cells progressed minimally toward the ventral midline. In these cases, blocks in P9/10 migration often occurred at borders between scaffold cells (Figure 2E). In other embryos there was substantial progression of P9/10 cells toward the midline, but this was slower than in wildtype embryos, and involved movement of the entire leading edge of the presumptive bridge cells toward the midline just in advance of the P9/10 leading edge (see Discussion).
Five of eight P9/10 cells in kinase dead embryos and only 8 of 28 in null embryos reached the midline before embryo elongation began. There were also few if any kinase dead embryos in which a P9/10 cell failed to migrate at all or minimally toward the midline (0 of 8 compared to 12 of 28 for null embryos). Among the types of mutant embryos observed, only those in which both P9/10 cells fail to migrate (5 of 14 null and 0 of 4 kinase dead) have a severe open pocket defect at the time embryo elongation begins, while others have a small open pocket defect (Figure 2E). These results suggest that the ability of P9/10 cells to migrate over an obstructed pocket bridge is efficient enough in vab-1 null embryos to account for their 60% embryonic viability but even more efficient in vab-1 kinase dead embryos accounting for their 94% viability.
Cell type-specific rescue of P cell midline alignment defects of the vab-1 null mutant
We sought to distinguish a P cell midline alignment function for the VAB-1 kinase and extracellular domains in plexin expressing band cells and P9/10 cells (Figure 6). We did this by first identifying plx-2 regulatory sequences able to drive expression of GFP (Figure S4) in the plexin band or separately in P9/10. Using these promoters to drive vab-1expression, we found that P9/10 cell expressed vab-1(+) does not rescue P cell midline alignment defects of the vab-1 null whereas plexin band expressed vab-1(+) or vab-1(delC) does rescue (Table S3). These results demonstrate that the VAB-1 extracellular domain on the surface of plexin band cells can mediate P cell midline alignment even in the presence of an obstructed pocket bridge. This is consistent with the ability of wildtype and kinase dead alleles to mediate normal P cell alignment.
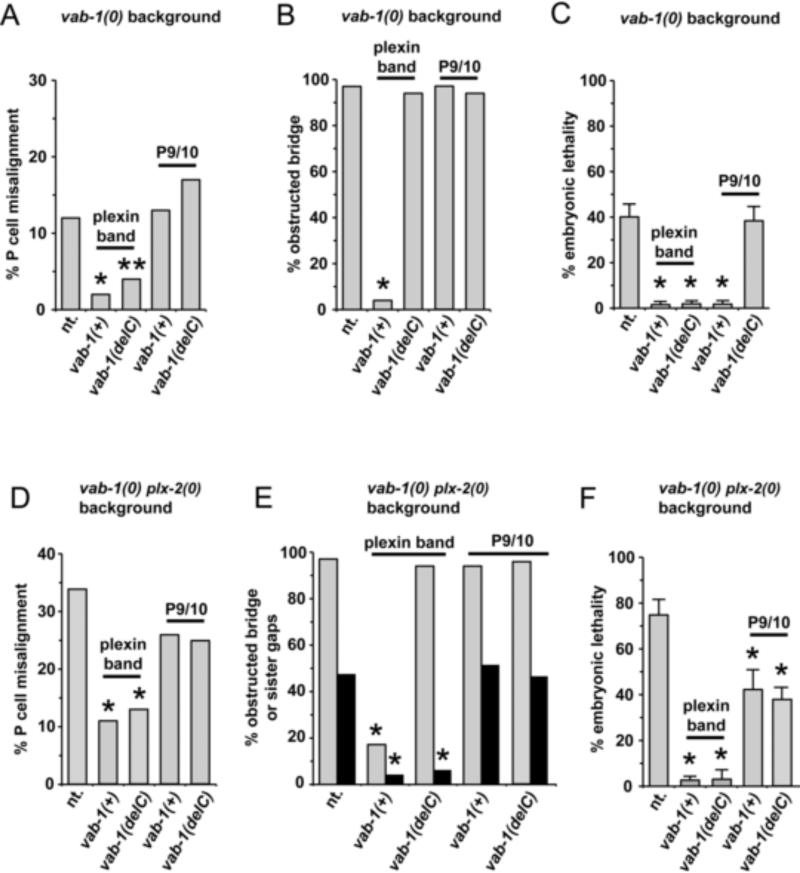
Figure 6. Cell-specific rescue of vab-1 null and vab-1 plx-2 double null mutant embryos by vab-1(+) or vab-1(delC).
Data are from highlighted transgenic lines in Table S3. Panels (A-F) show the fraction of embryos (A,D) with P cell midline ventral alignment defects, (B,E) with an obstructed bridge (gray bars) or with gaps between sister plexin band cells (black bars) at 340’ PFC, and (C,F) that die as embryos from an open pocket defect, for vab-1 null mutant animals (A-C) or for vab-1 plx-2 double null mutant animals (D-F) that carry no vab-1 transgene (abbreviated nt. on abscissa) or carry the transgene indicated on the abscissa expressed by the cell type indicated above the data bars. Error bars represent standard error of the mean (SEM). Data presented without error bars represent a single sample of size n (see Table S3).
PLX-2 and MAB-20 function in P cell guidance and alignment
The penetrance of mab-20 and plx-2 null mutant P cell midline alignment defects are 86% and 8%, respectively (Figure 5A, Table S2). mab-20(0) and plx-2(0) embryos do not have obvious open pocket defects, yet mab-20(0) embryos extrude internal cells from the ventral side [6] suggesting that severe P cell alignment defects weaken the midline causing it to rupture when circumferential constrictive forces of embryo elongation occur.
Despite more severe (Figure S3) and eight times more penetrant midline alignment defects in the mab-20 null (Figures 5, S3A and Table S2), the penetrance of lethality among mab-20(0) embryos is significantly lower than for vab-1(0) embryos (Figure 5C). These data suggest that the P cell alignment defects per se do not contribute significantly to vab-1(0) mutant lethality, instead the lethality results from a more severe effect on pocket closure, including failure of P cells to migrate sufficiently to the midline before embryo elongation occurs or failure to form a proper midline seal.
The plx-2(0) mutation was unable to significantly enhance either the P cell midline alignment defect or the embryonic lethality of the mab-20 null (Figure 5A,C). This is consistent with PLX-2 acting in the same pathway as MAB-20 to prevent these two defects and with the finding by Nakao et al. (2007), that MAB-20 binds PLX-2. The greater lethality and P cell alignment defects of the mab-20(0) suggest that MAB-20 also functions through at least one PLX-2-independent pathway to prevent the two defects (Figure 5D). LAD-2 is another reported receptor for MAB-20 that could mediate the PLX-2 independent functions of MAB-20 [12]. We examined lad-2 mutants and doubles of lad-2 with the plx-2, mab-20 and vab-1 mutant alleles and found very little lethality or enhancement of lethality in any of these strains (Figure S5), ruling out LAD-2 as an important component of ventral enclosure.
Two of five mab-20(0) embryos exhibited a detectable skewing of the P cell leading edges from the D/V body wall axis as they migrate toward the midline (Figure 1D, 370’ inset), not observed in the wild type (n=20). In contrast to vab-1 mutants, none of the mab-20 embryos showed a significant delay in either the onset or rate of P cell migration (n=5). For this reason we suggest that P cell misalignment in mab-20 and plx-2 mutants is caused by a defect in P cell polarity or guidance, or abnormal adhesions of P cell leading tips, but not by a defect in P cell progression toward the midline.
Cell type-specific rescue of pocket closure and P9/10 cell migration over an obstructed vab-1 mutant pocket bridge
We also used the plx-2 regulatory regions to drive vab-1(+) or vab-1(delC) expression in the plexin band cells or in P9/10 to identify in which cell type these genes are able to rescue (1) vab-1 null mutant pocket bridge formation, and (2) the ability of P9/10 to migrate over an obstructed bridge (as measured by prevention of embryonic lethality). At one extreme, P9/10 cell expression of vab-1(delC) did not rescue any of the pocket closure defects of the vab-1 null, and at the other extreme, plexin band expression of vab-1(+) rescued all pocket closure defects of the vab-1 null including formation of an unobstructed bridge and P cell alignment (Figure 6 A-C and Table S3). The latter finding is consistent with a requirement for VAB-1 kinase activity in either the bridge or scaffold cells to extend or maintain the narrow protrusions required for pocket bridge assembly.
Although pocket bridge formation was not significantly rescued by P9/10 expression of vab-1(+), this expression was able to rescue the embryonic lethality of the vab-1 null, as was plexin band expression of vab-1(+) or vab-1(delC) (Figure 6C and Table S3) by facilitating P9/10 cell migration over an obstructed bridge. Thus, vab-1(+) activity in P9/10 cells appears to be equivalent to vab-1(delC) or vab-1(+) activity in the plexin band in rescuing pocket closure defects. This raises the possibility that reverse signaling from VAB-1(delC) on the plexin band to P9/10 cells and forward signaling from the plexin band to VAB-1(+) on the P9/10 cells are equivalent in their ability to facilitate P cell migration over an obstructed pocket bridge, although other explanations are also feasible (see Discussion).
PLX-2 and VAB-1 function redundantly to prevent ‘gaps’ between sister plexin band cells
VAB-1 and PLX-2 appear to have distinct functions in ventral enclosure. For example, unlike vab-1 mutations, plx-2 and mab-20 mutations do not affect pocket bridge formation or the rate of P cell migration to the midline. However, we did observe some unexpected genetic enhancement of embryonic lethality caused by pocket closure defects in vab-1 plx-2 and mab-20; vab-1 double null mutants.
There are at least three patterns of enhancement possible in double mutants. Additive enhancement of a specific defect shared by mutants of two genes (when both cause a loss of function and at least one is a null) suggests that the two genes function in parallel to prevent the defect. Synergistic enhancement by mutants of two genes suggests that the genes may also function partially redundantly to prevent the defect. Synthetic defects are an extreme version of synergistic enhancement that occurs when a defect manifests in the double that was not obvious in either single mutant. This usually indicates that function of each gene is entirely redundant with the other, especially if the defect is specific, unlike a poorly defined defect such as ‘lethality’ or ‘sterility’.
A block or delay in bridge assembly, causing an obstructed bridge, occurs in over 90% of vab-1 null and kinase dead embryos (Figures 5B, S2 and Table S2), so enhancement by plx-2 or mab-20 mutations cannot be determined accurately for this phenotype. However, the few P cell alignment defects of the vab-1 kinase dead mutant are enhanced additively by plx-2 and synergistically by mab-20 null mutations, while the alignment defects of the vab-1 null are enhanced largely additively by the plx-2 null (Figure 5A). These results suggest that the kinase and non-kinase functions of VAB-1 act in a molecular pathway that functions largely in parallel with the PLX-2 pathway, whereas, VAB-1 acts redundantly with MAB-20 to regulate P cell alignment.
The genetic interactions of plx-2 and mab-20 with vab-1 mutations in preventing pocket closure defects are more complex. For example, the vab-1 null mutation enhances both the plx-2 null and the mab-20 null synergistically for embryonic lethality (this enhancement is largely synthetic) (Figure 5C). By contrast, the kinase-deficient vab-1(e2) mutation enhances the embryonic lethality of the plx-2 null additively if at all (Figure 5C), but enhances the mab-20 null synergistically. These results suggest that the kinase function of VAB-1 is redundant with the PLX-2-independent function of MAB-20 and the kinase-independent function of VAB-1 is redundant with the PLX-2-dependent function of MAB-20 in preventing pocket closure defects (see Figure 5D and Discussion).
All plexin band cells (i.e., bridge plus scaffold cells) are present in roughly normal positions in the vab-1 plx-2 double null mutant embryos. However, time lapse analysis shows that double mutant embryos unexpectedly have narrow gaps between sister plexin band cells (hereafter referred to as sister cell gaps) that are not present in the vab-1 null or the wild type (see Figure 2D 340’) in addition to the gaps between non-sister plexin band cells that are found in 97% of vab-1 null embryos. The gaps between sister plexin band cells in the vab-1 plx-2 double null are not as severe as in the mab-20; vab-1 double null since the former have some aggregates of sister cells that are absent in the latter (not shown). These results suggest that PLX-2 and MAB-20 have redundant functions with VAB-1 in forming or maintaining adhesions between sister plexin band cells, just as they have redundant functions with VAB-1 in preventing embryonic lethality.
The pattern of redundancies governing prevention of lethality (last four gray data bars to the right in Figure 5C) is nearly identical to the pattern of redundancies governing the prevention of gaps between sister plexin band cells (last four black data bars to the right in Figure 5B). This strongly suggests that the synergistic enhancement of vab-1 mutant embryonic lethality by plx-2 mutations is caused by the persistence of gaps between sister plexin band cells, which block migrating P9/10 cells from completing pocket closure. This interpretation is supported by cell type-specific rescue data presented below and considered further in the Discussion.
Cell type-specific rescue of the vab-1 plx-2 double null mutant pocket closure phenotypes
There are two obvious contributions to embryonic lethality of the vab-1 plx-2 double null. One is the presence of gaps between sister bridge and scaffold cells, which (unlike gaps between non-sister cells) appear to perdure and block P cell progression to the midline. The other is the impeded ability of P9/10 cells to migrate over an obstructed pocket bridge caused by a vab-1 deficit. We determined the degree of rescue of each of the vab-1 plx-2 double null ventral enclosure phenotypes by plexin band or P9/10 expressed vab-1(+) or vab-1(delC) just as we did for the vab-1 single null. The obstructed bridge phenotype of the double is rescued by plexin band expressed vab-1(+), but not by the other three expression constructs (Figure 6E). This is precisely what was found for rescue of the vab-1 single null (Figure 6B). Double mutant gaps between sister plexin band cells were rescued by expression of vab-1(+) or vab-1(delC) in the plexin band, but not by either P9/10-specific expression construct (Figure 6E). This suggests that gaps between sister plexin band cells may normally be prevented by an adhesive function between these cells provided by the VAB-1 extracellular domain, although kinase independent signaling through the largely resected cytoplasmic domain of VAB-1(delC) cannot be ruled out.
With one exception, the cell type-specific rescue results for the vab-1 plx-2 double mutant defects are as expected based on the rescue of the vab-1 single null using the same transgene arrays. The exception is P9/10 expressed vab-1(delC), which does not rescue lethality of the vab-1 single null (Figure 6C) but unexpectedly does partially rescue the lethality of the double null caused by an obstructed pocket bridge (Figure 6F). A plausible explanation for this result is presented in the Discussion.
DISCUSSION
Formation of the pocket bridge
In the last phase of epiboly in C. elegans, there is a hole or open pocket in the ventral epidermis that must be closed before embryo elongation begins. Epidermoblasts that line this open pocket, called P cells, mediate pocket closure by migrating to the ventral midline where they align with their contralateral analogues and form adherens junctions with them to seal the embryo in epidermis. Our data show that contralateral P cell analogues P9/10 right (R) and P9/10 left (L) can play a primary regulatory role in the pocket closure process by migrating directly toward the midline on a queue of four plx-2 and vab-1 expressing neuron cell bodies that comprise a bridge that spans the width of the open pocket.
The pocket bridge forms from the two most lateral (on each side) of a total of five sister pairs of plx-2 expressing cells that form a contiguous band of cells (the plexin band) that also spans the open pocket (Figures 1-4 and S1). All of these cells except the midline scaffold pair are neurons (Figure S1C), which later extend axons along the A/P body axis. How these neurons make the transition from extending a protrusion along the D/V axis to extending an axon along the A/P axis is unknown, but may depend on signals from the ventral epidermis, which they previously helped to organize.
Pocket bridge assembly involves formation of narrow protrusions by the most anterior of the sister pair of presumptive bridge cells on each side. These protrusions extend over scaffold cells and then pull the anterior bridge cell and its attached sister toward the midline. vab-1 null and kinase dead mutants fail to form or maintain these protrusions leaving the pocket bridge in an unassembled, obstructed state. The exclusive rescue of presumptive bridge cell protrusions by plexin band expressed vab-1(+) suggests that these protrusions require VAB-1 kinase function in the bridge cells or non-autonomously in the scaffold cells. Lacking appropriate promoters to test this, it remains possible that ‘guidance’ cue(s) for bridge cell rearrangements act directly through VAB-1 on the bridge cell surface.
Migration of P9/10 cells across the obstructed pocket bridge still occurs in vab-1 mutants, but is slower than movement across a mature pocket bridge. This movement does not involve the formation of distinct cellular protrusions, but rather appears to involve movement of the entire leading edge of the presumptive bridge cells in advance of the leading edge of the P9/10 cell by unknown molecular mechanisms such that P9/10 may not contact the scaffold cells. vab-1 null embryos are slower in this movement than kinase dead embryos accounting for the finding that 40% of the null and only 6% of the kinase dead embryos have an open pocket at the time embryo elongation occurs. During migration over an obstructed bridge, there is a tendency for P9/10 cells to become delayed or blocked at one of two positions on each side that correspond to the normal positions at which presumptive bridge cell protrusions are formed (Figure 2E). This raises the possibility that the hesitation at these positions is caused by the same signals needed to initiate the extension or retraction of these protrusions.
When both P9/10 cells become persistently blocked at the midline-distal positions this creates a large open pocket, whereas other blocks create a subtle open pocket defect (Figure 2E). For technical reasons we were unable to follow these embryos during embryo elongation, so we could not determine which of the time lapsed embryos extruded cells from their open pocket and died. However, five of fourteen (36%) of the mutant embryos appeared blocked with a large open pocket, which is roughly equal to the known penetrance of vab-1 null mutant embryonic lethality (40%). This is consistent with the idea that most embryos with large open pockets die and shows that some embryos with subtly open pockets are likely to survive their pocket defects.
VAB-1 kinase function in P9/10 can facilitate its migration over an obstructed bridge
VAB-1 kinase activity has two important cell type-specific functions. As described above, VAB-1 kinase function in the plexin band is required for normal bridge formation. However, P9/10 migration over an obstructed bridge (caused by a vab-1 null mutant defect) can be rescued by P9/10 expressed vab-1(+) (which rescues the narrow protrusions required for bridge formation) or by plexin band expressed vab-1(delC). The latter rescue can not be explained by rescue of bridge cell protrusions, but it could be explained by any of at least three molecular mechanisms that could facilitate P9/10 migration over an obstructed bridge. One is that VAB-1(delC) (which retains an 87 residue juxtamembrane domain that could be involved) is activated by an unknown ligand to transduce a signal in the plexin band cells that makes them a better substratum for P cell migration. A second is that the VAB-1 extracellular domain on the plexin band simply provides a change in adhesive function. A third possibility is that signaling from VAB-1(delC) on the plexin band to P9/10 (probably to an ephrin on P9/10) is equivalent to the forward signaling from an unknown ligand to VAB-1(+) on P9/10. The last possibility raises the speculation that the signal transduction mechanism activated by forward signaling from an ephrin (presumably on bridge cells) through VAB-1 kinase on the P9/10 cells could be functionally equivalent to the signal transduction cascade activated by reverse signaling of the VAB-1 extracellular domain on the bridge cells to an ephrin on P9/10. Examination of the role of cell type-specific expression of various ephrins in P cell motility and ventral enclosure will be required to shed light on these models.
P cell alignment is non-autonomously regulated by the VAB-1 extracellular domain on plexin band cells
The difference between the penetrance of P cell midline alignment defects in vab-1 null and kinase dead embryos raises the simple expectation that the VAB-1 extracellular domain mediates interactions between contralateral P cell partners that cause alignment. However, this is surprisingly not the case since only plexin band expression of vab-1(+) or vab-1(delC) rescues the P cell alignment defects of a vab-1 null, whereas, P9/10 expression of either form of vab-1 does not.
Plexin band expressed vab-1(+) and vab-1(delC) also rescue pocket closure defects, raising the possibility that closure defects are causal for the alignment defects of a vab-1 null. This is unlikely since there are arrays that rescue pocket closure, as measured by embryonic lethality, but do not rescue alignment (Figure 6A-C). These results suggest that P cell alignment requires the VAB-1 extracellular domain, non-autonomously, on the plexin band and that misalignments in vab-1 null embryos are not caused by migration over an obstructed pocket bridge (which happens in null and kinase dead mutants) or by impeded pocket closure per se. In principle, the VAB-1 extracellular domain on the plexin band cells may signal to P9/10 cells or mediate an adhesion between these two cell types for P cell alignment.
P9/10 cells as primary regulators of P cell migration to the midline
vab-1 mutant defects in bridge formation not only severely hinder P9/10 but also P3/4 and P5/6 migration to the ventral midline. Furthermore, any cell type-specific rescue of a vab-1 mutant that facilitates P9/10 migration to the midline also facilitates the migration of other P cells to the midline, including expression of vab-1(+) in P9/10. These results suggest that expression in P9/10 is sufficient to rescue migration of other P cells to the midline but may not be unique in this respect.
It is possible that coordinate blockage and rescue of neighboring P cell migrations to the midline requires their attachment to one another via, for example, the actin cable at the leading edge of the P cells [15]. By this model, other P cells would be pulled to the midline by P9/10, but once in the vicinity of the midline, their leading tips might be able to independently find and attach but not necessarily align to contralateral P cells. This would explain why late blocks in P9/10 cell migration do not affect the ability of P3/4 or P5/6 cells to reach the midline, whereas, early blocks do.
P9/10 cells also appear to play a central regulatory role in P cell alignment at the midline since plexin band expressed vab-1(delC) non-autonomously rescues P cell alignment defects along the entire midline axis even though only P9/10 have intimate contact with the obstructed pocket bridge in a vab-1 null. This suggests that tracking of P9/10 along even an obstructed pocket bridge can keep all the P cells in correct register with one another and cause them to align at the midline.
Semaphorin-2a/mab-20 mutations cause defects in the guidance of the leading edge of P cells
PLX-2 clearly functions in the same pathway as MAB-20 for pocket closure [7]. The milder and less penetrant effect of the plx-2 null compared to a mab-20 null suggests that MAB-20 plays an additional PLX-2-independent role in pocket closure, as in male ray cell sorting [16]. Our results suggest that MAB-20 and PLX-2 affect P cell midline alignment by a different mechanism than VAB-1. Nevertheless, the more than additive penetrance of the vab-1 plx-2 double null mutant suggests that semaphorin and Eph receptor signaling have parallel and possibly partially redundant functions in the alignment process.
VAB-1 has a redundant function with PLX-2 in regulating sister plexin band cell adhesions and a non-redundant function in facilitating P9/10 cell migration over an obstructed pocket bridge
There are two contributions to open pocket defects and consequent embryonic lethality in vab-1 plx-2 double mutants. One comes from the gaps that are present between sister plexin band cells. This defect, which has a penetrance of 48%, depends on a deficiency in both vab-1 and plx-2 function. Another contribution to double mutant lethality is the reduced ability of P9/10 to migrate over an obstructed pocket bridge. Although this defect can clearly be caused by a deficiency in vab-1 function alone, in principle, the plx-2 mutation in the double could further reduce the ability of P9/10 to migrate over an obstructed bridge. If there were no effect of the plx-2 mutation on migration over the double mutant obstructed bridge, this would predict that lethality would occur in all 48% of embryos with sister cell gaps plus 40% of those embryos without sister cell gaps (or 40% of 52% = 21%) or a total of 69% lethality for double mutant embryos, which is not significantly different from the observed lethality of 75% (Figure 5C and Table S2). Thus in the double mutant, sister cell gaps prevent P9/10 progression to the ventral midline, however a plx-2 deficit in embryos without sister cell gaps does not further hinder P9/10 progression over an obstructed bridge, rather this hindrance results from the vab-1 deficit alone. However, since the penetrance of obstructed bridges is nearly 100% in these double mutants, the sister cell gaps are almost never present alone, therefore we cannot yet be certain if the sister cell gaps alone cause open pocket defects or if they only do so only in combination with an obstructed bridge.
These two contributions to lethality in vab-1 plx-2 double mutant embryos represent two separable functions of VAB-1 as determined by cell-specific rescue experiments. For example, as described above, the inability of P9/10 to migrate over an obstructed bridge can be rescued by vab-1(+) or vab-1(delC) expressed in either P9/10 or plexin band cells, respectively. In contrast, only plexin band expressed vab-1(+) or vab-1(delC) can prevent sister plexin band cell gaps. This likely reflects a redundant adhesive function of the VAB-1 extracellular domain on the surface of the plexin band with PLX-2. In the future it will be interesting to determine whether the site of redundant PLX-2 function in preventing sister cell gaps is also on the surface of the pocket bridge.
Remarkably, the synergistic interactions identified by double mutant phenotypes are complex (Figure 5D). These results suggest that MAB-20 has PLX-2 independent functions that are redundant with the kinase activity of VAB-1 while its PLX-2 dependent functions are redundant with a non-kinase activity of VAB-1 to prevent the extra lethality observed in the vab-1 plx-2 double mutant caused by sister plexin band cell gaps. This is probably also the case for the redundancy of the VAB-1 kinase with the PLX-2 independent function of MAB-20 since the mab-20; vab-1 doubles are fully penetrant for gaps between sister plexin band cells. This complex pattern of redundancy highlights the utility of genetics in elucidating redundancies that might otherwise go unnoticed.
PLX-2 may normally inhibit a component of the VAB-1 signaling cascade in the P cells to regulate pocket closure
With one exception, all of the vab-1 plx-2 double mutant rescue results are completely consistent with expectations based on what is known about the non-redundant and plx-2-redundant functions of vab-1 in pocket closure. The exception is the finding that P9/10 expressed vab-1(delC) can partially rescue the embryonic lethality of the double null that is caused by a putative deficit in P cell motility over an obstructed bridge even though it is totally incapable of rescuing any ventral enclosure phenotype of the vab-1 single null. This suggests that loss of PLX-2 function somehow allows a function of VAB-1(delC) to compensate for loss of its cytodomain (e.g., kinase) activity. Such compensation could occur if, for example, PLX-2 normally antagonizes a function that is mediated by VAB-1(delC). Whether this or other molecular mechanisms are responsible for the unexpected rescue of the double mutant are important questions for future study.
Conclusions
Several features of ventral enclosure were identified in these studies including the sorting of cells by VAB-1 dependent protrusions and the redundant function of PLX-2/plexin and VAB-1/Eph receptors in regulating adhesion between sister plexin band cells to assemble a bridge of cells that serve as a substratum for P9/10 cell movements to the ventral midline. Entirely unexpected was the finding that the VAB-1 extracellular domain works from the surface of the underlying plexin band cells to mediate P cell alignment since one might predict that a simple adhesive mechanism between contralateral P cell partners could suffice. A second unexpected finding is that P cell migration can occur over an obstructed bridge, presumably as a fail-safe process. Surprisingly this can be facilitated by another VAB-1 function independent of its bridge-forming function. VAB-1 can enhance migration of P9/10 cells over an obstructed bridge in two different ways, one involving forward signaling from plexin band cells to P9/10 and the other involving kinase independent signaling (possibly reverse signaling from plexin band to P9/10 or kinase independent forward signaling from P9/10 to the plexin band). P9/10 cells can play a central regulatory role in basically all aspects of pocket closure as evidenced by cell type-specific rescue results that are not attributable to the direct rescue of other P cells. Together, these findings may represent a new basis for understanding body wall closure in mammals, which like C. elegans, use Eph receptor signaling [4, 5].
EXPERIMENTAL PROCEDURES
Caenorhabditis elegans strains and maintenance
Bristol strain was grown at 20°C and cultured as described previously [17]. The following genes and alleles were used in this study: LG I: mab-20(ev574); LG II: lin-7(e1413), vab-1(dx31), vab-1(e2), plx-2(ev773), mIn1[mIs14 dpy-10(e128)] (Edgley and Riddle, 2001); LG VI: jcIs1[AJM-1::GFP] [18].
DNA Constructs
plx-2 and vab-1 expression constructs were used to make the following transgene arrays: evIs190[Pvab-1::venus; pRF4]; evIs168[PLX-2::GFP; pRF4]; evIs136[Pplx-2::gfp; pRF4]; and evIs191[Pplx-2::cfp; pRF4] (summarized in Table S1). pRF4 encodes the dominant rol-6(su1006) co-transformation marker. Table S4 summarizes the derivation of constructs and corresponding transgene arrays used to identify plx-2 promoter regions to drive P9/10 and plexin band specific expression. Table S5 summarizes the constructs that use these promoter regions to test cell type specific rescue of mutant embryos.
Double mutant constructs
Multiply mutant strains were constructed by standard methods (Brenner, 1974). The plx-2(ev773) null mutation was passed into various strains using closely linked lin-7(e1413) as a balancer. A PCR assay was used to confirm the homozygosity of the plx-2(ev773) deletion.
Imaging and time lapse photomicroscopy
Gravid hermaphrodites were cut transversely through the vulva. Extruded embryos were mounted on 2% agarose pads in M9 solution. Transgenic reporters were observed by epifluorescence using a Leica DMRA2 microscope. Images were captured using a Hamamatsu camera and OpenLab™ software. Time lapse imaging of embryos employed multiphoton laser scanning microscopy using a SpectraPhysics Ti:Sapphire laser tuned to 900 nm as described previously [19]. Data acquisition and stereo-4D processing were performed as described in [20]. Expressing cells were identified by following division patterns, timing and position by time lapse relative to the reported cell lineage [21].
Embryonic lethality assays
Individual L4 larval stage animals were allowed to develop and lay eggs under standard growth conditions at 20°C until egg-laying ceased. Lethality was determined by counting hatched and un-hatched eggs. Ten to fourteen broods were followed for each genotype.
Statistical analyses
Embryonic lethality was expressed as a mean of a sample proportion of n broods of a single genotype and assumed to follow a binomial distribution. The confidence interval for the mean value was estimated as the standard error of the mean (SEM) and is shown in Figures 5 and 7 by the error bars. To test for significant genetic interactions in embryonic lethality, we used an unpaired t-test since these values represent continuous variable data. To test for significant genetic interactions for P cell alignment and pocket bridge defects, a two-sided Fisher's Exact Test utilizing a 2x2 contingency table was used since these values represent categorical data (absence vs. presence of defect). For all tests, a two-tailed P value of 0.05 was used as the cutoff for statistical significance (P < 0.05).
Supplementary Material
HIGHLIGHTS.
- Epidermal P cell migration to the ventral midline encloses the C. elegans embryo.
- VAB-1/Eph receptor is required to build a neuronal bridge for P cell migration.
- VAB-1 in bridge-forming or in P cells speeds P cell migration over mutant bridge.
- VAB-1 functions redundantly with semaphorin signaling in bridge formation.
ACKNOWLEDGEMENTS
We thank the assistance and advice of K. Eliceiri and J. White of the Laboratory for Optical and Computational Instrumentation (LOCI) supported by NIH grant RR00570. We also thank Y. Kohara (National Institute of Genetics, Mishima, Japan) for cDNAs, A. Fire (Stanford, CA) for GFP vectors, and N. Levy-Strumpf, T. Kawano, K. Fujisawa, and J. Plummer for their critical reading of the manuscript. Some worm strains were obtained from the Caenorhabditis Genetics Center, funded by the NIH National Center for Research Resources. This work was supported by an Ontario Graduate Scholarship to R.I. and by grants from the CIHR and the NIH (NS41397) to J.C. K.S. and J.H. were supported by NIH grant GM058038 awarded to J.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brewer S, Williams T. Finally, a sense of closure? Animal models of human ventral body wall defects. Bioessays. 2004;26:1307–1321. doi: 10.1002/bies.20137. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez BG, Arias AM, Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev. 2007;124:884–897. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 4.Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. Embo J. 1996;15:6035–6049. [PMC free article] [PubMed] [Google Scholar]
- 5.Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 6.Roy PJ, Zheng H, Warren CE, Culotti JG. mab-20 encodes Semaphorin-2a and is required to prevent ectopic cell contacts during epidermal morphogenesis in Caenorhabditis elegans. Development. 2000;127:755–767. doi: 10.1242/dev.127.4.755. [DOI] [PubMed] [Google Scholar]
- 7.Nakao F, Hudson ML, Suzuki M, Peckler Z, Kurokawa R, Liu Z, Gengyo-Ando K, Nukazuka A, Fujii T, Suto F, et al. The PLEXIN PLX-2 and the ephrin EFN-4 have distinct roles in MAB-20/Semaphorin 2A signaling in Caenorhabditis elegans morphogenesis. Genetics. 2007;176:1591–1607. doi: 10.1534/genetics.106.067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George SE, Simokat K, Hardin J, Chisholm AD. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell. 1998;92:633–643. doi: 10.1016/s0092-8674(00)81131-9. [DOI] [PubMed] [Google Scholar]
- 9.Chin-Sang ID, George SE, Ding M, Moseley SL, Lynch AS, Chisholm AD. The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell. 1999;99:781–790. doi: 10.1016/s0092-8674(00)81675-x. [DOI] [PubMed] [Google Scholar]
- 10.Chin-Sang ID, Chisholm AD. Form of the worm: genetics of epidermal morphogenesis in C. elegans. Trends Genet. 2000;16:544–551. doi: 10.1016/s0168-9525(00)02143-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Roy PJ, Holland SJ, Zhang LW, Culotti JG, Pawson T. Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol Cell. 1999;4:903–913. doi: 10.1016/s1097-2765(00)80220-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Zhang W, Cheever T, Schwarz V, Opperman K, Hutter H, Koepp D, Chen L. The C. elegans L1CAM homologue LAD-2 functions as a coreceptor in MAB-20/Sema2 mediated axon guidance. J Cell Biol. 2008;180:233–246. doi: 10.1083/jcb.200704178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priess JR, Hirsh DI. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol. 1986;117:156–173. doi: 10.1016/0012-1606(86)90358-1. [DOI] [PubMed] [Google Scholar]
- 14.Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 15.Williams-Masson EM, Malik AN, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124:2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami R, Zheng H, Ong SH, Culotti J. Integration of semaphorin-2A/MAB-20, ephrin-4, and UNC-129 TGF-beta signaling pathways regulates sorting of distinct sensory rays in C. elegans. Dev Cell. 2004;6:383–395. doi: 10.1016/s1534-5807(04)00057-7. [DOI] [PubMed] [Google Scholar]
- 17.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppen M, Simske JS, Sims PA, Firestein BL, Hall DH, Radice AD, Rongo C, Hardin JD. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3:983–991. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- 19.Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol Biol Cell. 1998;9:2037–2049. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohler WA, Simske JS, Williams-Masson EM, Hardin JD, White JG. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr Biol. 1998;8:1087–1090. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- 21.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.