Abstract
Serum amyloid A (SAA) has a number of proatherogenic effects including induction of vascular proteoglycans. Chronically elevated SAA was recently shown to increase atherosclerosis in mice. The purpose of this study was to determine whether a brief increase in SAA similarly increased atherosclerosis in a murine model. The recombination activating gene 1-deficient (rag1−/−) × apolipoprotein E-deficient (apoe−/−) and apoe−/− male mice were injected, multiple times or just once respectively, with an adenoviral vector encoding human SAA1 (ad-SAA); the injected mice and controls were maintained on chow for 12–16 weeks. Mice receiving multiple injections of ad-SAA, in which SAA elevation was sustained, had increased atherosclerosis compared with controls. Strikingly, mice receiving only a single injection of ad-SAA, in which SAA was only briefly elevated, also had increased atherosclerosis compared with controls. Using in vitro studies, we demonstrate that SAA treatment leads to increased LDL retention, and that prevention of transforming growth factor beta (TGF-β) signaling prevents SAA-induced increases in LDL retention and SAA-induced increases in vascular biglycan content. We propose that SAA increases atherosclerosis development via induction of TGF-β, increased vascular biglycan content, and increased LDL retention. These data suggest that even short-term inflammation with concomitant increase in SAA may increase the risk of developing CVD.
Keywords: apolipoproteins, extracellular matrix, lipoproteins, proteoglycans, vascular biology, free-form: biglycan, transforming growth factor beta, cardiovascular disease
Serum amyloid A (SAA) is a family of apoproteins highly expressed during an acute phase response (APR), the body’s initial response to infection or trauma. Humans and mice each have two acute phase isoforms of SAA synthesized primarily in the liver, but also expressed in vascular smooth muscle cells (VSMCs), adipocytes, and macrophages (1, 2). During an APR, SAA levels can increase up to 1,000-fold, during which SAA becomes the primary apoprotein on HDL (3). The increase in SAA during an APR is thought to play a role in normal host response to an immunogenic stimuli; however, diabetes and obesity as well as other chronic inflammatory diseases are characterized by having persistently elevated SAA expression (4, 5). SAA, like C-reactive protein, is a marker of inflammation and predictive of CVD events (6). The observation that SAA is increased in diabetes and obesity and that individuals with those diseases have an increased risk of developing CVD led to the question of whether SAA could play a causal role in CVD. SAA has several characteristics that make it potentially atherogenic. It can enhance monocyte recruitment by increasing expression of chemokine ligand 2 (CCL2) in a formyl peptide receptor-like dependent manner (7), directly stimulate foam cell formation by upregulating lectin-like oxidized LDL receptor 1 (8), stimulate chemotaxis of lymphocytes to subcutaneous sites of recombinant SAA injection (9), and facilitate the binding of HDL to vascular proteoglycans (10). SAA has been found in atherosclerotic lesions of both LDL receptor and apolipoprotein E-deficient (apoe−/−) mice colocalized with apoB- and apoA-I-containing lipoproteins (11). SAA mRNA has also been detected in many different cell types in human atherosclerotic lesions (2). Recently, Dong et al. (12) overexpressed murine SAA1 via a lentiviral vector in apoe−/− mice and demonstrated that modest but sustained elevation of SAA led to increased atherosclerosis through increased inflammatory cell infiltration into the lesion (12).
As outlined in the response to retention hypothesis (13), atherosclerosis is thought to be a disease of lipoprotein retention followed by inflammation. In the earliest stages of the disease, lipoproteins such as LDL are retained by extracellular matrix proteoglycans. The small leucine-rich proteoglycan biglycan is the proteoglycan most consistently found colocalized with LDL (14, 15). The retained LDL becomes chemically modified triggering an inflammatory response characterized by the infiltration of macrophages into the subendothelial space leading to foam cell formation. The lesion development progresses, ultimately leading to clinically significant complex atheroma (16). We have previously shown that stimulating VSMCs with physiologically relevant doses of SAA resulted in a dose-dependent increase in proteoglycan synthesis, especially biglycan. SAA-stimulated VSMCs secreted biglycan with longer glycosaminoglycan side chains and greater affinity for LDL suggesting another potentially proatherogenic role for SAA. Interestingly, the increase in biglycan synthesis was ameliorated when the transforming growth factor beta (TGF-β) inhibitory antibody 1D11 was given concurrently with SAA, suggesting that SAA acts through TGF-β to increase vascular biglycan. The apoe−/− mice injected with an adenoviral vector encoding human SAA1 (ad-SAA) had increased vascular biglycan content. The increase in vascular biglycan content was seen after only a brief increase in SAA (17). Thus, the present study was performed to determine whether a brief increase in SAA, such as that seen in an APR, was sufficient to increase atherosclerosis. Here we demonstrate that not only sustained but also brief increases in SAA caused increased atherosclerosis in a murine model. This novel finding is clinically relevant given that epidemiological data suggest that survivors of intensive hospitalization or injury with a concomitant APR have increased subsequent mortality primarily from CVD (18–20).
MATERIALS AND METHODS
All work was completed in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals and the University of Kentucky Animal Care and Use Committee guidelines.
Primary VSMCs were isolated from ∼3-week-old apoe−/− mice (stock # 002052) and grown to confluence without passaging. Cells were incubated with vehicle, sodium chlorate, and endotoxin free SAA (25 μg/ml) isolated from acute phase murine plasma (21) (kindly provided by Frederick and Maria de Beer, University of Kentucky) ± TGF-β-neutralizing antibody 1D11 (10 µg/ml, MAB1835; R and D Systems, Minneapolis, MN), control antibody 13C4 (10 µg/ml, Custom antibody; GenScript, Piscataway, NJ) or 10 mM sodium chlorate (Sigma-Aldrich, St. Louis, MO) (22), or TGF-β (T7039, Sigma-Aldrich) ± sodium chlorate for 24 h. The cells were washed and then labeled with Alexa-fluor 594 (A10237; Life Technology, Grand Island, NY) labeled LDL for 2 h at 4°C. Cells were washed twice in cold PBS then fixed in 4% formaldehyde and imaged and quantified using ImageJ software. LDL binding is expressed as Alexa-fluor 594 surface area normalized to 4,6-diamidino-2-phenylindole (DAPI) surface area from 5 to 10 20× microscope fields. Negative controls included wells with no cells and/or no LDL.
For all in vivo studies, apoe−/− (stock # 002052) and recombination activating gene 1-deficient (rag1−/−; stock # 003729) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The rag1−/− × apoe−/− mice were generated in our breeding colony. Beginning at 8 weeks of age, male rag1−/− × apoe−/− mice were injected via the lateral tail vein every 21 days with 1011 plaque forming units of an ad-SAA, a control adenoviral vector (ad-Null), or saline as previously reported (17). These mice were utilized as the rag1−/− made them tolerant to repeated viral injections facilitating chronic expression of SAA (model of sustained elevation in SAA). Eight-week-old apoe−/− male mice, also from the Jackson Laboratory, received a single lateral tail vein injection of ad-SAA, ad-Null, or saline (model of brief elevation in SAA). In some experiments, mice were concurrently injected with 1D11 or 13C4 (2 mg/kg, ip). All studies were performed on male mice housed in specific pathogen-free caging systems with 12 h light/dark cycles and ad lib access to normal rodent chow (Harlan Teklad) and water. After 12 weeks (rag1−/− × apoe−/−) or 16 weeks (apoe−/−), mice were euthanized and tissues collected. Plasma levels of human SAA and murine SAA were measured by species-specific ELISA kits from Anogen (EL10015; Mississauga, Ontario, Canada) and Life Technologies (KMA0021; Grand Island, NY), respectively. Atherosclerosis was measured on the aortic intimal surface, the aortic root, and the brachiocephalic artery as previously described (23). Aortic root sections from ad-SAA, ad-Null, and saline groups were double labeled for biglycan (AF2667; R and D Systems) and apoB (K23300R; BioDesign International, Saco, ME), apoB and perlecan (MAB458; Millipore, Billerica, MA), biglycan and SAA (kindly provided by the de Beer laboratory, University of Kentucky), or isotype controls and visualized using confocal or fluorescent microscopy as previously described (24). Aortas and/or carotid arteries were collected from apoe−/− mice and analyzed for vascular biglycan content by Western blot for biglycan or actin and analyzed by densitometry using ImageJ software (National Institutes of Health). Total plasma cholesterol, triglycerides, TGF-β, and lipoprotein distribution by fast-performance liquid chromatography were measured as previously described (24). Statistical differences were assessed by one-way ANOVA to assess effects of treatment with ad-SAA, ad-Null, or saline; or ad-SAA and 1D11 or 13C4. P < 0.05 was considered statistically significant.
RESULTS
The response to retention hypothesis states that lipoprotein retention by vascular matrix proteoglycans is an initiating event in atherosclerosis (16). To determine whether SAA increased the binding of apoB-containing lipoproteins to vascular matrix, primary apoe−/− VSMCs were incubated with vehicle or SAA for 24 h followed by incubation with Alexa-594-labeled LDL for 2 h. Our previous work revealed that SAA acted through TGF-β to stimulate biglycan synthesis in VSMCs (17). To determine whether SAA acted via TGF-β, parallel wells were treated with TGF-β, the TGF-β neutralizing antibody 1D11, or irrelevant control antibody 13C4. Proteoglycans interact with apoB-containing lipoproteins via ionic interactions between positively charged residues on apoB and negatively charged sulfate ions on the proteoglycan glycosaminoglycan side chains (25). To determine whether the LDL binding was the direct result of proteoglycan/lipoprotein interaction, apoe−/− VSMCs were treated with the sulfate biosynthesis inhibitor sodium chlorate, resulting in glycosaminoglycan side chains with a neutral charge thereby inhibiting the proteoglycan/lipoprotein interaction. Cells treated with SAA or TGF-β, known to stimulate proteoglycan synthesis, had increased LDL retention compared with vehicle-treated cells (P < 0.001; Fig. 1); however, the presence of sodium chlorate attenuated this effect, implying that the increased LDL binding was due to increased proteoglycan synthesis and/or sulfation. When cells were coincubated with SAA and the TGF-β inhibitory antibody 1D11, LDL binding was reduced compared with SAA alone; however, there was no effect of the control antibody 13C4, further supporting the role for TGF-β in SAA-mediated lipid retention.
Fig. 1.
SAA-stimulated VSMC-secreted matrix proteoglycans from apoe−/− mice had increased LDL binding, which was dramatically reduced when TGF-β was inhibited. VSMCs were treated with vehicle, SAA, or TGF-β for 24 h, then washed and incubated with Alexa-594-labeled LDL for 2 h. SAA- and TGF-β-treated cells had increased LDL binding compared with vehicle treatment. To determine the necessity of TGF-β in SAA-mediated LDL retention, VSMCs were treated with SAA and either the TGF-β-neutralizing antibody 1D11 or control antibody 13C4. The 1D11 prevented the SAA increase in LDL binding; there was no effect of 13C4. To determine the role of proteoglycans in the SAA-mediated LDL binding, cells were treated with sodium chlorate, which prevents sulfation of proteoglycan glycosaminoglycan side chains. Sodium chlorate prevented both the SAA- and TGF-β-dependent increase in LDL binding. LDL binding is expressed as Alexa-fluor 594 surface area normalized to DAPI surface area quantified by fluorescent microscopy using ImageJ software (NIH). Data are presented as mean ± SEM from 5 to 10 20× regions/condition. * P < 0.001.
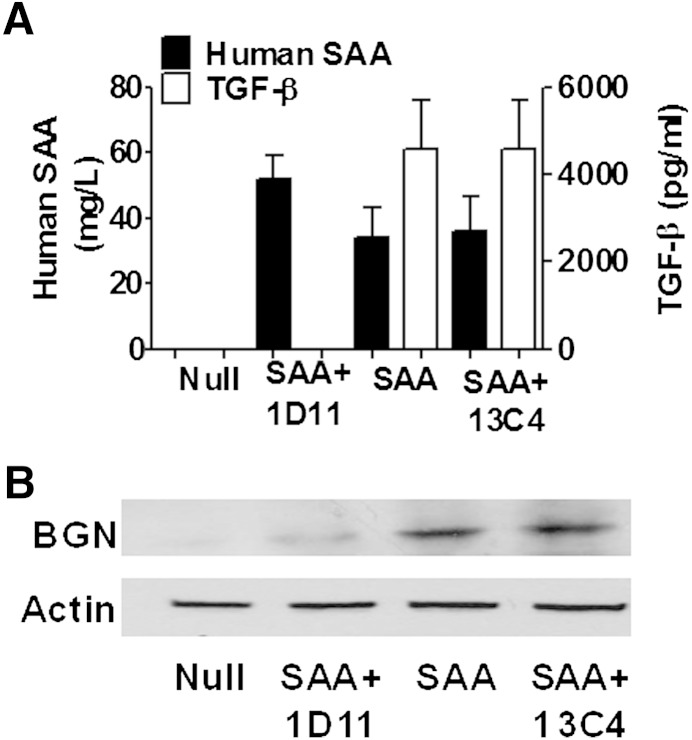
We previously demonstrated in apoe−/− mice that a brief increase in SAA led to increased vascular biglycan content 28 days later (17). To determine whether TGF-β is required in vivo for SAA induction of biglycan, apoe−/− mice were injected with ad-Null, ad-SAA, or ad-SAA concurrently with 1D11 or 13C4. Plasma was collected 24 h after the injections and assayed for human SAA and TGF-β. As expected, mice receiving ad-Null had no detectable SAA or induction of TGF-β. Mice that received ad-SAA alone or in combination with 13C4 had dramatic induction of both SAA and TGF-β. However, mice receiving ad-SAA + 1D11 antibody had the same dramatic increase in plasma SAA observed in the ad-SAA and ad-SAA + 13C4 groups, yet the SAA-induced increase in plasma TGF-β was completely inhibited (Fig. 2A). Mice receiving ad-SAA or ad-SAA with 13C4 had an increase in vascular biglycan content 28 days after injections compared with ad-Null-injected mice; however, 1D11 strikingly attenuated SAA- induced vascular biglycan content (Fig. 2B).
Fig. 2.
Inhibition of TGF-β prevented ad-SAA increase in vascular biglycan content. The apoe−/− mice were injected with ad-Null, ad-SAA, or ad-SAA with the TGF-β-neutralizing antibody 1D11 or control antibody 13C4. A: Human SAA and TGF-β were measured in plasma collected 24 h after injections. 1D11 prevented the ad-SAA induction of TGF-β but had no effects on plasma SAA; N = 4–5/group. B: Aortas were collected 28 days after injections and immunoblotted for biglycan (BGN) or actin. The 1D11 prevented the ad-SAA induction of vascular biglycan, but 13C4 had no effect. Each lane shows protein from an individual mouse representative of 4–5 mice per group.
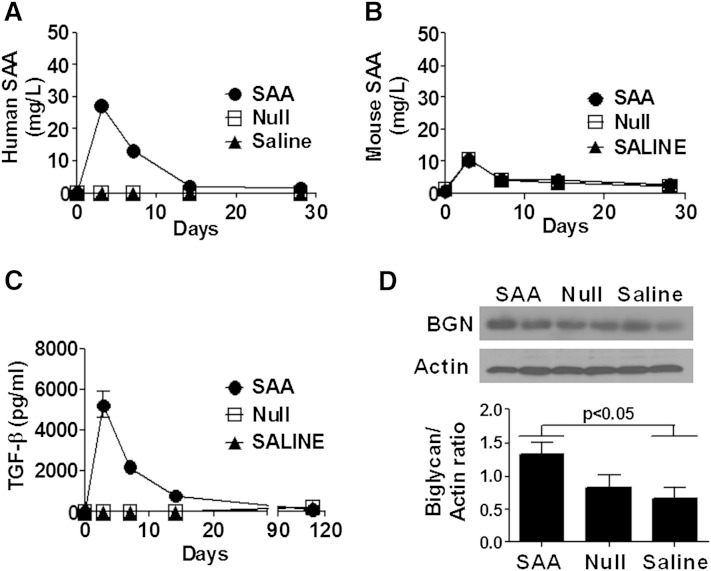
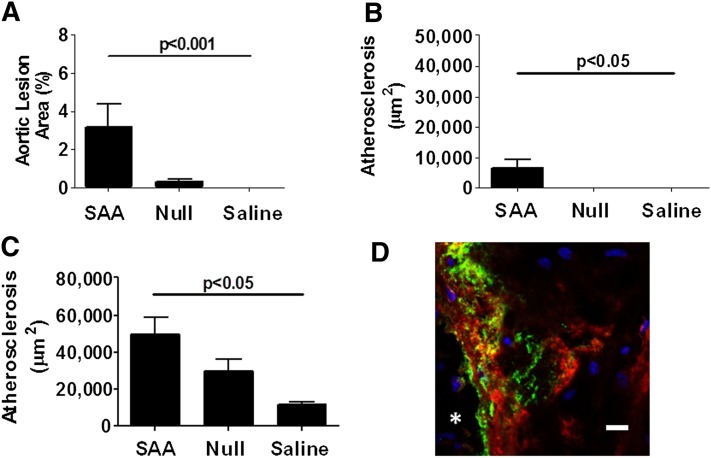
To determine whether a brief increase in SAA resulted in increased atherosclerosis, 8-week-old apoe−/− male mice received a single injection of ad-SAA, ad-Null, or saline and were maintained on normal rodent chow for 16 weeks. The mice receiving ad-SAA had a dramatic increase in human SAA that returned to baseline levels in less than 2 weeks (Fig. 3A). Murine SAA did not differ between groups at any time point (Fig. 3B). TGF-β increased briefly in ad-SAA-injected mice in concordance with plasma SAA expression, with no changes observed in ad-Null or saline groups (Fig. 3C). To determine the persistence of increased vascular wall biglycan induced by increased plasma SAA, carotid arteries were collected 16 weeks after a single injection of ad-SAA, ad-Null, or saline, and Western blot analysis was performed. Remarkably, vascular biglycan content remained increased 16 weeks after a brief increase in plasma SAA (Fig. 3D, P < 0.05). Despite only the brief increase in human SAA levels, ad-SAA mice developed significantly more atherosclerosis on the aortic intimal surface (Fig. 4A, P < 0.001), the brachiocephalic artery (Fig. 4B, P < 0.05), and the aortic root (Fig. 4C, P < 0.05) compared with ad-Null- or saline-treated mice. To assess the interaction between vascular biglycan and apoB-containing lipoproteins, aortic root sections from ad-SAA-, ad-Null-, and saline-injected mice were double stained for biglycan and apoB, demonstrating colocalization of biglycan with apoB in atherosclerotic lesions (Fig. 4D). Aortic root sections from ad-SAA-, ad-Null-, and saline-injected mice were double stained for perlecan and apoB as perlecan is known to be upregulated in murine atherosclerotic lesions (26). No difference in apoB retention was observed between the three groups (supplementary Fig. 1). Furthermore, murine aortic root sections from ad-SAA-, ad-Null-, and saline-injected mice were double stained for biglycan and SAA demonstrating colocalization in mice injected with ad-SAA (supplementary Fig. 1). The induction of human SAA had no effect on plasma lipids or body weight (Table 1). Lipoprotein distribution was measured using fast-performance liquid chromatography with no differences observed between groups (data not shown).
Fig. 3.
Increase in vascular biglycan content after a brief elevation of SAA persists at least 16 weeks. Mice were injected with ad-SAA (black circles), ad-Null (open squares), or saline (black triangles) and fed normal rodent chow for 16 weeks. A: Mice receiving a single injection of ad-SAA had a dramatic albeit transient increase in human SAA. B: All groups had a small, transient increase in murine SAA. C: TGF-β was transiently but dramatically increased in ad-SAA mice compared with ad-Null or saline mice. Shown are means ± SEM from n = 2–6 mice/group per time point. D: After 16 weeks, carotid arteries were collected and immunoblotted for biglycan (BGN) or actin, then analyzed by densitometry using ImageJ software. Each lane shows protein from an individual mouse; densitometry shows means ± SEM for 5–7 mice per group.
Fig. 4.
Atherosclerosis is increased in apoe−/− mice after only a brief increase in plasma SAA. Mice were injected with ad-SAA, ad-Null, or saline and fed normal rodent chow for 16 weeks. Atherosclerosis was measured at three sites: the aortic intimal surface (A), the brachiocephalic artery (B), and the aortic sinus (C). Atherosclerosis data presented as mean ± SEM; n = 3–20/ group analyzed by one-way ANOVA. D: An atherosclerotic lesion in an aortic root from an ad-SAA-injected apoe−/− mouse was double stained for apoB (green) and biglycan (red). Colocalization is indicated by yellow. Shown is a confocal image magnified 63×, representative of four mice. The asterisk indicates the lumen of the aortic sinus. Scale bar indicates 10 mm.
TABLE 1.
Metabolic parameters known to confer cardiovascular risk did not differ between groups in either study
| Brief SAA Expression apoe−/− | Sustained SAA Expression rag1−/− × apoe−/− | |||||
| SAA | Null | Saline | SAA | Null | Saline | |
| Cholesterol (mg/dl) | 421 ± 74 | 409 ± 34 | 343 ± 49 | 437 ± 53 | 422 ± 50 | 400 ± 52 |
| Triglycerides (mg/dl) | 99 ± 17 | 77 ± 10 | 56 ± 11 | 70 ± 12 | 97 ± 18 | 76 ± 15 |
| Body weights (g) | 31.4 ± 0.63 | 32.4 ± 0.68 | 32.6 ± 1.19 | 27.0 ± 0.63 | 28.8 ± 0.84 | 29.5 ± 1.4 |
The apoe−/− mice were injected once, or rag1−/− × apoe−/− were injected every 21 days with ad-SAA, ad-Null, or saline and maintained on normal rodent chow for 16 weeks for apoe−/− or 12 weeks for rag1−/− × apoe−/−.
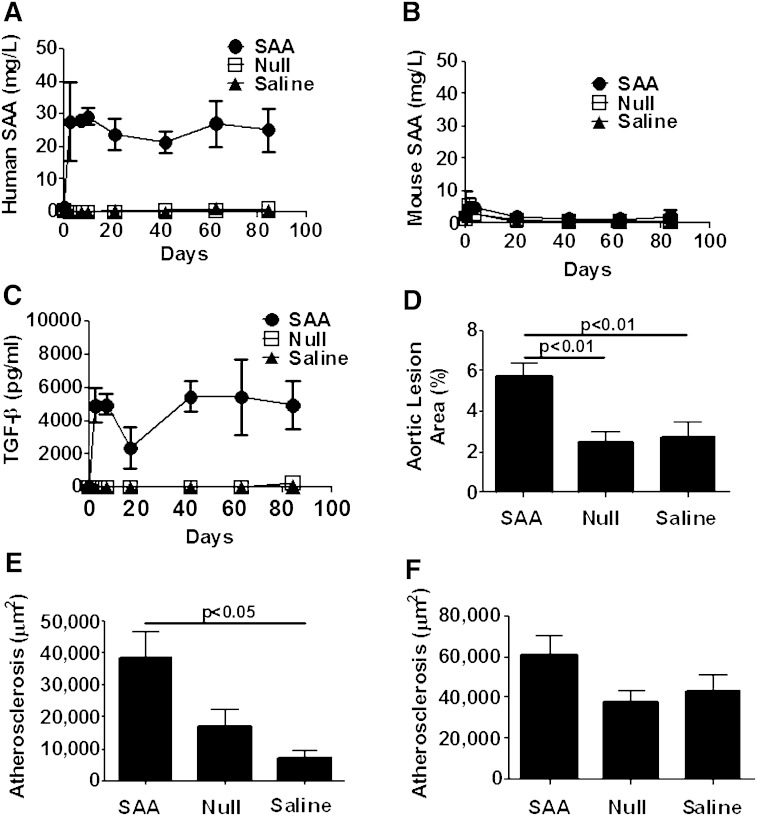
SAA is persistently elevated in a number of chronic inflammatory conditions such as diabetes, obesity, metabolic syndrome, rheumatoid arthritis, and so forth, all of which are associated with increased CVD. To determine the impact of sustained elevations of SAA on atherosclerosis development, 8-week-old male rag1−/− × apoe−/− mice were injected with ad-SAA, ad-Null, or saline once every 21 days and maintained on normal rodent chow for 12 weeks. As immunocompetent mice are intolerant of repeated adenoviral injections, rag1−/− × apoe−/− mice were used. The injection of ad-SAA led to a similar induction of SAA as seen in apoe−/− mice, but the plasma SAA elevation was sustained over 12 weeks (Fig. 5A). There were no elevations in murine SAA (Fig. 5B) in any group at any time point. Mice injected with ad-SAA had increased TGF-β compared with ad-Null- or saline-injected mice throughout the study (Fig. 5C). Ad-SAA-injected mice had increased atherosclerosis on the aortic intimal surface (Fig. 5D, P < 0.001) and within the brachiocephalic artery (Fig. 5E, P < 0.05) compared with ad-Null or saline groups. There was a trend toward increased atherosclerosis in the aortic root; however, it did not reach statistical significance (Fig. 5F). Similar to the apoe−/− model, there were no differences between groups in plasma lipids, body weight (Table 1), or lipoprotein distribution (not shown).
Fig. 5.
Atherosclerosis was increased after sustained elevation of human SAA in rag1−/− × apoe−/− mice. Mice were injected with ad-SAA (black circles), ad-Null (open squares), or saline (black triangles) every 21 days and fed normal rodent chow for 12 weeks. A: Mice receiving ad-SAA had a significant, persistent elevation of human SAA. B: Murine SAA did not increase nor did it differ between groups throughout the study. C: TGF-β was increased only in mice injected with ad-SAA and remained elevated throughout the study. Shown are means ± SEM from n = 2–6 mice/group per time point. Ad-SAA-injected mice had increased atherosclerosis on the aortic intimal surface (D) and in the brachiocephalic artery (E). There was a trend toward increased atherosclerosis in the aortic root that did not reach significance (F). Data are presented as mean ± SEM; n = 4–16/group analyzed by one-way ANOVA.
DISCUSSION
We previously demonstrated that SAA increased VSMC proteoglycan synthesis. Further, proteoglycans synthesized in response to elevated SAA had increased LDL binding affinity (17). We confirmed this in vivo by demonstrating that vascular biglycan content was increased in apoe−/− mice 28 days after ad-SAA. We now demonstrate that SAA’s induction of vascular biglycan content persists up to 16 weeks after a single brief increase in SAA. Strikingly, apoe−/− mice given only a single injection of ad-SAA also had increased atherosclerosis even though SAA levels were back to baseline in less than 10 days. Using in vitro studies, we demonstrate that SAA treatment leads to increased LDL retention, and that prevention of TGF-β signaling prevents SAA-induced increases in LDL retention and SAA-induced increases in vascular biglycan content. We further demonstrate that SAA-mediated LDL binding is proteoglycan specific as inhibition of sulfation of the glycosaminoglycan side chains resulted in reduced LDL binding. In the LDL binding studies presented here, the cells were treated with lipid-free murine SAA. There is still debate as to the biological activity of acute SAA in the lipid-free versus HDL bound states. In vivo SAA is almost exclusively associated with HDL, and SAA displaces apoA-I as the predominant apoprotein on HDL during an APR (3). However, the exact physiological effect of SAA-rich acute phase HDL on reverse cholesterol transport is still being debated. Interestingly, in mice lacking both forms of acute SAA, macrophage reverse cholesterol transport was still reduced in lipopolysaccharide-induced acute inflammation suggesting that inflammation and not SAA loading alters HDL sterol efflux capacity (27). Furthermore, in previous work we demonstrated that lipid-free, but not HDL bound SAA increased biglycan synthesis in vitro; yet in vivo SAA is found only associated with HDL, and biglycan synthesis still increased. This suggests that somewhere within the milieu of the vessel wall the SAA is delipidated prior to stimulating biglycan synthesis (17). Thus, we propose that SAA increases atherosclerosis development via induction of TGF-β, increased vascular biglycan content, and increased LDL retention.
In these murine models, either brief or sustained increases in SAA led to increased atherosclerosis development. In both murine models studied, human SAA was increased with no change in murine SAA implying the absence of any inflammatory response to the adenoviral vectors or experimental manipulation of the mice. Furthermore, injection of ad-SAA led to an increase in TGF-β, which correlated with the duration of increased human SAA. In both models, increased SAA had no effect on lipids or body weight. Our model of sustained elevation in SAA supports the previous findings from Dong et al. (12) that SAA is proatherogenic. In that study, murine SAA was overexpressed by lentiviral vector leading to significantly increased atherosclerosis on the aortic intimal surface and the aortic sinus, which the authors attributed to upregulation of vascular adhesion molecules and chemotactic factors capable of increasing leukocyte migration into atherosclerosis-susceptible arteries. The study presented here addresses events prior to vascular wall leukocyte infiltration, namely vascular wall proteoglycan synthesis and lipoprotein retention. Our data demonstrate that at least one mechanism by which SAA is proatherogenic is through the induction of vascular biglycan and subsequent retention of atherogenic lipoproteins. We recently demonstrated correlation between the extent of increased vascular biglycan content and increased atherosclerosis development in the LDL receptor-deficient murine model (24), further supporting that increased vascular biglycan content contributes to increased atherosclerosis. The experiments presented here, as well as the work by Dong et al., utilized the SAA1 isoform specifically. However, acute SAA exists in multiple isoforms, and SAA2 has been shown to increase reverse cholesterol transport, which would be considered atheroprotective (28). Within human and murine physiology, the acute SAAs increase together and to about the same magnitude after inflammatory insult (1). Diabetic and obese individuals have chronically elevated SAA, both 1 and 2, and a greater risk of developing CVD suggesting that the increase in both acute SAAs is predominantly pathologic, even if increased SAA2 can increase reverse cholesterol transport (29, 30). However, further studies are needed to explicitly define the role of increased SAA2.
Using the rag1−/− × apoe−/− murine model, which is tolerant of repeated adenoviral injections, we demonstrate that sustained elevations in SAA increased atherosclerosis to a greater extent than a single brief elevation of SAA. Although direct comparisons of apoe−/− and rag1−/− × apoe−/− are limited, the literature reports that rag1- or rag2-deficient apoe−/− mice appear to have decreased lesion size compared with their immunocompetent littermates (31, 32). Thus, even with our findings of a 2-fold (aortic intimal surface) and a 5-fold (aortic sinus) increase in atherosclerosis compared with apoe−/− mice, the extent of disease development is still likely underestimated given the relative resistance to the development of atherosclerosis in rag1−/− × apoe−/− mice. The underestimation of the effect of sustained SAA expression in the rag1−/− × apoe−/− mice is further exacerbated by the fact that this model was on study for only 12 weeks while the apoe-deficient mice were on study for 16 weeks.
In both models presented here, the administration of ad-SAA had the immediate effect of increasing TGF-β levels, which then mirrored the human SAA levels throughout the studies. Inhibition of TGF-β in VSMCs stimulated with SAA attenuated the increase in biglycan synthesis (17). Here, we further demonstrate in apoe−/− mice that concurrent injection of ad-SAA and 1D11 antibodies resulted in no induction of vascular biglycan 28 days after the injection. Thus, we propose that SAA is acting through TGF-β to drive an increase in vascular biglycan content, predisposing the vessel wall to increased lipid retention. However, the role of TGF-β in atherosclerosis is controversial. Mallat et al. (33) used a TGF-β inhibitory antibody and demonstrated that suppression of TGF-β led to increased inflammatory cell infiltrate into lesions and increased atherosclerotic lesion area. Conversely, Lutgens et al. (34) reported that TGF-β inhibition with a soluble receptor resulted in increased inflammatory cell infiltrate, but the atherosclerotic lesion area decreased. We have previously shown in an angiotensin (ang) II model of atherosclerosis that inhibition of TGF-β attenuated angII-induced atherosclerotic lesion development; however, there was no change in inflammatory cell content within those lesions (23). Therefore, we propose that prevention or attenuation of elevations of TGF-β can limit induction of vascular biglycan, thus limiting atherosclerosis. Further study is necessary to determine whether increased TGF-β is necessary and/or required for SAA-induced atherosclerosis.
We propose that SAA increases susceptibility to early atherosclerosis by directing the proatherogenic remodeling of the vessel wall with increased biglycan content resulting in increased lipoprotein retention. In support of this model, the current studies provide evidence that biglycan colocalizes with apoB-containing lipoproteins within the lesion. However, SAA likely contributes to atherosclerosis through multiple mechanisms, including the induction of biglycan presented here. Though we have clearly defined a role for elevated SAA in both brief and sustained models of atherosclerosis, endogenous SAA is not absolutely required for atherosclerosis development. Recently, we reported that apoe−/− mice deficient in both SAA1 and SAA2 have no difference in the degree of atherosclerotic lesion formation compared with apoe−/− controls (35). However, the studies investigating deletion of the endogenous acute SAAs differ in several key aspects compared with the study presented here and the work of Dong et al. The investigation of atherosclerosis in apoe−/− mice lacking acute SAA was investigated after 50 weeks of chow diet or 12 weeks of Western diet. We have demonstrated a remarkable effect of elevated SAA to accelerate early atherosclerosis development that may be less relevant in later stages of the disease. Similarly, Skålén et al. reported that mice transgenic for human apoB100 in which the proteoglycan binding domain was mutated had decreased early atherosclerosis (36) but not later atherosclerosis (37), suggesting that other mechanisms were responsible for late atherosclerosis to develop. Taken together, the data suggest that atherosclerosis therapies targeted at SAA need only reduce its levels to those observed in a noninflammatory state and not silence its expression completely.
Our findings have significant implications in Westernized societies. It is currently estimated that 347 million people worldwide have diabetes (38), and almost half a billion people are obese (39), with the prevalence of both continuing to rise. These inflammatory diseases are characterized by chronically and significantly elevated plasma SAA among other clinical findings, and individuals with these diseases have an increased risk of developing atherosclerosis (29, 30). Furthermore, acute bouts of inflammation with increased SAA such as seen in patients hospitalized for diseases including sepsis, renal failure, or community-acquired pneumonia also confer increased mortality once discharged, often from poor cardiovascular outcomes (18–20). Taken together with our current data on short-term increases in SAA, acute but significant bouts of inflammation may need to be considered a risk factor for increased cardiovascular events.
Although elevations in SAA are well validated as a predictor of cardiovascular risk, we propose that beyond indicating risk, elevated plasma SAA plays a causal role in atherosclerosis development, and prevention of increased SAA may be a therapeutic target. A number of currently available agents can lower SAA including statins. Individuals with a history of myocardial infarction with elevated SAA are at greater risk for a second cardiovascular event than similar patients with lower SAA; however, treatment with pravastatin reduces this increased risk, perhaps in part by lowering SAA (40). Given the pleiotropic effects of statins, it is difficult to dissect the mechanisms by which they lower CVD risk. However, it is possible that beyond lowering LDL cholesterol, statins may have beneficial effects by lowering SAA. Beyond statins, other approaches have been found to lower SAA, such as weight loss (5) and aspirin-based therapies (41). However, further clinical research is necessary to validate SAA as a therapeutic target in humans and to determine whether therapies targeted to elevated SAA in humans can be protective.
Supplementary Material
Footnotes
Abbreviations:
- ad-Null
- control adenoviral vector
- ad-SAA
- adenoviral vector encoding human SAA1
- apoe−/−
- apolipoprotein E deficient
- APR
- acute phase response
- rag1−/−
- recombination activating gene 1 deficient
- SAA
- serum amyloid A
- TGF-β
- transforming growth factor beta
- VSMC
- vascular smooth muscle cell
This work was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under Grant RO1HL096589 (L.R.T.); by a Predoctoral Fellowship from the American Heart Association, Great Rivers Affiliate (12PRE12060285) (J.C.T.); and through cores supported by National Institutes of Health (8 P20 GM103527). The authors have no conflicts of interest to disclose.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Uhlar C. M., Burgess C. J., Sharp P. M., Whitehead A. S. 1994. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 19: 228–235. [DOI] [PubMed] [Google Scholar]
- 2.Meek R. L., Urieli-Shoval S., Benditt E. P. 1994. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc. Natl. Acad. Sci. USA. 91: 3186–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., de Beer F. C. 1986. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J. Biol. Chem. 261: 9644–9651. [PubMed] [Google Scholar]
- 4.Leinonen E., Hurt-Camejo E., Wiklund O., Hulten L. M., Hiukka A., Taskinen M. R. 2003. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 166: 387–394. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien K. D., Brehm B. J., Seeley R. J., Bean J., Wener M. H., Daniels S., D’Alessio D. A. 2005. Diet-induced weight loss is associated with decreases in plasma serum amyloid A and C-reactive protein independent of dietary macronutrient composition in obese subjects. J. Clin. Endocrinol. Metab. 90: 2244–2249. [DOI] [PubMed] [Google Scholar]
- 6.Johnson B. D., Kip K. E., Marroquin O. C., Ridker P. M., Kelsey S. F., Shaw L. J., Pepine C. J., Sharaf B., Bairey Merz C. N., Sopko G., et al. 2004. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 109: 726–732. [DOI] [PubMed] [Google Scholar]
- 7.Lee H. Y., Kim S. D., Shim J. W., Lee S. Y., Lee H., Cho K. H., Yun J., Bae Y. S. 2008. Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes. J. Immunol. 181: 4332–4339. [DOI] [PubMed] [Google Scholar]
- 8.Lee H. Y., Kim S. D., Baek S. H., Choi J. H., Cho K. H., Zabel B. A., Bae Y. S. 2013. Serum amyloid A stimulates macrophage foam cell formation via lectin-like oxidized low-density lipoprotein receptor 1 upregulation. Biochem. Biophys. Res. Commun. 433: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badolato R., Wang J. M., Murphy W. J., Lloyd A. R., Michiel D. F., Bausserman L. L., Kelvin D. J., Oppenheim J. J. 1994. Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J. Exp. Med. 180: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba T., Chang M. Y., Wang S., Wight T. N., McMillen T. S., Oram J. F., Vaisar T., Heinecke J. W., De Beer F. C., De Beer M. C., et al. 2011. Serum amyloid A facilitates the binding of high-density lipoprotein from mice injected with lipopolysaccharide to vascular proteoglycans. Arterioscler. Thromb. Vasc. Biol. 31: 1326–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien K. D., McDonald T. O., Kunjathoor V., Eng K., Knopp E. A., Lewis K., Lopez R., Kirk E. A., Chait A., Wight T. N., et al. 2005. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25: 785–790. [DOI] [PubMed] [Google Scholar]
- 12.Dong Z., Wu T., Qin W., An C., Wang Z., Zhang M., Zhang Y., Zhang C., An F. 2011. Serum amyloid A directly accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Mol. Med. 17: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams K. J., Tabas I. 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien K. D., Olin K. L., Alpers C. E., Chiu W., Ferguson M., Hudkins K., Wight T. N., Chait A. 1998. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 98: 519–527. [DOI] [PubMed] [Google Scholar]
- 15.Little P. J., Osman N., O’Brien K. D. 2008. Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr. Opin. Lipidol. 19: 448–454. [DOI] [PubMed] [Google Scholar]
- 16.Williams K. J., Tabas I. 1998. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 9: 471–474. [DOI] [PubMed] [Google Scholar]
- 17.Wilson P. G., Thompson J. C., Webb N. R., de Beer F. C., King V. L., Tannock L. R. 2008. Serum amyloid A, but not C-reactive protein, stimulates vascular proteoglycan synthesis in a pro-atherogenic manner. Am. J. Pathol. 173: 1902–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coca S. G., Yusuf B., Shlipak M. G., Garg A. X., Parikh C. R. 2009. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am. J. Kidney Dis. 53: 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quartin A. A., Schein R. M., Kett D. H., Peduzzi P. N.; for the Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. 1997. Magnitude and duration of the effect of sepsis on survival. J. Am. Med. Assoc. 277: 1058–1063. [PubMed] [Google Scholar]
- 20.Koivula I., Sten M., Makela P. H. 1999. Prognosis after community-acquired pneumonia in the elderly: a population-based 12-year follow-up study. Arch. Intern. Med. 159: 1550–1555. [DOI] [PubMed] [Google Scholar]
- 21.Kim M. H., de Beer M. C., Wroblewski J. M., Webb N. R., de Beer F. C. 2013. SAA does not induce cytokine production in physiological conditions. Cytokine. 61: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyanovsky B. B., van der Westhuyzen D. R., Webb N. R. 2005. Group V secretory phospholipase A2-modified low density lipoprotein promotes foam cell formation by a SR-A- and CD36-independent process that involves cellular proteoglycans. J. Biol. Chem. 280: 32746–32752. [DOI] [PubMed] [Google Scholar]
- 23.Tang T., Wilson P. G., Thompson J. C., Nelson C., Yoder M. H., Tannock L. R. 2013. Prevention of TGFbeta induction attenuates angII-stimulated vascular biglycan and atherosclerosis in Ldlr−/− mice. J. Lipid Res. 54: 2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson J. C., Tang T., Wilson P. G., Yoder M. H., Tannock L. R. 2014. Increased atherosclerosis in mice with increased vascular biglycan content. Atherosclerosis. 235: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borén J., Olin K., Lee I., Chait A., Wight T. N., Innerarity T. L. 1998. Identification of the principal proteoglycan-binding site in LDL. A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J. Clin. Invest. 101: 2658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vikramadithyan R. K., Kako Y., Chen G., Hu Y., Arikawa-Hirasawa E., Yamada Y., Goldberg I. J. 2004. Atherosclerosis in perlecan heterozygous mice. J. Lipid Res. 45: 1806–1812. [DOI] [PubMed] [Google Scholar]
- 27.de Beer M. C., Wroblewski J. M., Noffsinger V. P., Ji A., Meyer J. M., van der Westhuyzen D. R., de Beer F. C., Webb N. R. 2013. The impairment of macrophage-to-feces reverse cholesterol transport during inflammation does not depend on serum amyloid A. J. Lipids. 2013: 283486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam S. P., Flexman A., Hulme J., Kisilevsky R. 2002. Promoting export of macrophage cholesterol: the physiological role of a major acute-phase protein, serum amyloid A 2.1. J. Lipid Res. 43: 1410–1420. [DOI] [PubMed] [Google Scholar]
- 29.Kannel W. B., McGee D. L. 1979. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 2: 120–126. [DOI] [PubMed] [Google Scholar]
- 30.Rabkin S. W., Mathewson F. A., Hsu P. H. 1977. Relation of body weight to development of ischemic heart disease in a cohort of young North American men after a 26 year observation period: the Manitoba Study. Am. J. Cardiol. 39: 452–458. [DOI] [PubMed] [Google Scholar]
- 31.Reardon C. A., Blachowicz L., White T., Cabana V., Wang Y., Lukens J., Bluestone J., Getz G. S. 2001. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 32.Dansky H. M., Charlton S. A., Harper M. M., Smith J. D. 1997. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. USA. 94: 4642–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallat Z., Gojova A., Marchiol-Fournigault C., Esposito B., Kamate C., Merval R., Fradelizi D., Tedgui A. 2001. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89: 930–934. [DOI] [PubMed] [Google Scholar]
- 34.Lutgens E., Gijbels M., Smook M., Heeringa P., Gotwals P., Koteliansky V. E., Daemen M. J. 2002. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 22: 975–982. [DOI] [PubMed] [Google Scholar]
- 35.De Beer M. C., Wroblewski J. M., Noffsinger V. P., Rateri D. L., Howatt D. A., Balakrishnan A., Ji A., Shridas P., Thompson J. C., van der Westhuyzen D. R., et al. 2014. Deficiency of endogenous acute phase serum amyloid A does not affect atherosclerotic lesions in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 34: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skålén K., Gustafsson M., Rydberg E. K., Hulten L. M., Wiklund O., Innerarity T. L., Boren J. 2002. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 417: 750–754. [DOI] [PubMed] [Google Scholar]
- 37.Gustafsson M., Levin M., Skålén K., Perman J., Friden V., Jirholt P., Olofsson S. O., Fazio S., Linton M. F., Semenkovich C. F., et al. 2007. Retention of low-density lipoprotein in atherosclerotic lesions of the mouse: evidence for a role of lipoprotein lipase. Circ. Res. 101: 777–783. [DOI] [PubMed] [Google Scholar]
- 38.Danaei G., Finucane M. M., Lu Y., Singh G. M., Cowan M. J., Paciorek C. J., Lin J. K., Farzadfar F., Khang Y.-H., Stevens G. A., et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 378: 31–40. [DOI] [PubMed] [Google Scholar]
- 39.Finucane M. M., Stevens G. A., Cowan M. J., Danaei G., Lin J. K., Paciorek C. J., Singh G. M., Gutierrez H. R., Lu Y., Bahalim A. N., et al. 2011. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 377: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker P. M., Rifai N., Pfeffer M. A., Sacks F. M., Moye L. A., Goldman S., Flaker G. C., Braunwald E.; for the Cholesterol, and Recurrent Events (CARE) Investigators. 1998. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 98: 839–844. [DOI] [PubMed] [Google Scholar]
- 41.El Kebir D., Jozsef L., Khreiss T., Pan W., Petasis N. A., Serhan C. N., Filep J. G. 2007. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J. Immunol. 179: 616–622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.