Abstract
Implication of the long-chain fatty acid (LCFA) receptor GPR120, also termed free fatty acid receptor 4, in the taste-guided preference for lipids is a matter of debate. To further unravel the role of GPR120 in the “taste of fat”, the present study was conducted on GPR120-null mice and their wild-type littermates. Using a combination of morphological [i.e., immunohistochemical staining of circumvallate papillae (CVP)], behavioral (i.e., two-bottle preference tests, licking tests and conditioned taste aversion) and functional studies [i.e., calcium imaging in freshly isolated taste bud cells (TBCs)], we show that absence of GPR120 in the oral cavity was not associated with changes in i) gross anatomy of CVP, ii) LCFA-mediated increases in intracellular calcium levels ([Ca2+]i), iii) preference for oily and LCFA solutions and iv) conditioned avoidance of LCFA solutions. In contrast, the rise in [Ca2+]i triggered by grifolic acid, a specific GPR120 agonist, was dramatically curtailed when the GPR120 gene was lacking. Taken together, these data demonstrate that activation of lingual GPR120 and preference for fat are not connected, suggesting that GPR120 expressed in TBCs is not absolutely required for oral fat detection in mice
Keywords: lipids, diet and dietary lipids, nutrition, receptors, G-protein, fat taste, feeding behavior, mouse
A growing number of studies strongly suggest that, in addition to textural, olfactory, and post-ingestive cues, the oro-sensory detection of lipids involves a taste component dependent on long-chain fatty acids (LCFAs). LCFAs are mainly released from triglycerides by the salivary lipases (1–3). The plasma membrane receptor CD36 is thought to play a significant role in this taste detection system. First, CD36 displays a high affinity for LCFAs (4) and its expression in lingual epithelium is restricted to the gustatory papillae in rats (5), mice (6), and humans (7). Second, a LCFA-dependent signaling cascade leading to a huge rise in intracellular calcium levels ([Ca2+]i) was specifically found in mouse CD36-positive taste bud cells (TBCs), associated with neurotransmitter release (8, 9). Third, the activation of the nucleus of the solitary tract in the brainstem after an oral fat deposition is CD36-dependent (8). Finally, there is a tight correlation between CD36 gene expression and oral fat detection sensitivity: CD36 disruption rendered mice unable to properly detect, and prefer, low concentrations of lipids (6, 10, 11). Similarly, siRNA-targeted depletion of CD36 expression in gustatory papillae decreased oral lipid detection performance in rats (12). In humans, subjects expressing the A/A alleles at the gene variant rs1761667, which reduces CD36 expression (13), displayed a higher detection threshold (i.e., a lower sensitivity) for lipids than G/G controls (2) and showed a greater attraction for added fats and oils (14).
Recently, two members of the G-protein coupled receptor (GPCR) family, GPR40 , [also known as free fatty acid receptor (FFAR) 1] and GPR120 (FFAR4), known to bind and be activated by medium- and long-chain fatty acids, were reported also to be implicated in oral lipid sensing in mice (15). A low gustatory nerve response to LCFAs and a decreased preference for linoleic or oleic acids (LAs, OLAs) were found both in GPR40−/− and GPR120−/− mice (15). Nevertheless, a role of GPR40 as gustatory lipid sensor is questionable because this GPCR has not been found by others in mouse (16), rat (17), or human (18) taste buds. In contrast, GPR120 was systematically detected in murine and human gustatory papillae (11, 15, 18) in which it was frequently coexpressed with CD36 (11, 19). This last observation raises the question of the respective role(s) of these two LCFA receptors in the coding mechanisms eliciting the oro-sensory detection of dietary lipids at the periphery. Recently, it was shown that CD36 and GPR120 have non-overlapping roles in the LCFA-mediated signaling in human TBCs (19). Whether GPR120 plays a primary role in oral fat detection and preference remains a subject of debate. In contrast to prior results (15), a recent study reported that GPR120−/− mice did not differ from wild-type mice in their preference for Intralipid (20), a fat emulsion composed of soy bean oil, egg phospholipids, and glycerin, suggesting that GPR120 might not be essential for oral fat detection. Although these two studies were conducted using the same GPR120 KO mouse model, a direct comparison between these two sets of data is difficult because of major differences in the lipid stimuli used, which were either free LCFAs (15) or lipid micelles (20). In another report, despite a specific activation of their afferent gustatory fibers, wild-type mice were unable to detect and prefer nonfatty acid GPR120 agonists during behavioral tests (21). Consistent with this behavioral data, healthy humans subjected to a two-alternative forced choice test were also unable to detect these GPR120 agonists (21). Altogether, these new findings suggest that the ligand binding activation of lingual GPR120 is not sufficient to generate a typical food choice. These contradictory reports confuse the role played by GPR120 in the preference for fat. To unravel the GPR120 function in the lingual gustatory epithelium, a set of behavioral tests (two-bottle preference tests, licking tests, and conditioned taste aversion) associated with functional experiments (calcium imaging studies on freshly isolated TBCs) were conducted in GPR120−/− mice and their wild-type littermates.
Materials and Methods
Animals
French guidelines for the use and care of laboratory animals were followed and experimental protocols were approved by the Animal Ethic Committee of the University of Burgundy. GPR120-deficient mice on a C57Bl/6 background were generated by homologous recombination leading to the replacement of exon 1 by a PGK-neo cassette (21). Animals were individually housed in a controlled environment (constant temperature and humidity, dark period from 7 PM to 7 AM). The mice had free access to regular chow and tap water during the experiments, unless otherwise specified. GPR120−/− mice and GPR120+/+ littermate controls were locally generated by mating of heterozygous animals. Genotypes of animals were determined by PCR using previously published procedures (21).
Tissues and blood samples
Mouse circumvallate papillae (CVPs) were isolated according to the procedure described elsewhere (6). Briefly, lingual epithelium was separated from connective tissue by enzymatic dissociation (elastase and dispase mixture, 2 mg/ml each in Tyrode buffer) and the unique CVP was dissected under a binocular microscope. Heparinized blood samples were obtained from retro-orbital sinus and plasmas were obtained after centrifugation of blood (5,000 g for 10 min, 4°C). Plasma insulin levels were assayed by using a commercial ELISA kit (Mercodia, Sweden) and plasma glucose levels were determined using enzymatic reaction kits (Biomerieux, France).
Behavioral experiments
Two-bottle preference tests.
Mice were offered simultaneously two bottles for 12 h at the beginning of the dark period (22). Animals were subjected to a choice between control or experimental solutions (each test was separated by a rest period of at least 48 h). In one set of experiments, both solutions contained 0.3% xanthan gum w/v (Sigma-Aldrich) in water in order to produce an oil suspension and to minimize textural cues between the two bottles. Experimental solutions contained, in addition to xanthan gum, either 0.02%, 0.2%, or 2.0% rapeseed oil (w/v, Fleur de Colza, Lesieur, France), 0.5% or 1% LA, 2% sucrose (w/v), or 0.3 mM quinine (Sigma-Aldrich). In another set of experiments, Intralipid (20% Fresenius kabi a.b., Sweden) solutions were tested versus deionized water (0.25% and 2.5% Intralipid, v/v) (20). To avoid the development of side preferences, the position of each bottle was randomized and reversed for each test. At the end of the tests, the consumption of control and experimental solutions was analyzed by weighing the bottles and the percentage of preference for the experimental solution was calculated (ratio of the consumption of the experimental solution relative to total consumption).
Licking tests.
Licking tests were performed in order to analyze the short-term (1 min) attraction for a LCFA by using computer-controlled lickometers (Med Associates). Mice were food and water deprived at the beginning of the dark period and for 6 h before the tests. Naive animals were trained to learn the procedure. The first day, mice were successively subjected to a bottle containing either water or a more attractive solution (4% sucrose) for 15 min sessions. The following day, the procedure was repeated, except that the mice were given the bottles in the reverse order. On the third day, mice were given mineral oil (Cooper, France) for both sessions so that the mice were not neophobic to the oily texture.
On test days, animals were successively and randomly subjected to the control (mineral oil) or experimental (mineral oil + 0.5% OLA or 0.5% LA w/v; Sigma-Aldrich) solutions and the number of licks on each bottle was determined for 1 min from the first lick to minimize post-ingestive signals (11). LA and OLA were tested on 2 different days.
CTA tests.
Conditioned taste aversion (CTA) was performed on wild-type and GPR120−/− mice. To train the animals to drink during a short period, access to water (containing 0.3% xanthan gum) was restricted to 10:00 to 11:00 AM for 3 days before conditioning. Water restriction was followed during the conditioning and the preference tests. To avoid dehydration, animals also had access to drinking water from 3:00 to 3:30 PM. On the day of conditioning, mice were given 2% LA in 0.3% xanthan gum (conditioned stimulus) from 10:00 to 11:00 AM, then were intraperitoneally injected with 0.15M LiCl (16.5 µl/g body weight, conditioned mice) or saline solution (16.5 µl/g body weight, controls). To reinforce the CTA, a second conditioning was performed 3 days later, as described previously, with 0.5% LA. Preference tests were performed with 0.5% LA versus vehicle (0.3% xanthan gum in water). The specificity of aversion to LA was assessed using an additional two-bottle preference test with 2% sucrose versus vehicle.
For all double-choice tests, the preference for the experimental solution (LA or sucrose) was calculated as the ratio of the consumption of the experimental solution to total consumption.
Histology and immunohistochemistry
CVPs from fasted male wild-type and GPR120−/− mice were harvested and tissues were fixed by immersion in 4% paraformaldehyde in PBS for 4 h on ice. CVPs were transferred to 15% sucrose in PBS for 2 h then placed in 30% sucrose overnight before being embedded in OCT medium (Tissue-Tek, Sakura Finetek). Cryostat sections (10 µm) were air dried for 2 h at room temperature and then rehydrated in PBS for 10 min. Rehydrated sections were incubated during 1 h with PBS containing 0.3% Triton X-100 and 10% fatty acid-free BSA. Then, the slices were incubated overnight at 4°C with an anti-rabbit α-gustducin antibody (1/50; Santa Cruz Biotechnology Inc., sc-395) or an anti-rabbit Ki67 antibody (1/50; Abcam, ab16667). α-gustducin was used as a marker of type II TBCs and Ki67 as a proliferative cells marker. After washing, sections were incubated for 1 h at room temperature with a fluorescent anti-rabbit secondary antibody (Alexa 568, 1:500 dilution; Invitrogen) for α-gustducin staining or for 30 min with an HRP-conjugated anti-rabbit secondary antibody (JIR, 111-035-144) for Ki67, then were revealed with an Alexa fluor 568 tyramide kit (Invitrogen, T20934). The nuclei were counterstained with Hoechst 33258 (0.05 mg/ml; Sigma-Aldrich). Negative control tests without primary antibodies were performed (data not shown). Slices were analyzed under an epifluorescence microscope (Cell Observer Zeiss). For hematoxilin-eosin staining, the tissues containing CVP were fixed in formalin, dehydrated, and then embedded in paraffin. Colorations were performed with Autostainer XL robot (Leica Biosystems, Germany).
Real-time RT-PCR
Total RNAs from CVP were extracted using the Total RNA Purification Kit (Norgen Biotek, Canada) according to the manufacturer’s instructions. Briefly, nitrogen-frozen CVPs were homogenized in the lysing buffer with an RNase free piston pellet. After homogenization, RNAs were bound on purification columns and treated with an amplification grade DNase (RNase-Free DNase I kit, Norgen Biotek, Canada) to ensure genomic DNA removal. After purification, RNAs were resuspended in RNase free water. RNA concentrations were measured with a Nanodrop® (Thermo Fisher Scientific) spectrophotometer. Reverse transcription reactions were carried out using the High Capacity cDNA Reverse Transcription kit (LifeTechnology) according to the manufacturer’s recommendations with 50 ng of total RNA from CVP and random primers. Real-time PCR reactions were performed with a StepOne Plus (LifeTechnology) device with the use of TaqMan Gene Expression Master Mix (LifeTechnology) according to the manufacturer’s instructions. To assay transcript quantities, commercially available Taqman Gene Expression Assays (LifeTechnology) were used: ffar4 (GPR120) Mm00725193_m1, GPR40 Mm_00809442_S1, Hprt1 (housekeeper) Mm01545399_m1; and CD36 and α-gustducin as described in (22). The comparative 2-ΔΔCT method was used for relative quantification.
Measurement of Ca2+ signaling
CD36-positive gustatory taste buds cells were freshly isolated from mouse CVP as described previously (23) and further cultured for 24 h in Willico-Dish wells, containing RPMI-1640 medium, supplemented with 10% fetal calf serum, 200 U/ml penicillin, and 0.2 mg/ml streptomycin. The next day, the CD36-positive TBCs were gently washed with a buffer containing the following: 3.5 mM KH2PO4; 17.02 mM Na2HPO4; 136 mM NaCl, pH 7.4. The cells were then incubated with Fura-2/AM (1 µM) for 60 min at 37°C in loading buffer, which contained the following: 110 mM NaCl; 5.4 mM KCl; 25 mM NaHCO3; 0.8 mM MgCl2; 0.4 mM KH2PO4; 20 mM Hepes-Na; 0.33 mM Na2HPO4; 1.2 mM CaCl2, pH 7.4.
After loading, the cells (2 × 105/ml) were washed three times and remained suspended in the identical buffer. After adding LA or specific agonist grifolic acid (GA) at a concentration of 20 µmol/l, or both successively in the medium, the changes in intracellular Ca2+ (F340/F380) were monitored under the Nikon microscope (TiU) by using S-fluor 40× oil immersion objective. The planes were taken at Z intervals of 0.3 μm, and NIS-Elements software was used to deconvolve the images. The microscope was equipped with EM-CCD (Lucas) camera for real-time recording of 16-bit digital images. The dual excitation fluorescence imaging system was used for studies of individual cells. The changes in intracellular Ca2+ were expressed as ΔRatio, which was calculated as the difference between the peak F340/F380 ratio. The data were summarized from the large number of individual cells (20–40 cells in a single run, with 3–9 identical experiments done in at least three cell preparations). Each experiment was repeated 3 times.
Statistics
Results are expressed as means ± SEM. The significance of differences between groups was evaluated with GraphPad Prism (GraphPad Software). We first checked that the data for each group were normally distributed and that variances were equal. We then carried out two-tailed Student’s t-test or two-way ANOVA corrected for multiple comparison with the Tukey posthoc test.
Results
The lack of GPR120 does not affect CVP organization
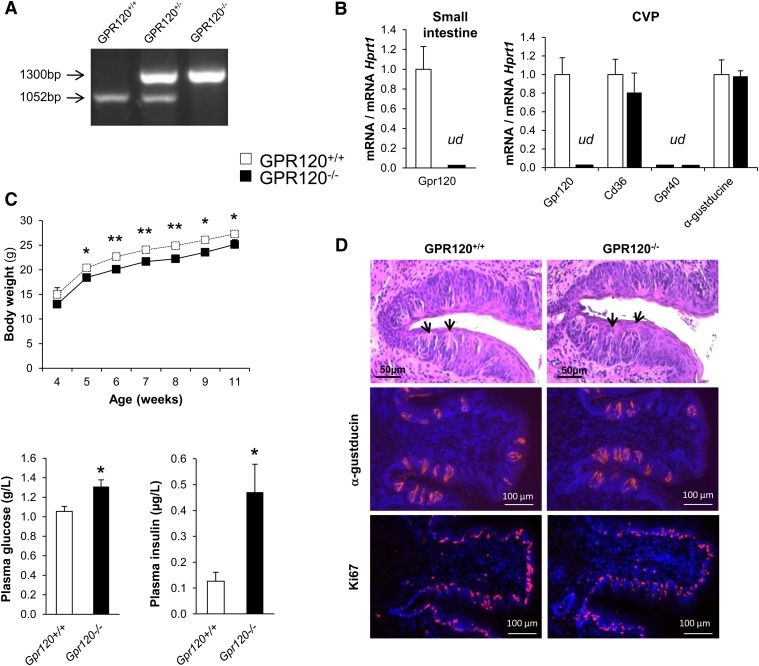
According to the genotyping profile (Fig. 1A), no GPR120 mRNA was detected in small intestine or in CVP from GPR120−/− animals (Fig. 1B). GPR120 gene invalidation did not interfere on CD36 and α-gustducin expression levels in taste buds as similar mRNA levels were found in CVP from GPR120+/+ and GPR120−/− mice. According to data found in different species (16–18), GPR40 mRNA was undetectable in mouse CVP (Fig. 1B). In young GPR120−/− animals, evolution of body mass is slightly lower than in littermate controls (Fig. 1C). This small difference is due to a lower lean mass, which might explain the weaker energy expenditure previously found in GPR120−/− mice during the light-inactive phase (21). In agreement with published data obtained in different GPR120 KO mouse models (21, 24), our GPR120−/− mice displayed higher fasted blood glucose and plasma insulin levels than wild-type counterparts, suggesting a latent insulin resistance (Fig. 1C). In brief, the GPR120−/− mice used in this study share the classical phenotyping characteristics previously evidenced by others (21, 24).
Fig. 1.
Impacts of GPR120 gene invalidation on body weight, blood glucose, plasma insulin levels and circumvallate papilla morphology. A: Genotyping of studied mice: agarose gel electrophoresis of PCR products. B: RT-quantitative PCR of CVP (n = 5, ud, undetectable). C: Typical GPR120−/− phenotypes compared with littermates GPR120+/+ mice: evolution of body weight in mice fed a standard chow (n = 7), fasted blood glucose and plasma insulin levels (n = 8–12). Means ± SEM. *, P < 0.05; **, P < 0.01. D: Morphology of CVP from wild-type and GPR120−/− mice. Gross anatomy: hematoxylin-eosin-stained CVP. Black arrows show apical taste pore areas of taste buds. Identification of taste buds cells by α-gustducin staining and visualization of taste bud cells proliferation by Ki67 staining.
The GPR120 gene is flanked by two genes known to be involved in the regulation of cell cycle and cell proliferation (i.e., Centrosomal Protein 55 and Retinol Binding Protein 4, respectively). To verify whether the genic deletion used to disrupt GPR120 gene expression interfered with the organization of the gustatory tissue, histology and immunostaining using specific markers of type-II cells (i.e., α-gustducin), in which GPR120 is mainly expressed (17), and of cell proliferation (i.e., Ki67) were undertaken in CVP from controls and GPR120−/− mice. No obvious change in the gross anatomy of CVP, taste bud structure, or Ki67 labeling was elicited when GPR120 was lacking (Fig. 1D).
GPR120-null mice detect and prefer lipid solutions
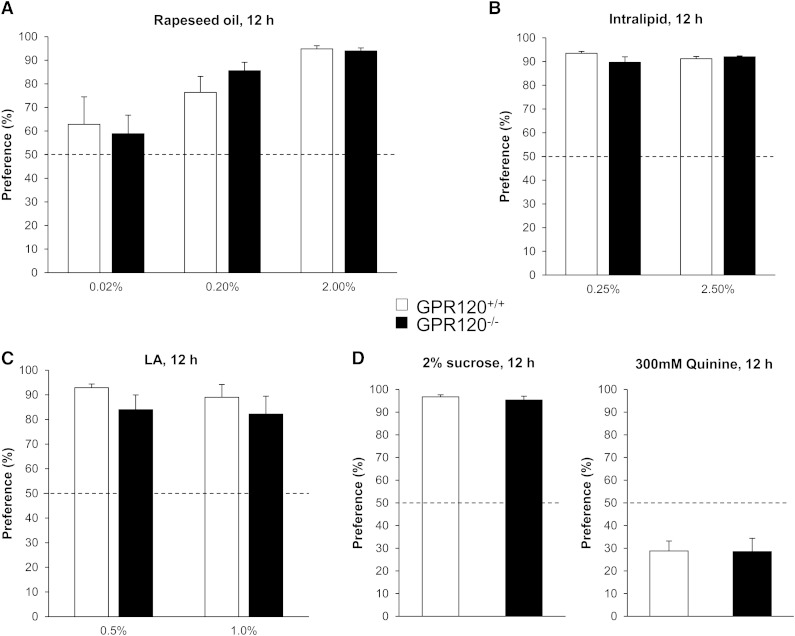
We have previously shown, using 12 h two-bottle preference tests performed with different concentrations of rapeseed oil, that mice become able to detect and prefer oily solutions when concentration reaches 0.02% oil, the maximal preference being observed with 2.0% oil in solution (22). According to this previous result, preference tests were performed in wild-type and GPR120−/− mice in presence of 0.02, 0.2, and 2% rapeseed oil mixed with 0.3% xanthan gum. Rapeseed oil was chosen by reason of its LCFA composition including saturated, mono-unsaturated (OLA) and poly-unsaturated ω6 (LA) and ω3 fatty acids known to be GPR120 ligands (25). Surprisingly, GPR120−/− mice displayed the same behavior as their littermate controls and were able to properly detect the different concentrations of oil (Fig. 2A). This behavior is not gender-dependent as similar results were found in age-matched males and females (data not shown). Consistent with data from Sclafani et al. (20), GPR120−/− mice displayed an attraction to Intralipid similar to that of controls (Fig. 2B). Also, in contrast to prior results (15), the GPR120 gene invalidation was not associated with a decrease in the detection and preference for LA during two-bottle preference tests (Fig. 2C). No difference between the responses of control and GPR120-null mice was detected for sucrose (sweet) and quinine (bitter) (Fig. 2D).
Fig. 2.
Effect of GPR120 gene invalidation on the preference for lipids. Two bottles (control and experimental solutions) were simultaneously offered to wild-type and GPR120−/− mice for 12 h. Experimental solutions contained: A: 0.02%, 0.2% or 2.0% of rapeseed oil (w/v), n = 10-12. B:2.5%, 0.25% of Intralipid (v/v), n = 10. C: 0.5% or 1% LA, n = 7. D: 2% sucrose (w/v) or 0.3 mM quinine (bitter taste), n = 10–12. Means ± SEM. Dotted line represents the absence of preference.
GPR120−/− and GPR120+/+ mice display similar conditioned aversion to LA
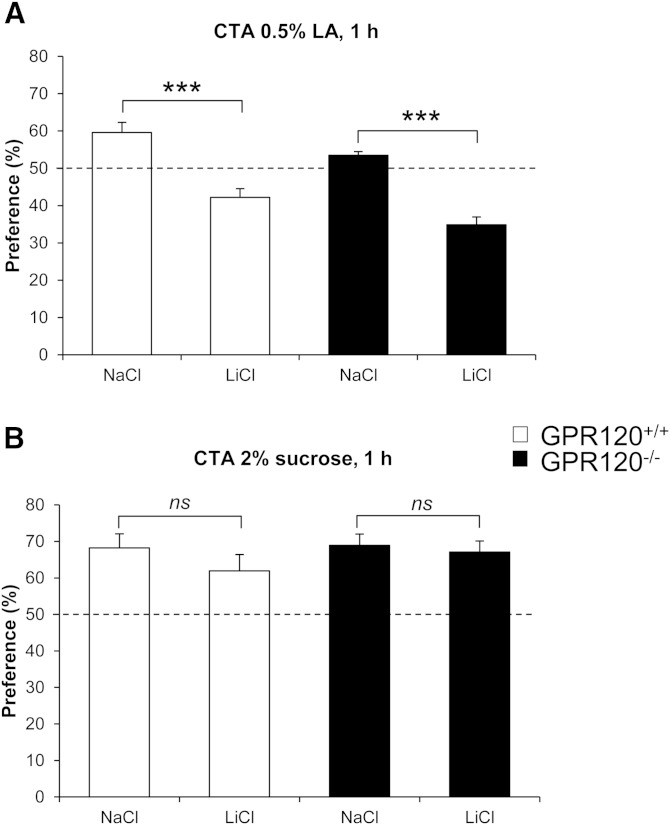
CTA is a learned reduction of hedonic value of a taste occurring when the taste experience is paired with a digestive malaise. This paradigm was used to further explore the putative involvement of GPR120 in oral LCFA detection. Consistent with previously published data (8), control mice developed a clear aversion for LA when this conditioned stimulus was previously paired with a LiCl-induced digestive pain (Fig. 3A). A similar behavior was maintained in GPR120−/− mice (Fig. 3A). This avoidance was specific as the sweet preference was not affected in LA-conditioned GPR120+/+ and GPR120−/− mice (Fig. 3B).
Fig. 3.
Effect of GPR120 gene invalidation on the conditioned avoidance of LA. Conditioned taste aversion (CTA) performed by pairing a conditioned stimulus, i.e., LA with a digestive pain obtained by intraperitoneal (i.p.) injection of LiCl (150 mM, 16µl/g of body weight), was assessed by using 1 h two-bottle preference tests. Unconditioned controls (GPR120+/+ and GPR120−/− mice) received an i.p. NaCl injection. A: LA-mediated CTA. Data shown are the average of two successive tests (48 h interval). There was no interaction between the tests and no effect of the time. Means ± SEM (n = 7–9). ***, P ≤ 0.001. B: To demonstrate the specificity of LA-taste aversion, two-bottle preference test was performed with sucrose (n = 7–9). Dotted line represents the absence of preference.
GPR120−/− and GPR120+/+ mice display similar reward behavior for LCFAs
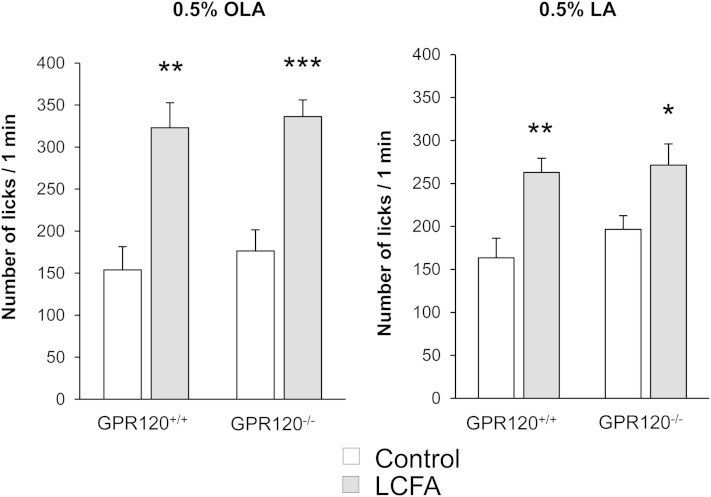
The behavior observed during long-term two-bottle preference tests is greatly influenced by post-ingestive regulatory feedback (26). The brief-access lick paradigm, by limiting post-ingestive cues, is believed to primarily measure taste-guided reward behavior [i.e., hedonic value and motivation (27)]. Therefore, computer-controlled lickometers were used to measure the number of licks for 1 min and determine the attraction for 0.5% OLA or 0.5% LA solubilized in mineral oil. As shown in Fig. 4, the number of licks given by GPR120−/− or GPR120+/+ mice is significantly higher for the LA or OLA solutions than for the control solution; thus, the lack of GPR120 did not affect the reward behavior for these two LCFAs.
Fig. 4.
Effect of GPR120 gene invalidation on the attraction for LCFAs. Short-term (1 min) licking tests in GPR120−/− and their littermate controls (GPR120+/+). Animals were subjected successively in a randomized manner to a control solution (mineral oil) and an experimental solution of 0.5% OLA or LA in mineral oil. Means ± SEM (n = 12). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
LA triggers the Ca2+ signaling pathway in TBCs from GPR120-null mice
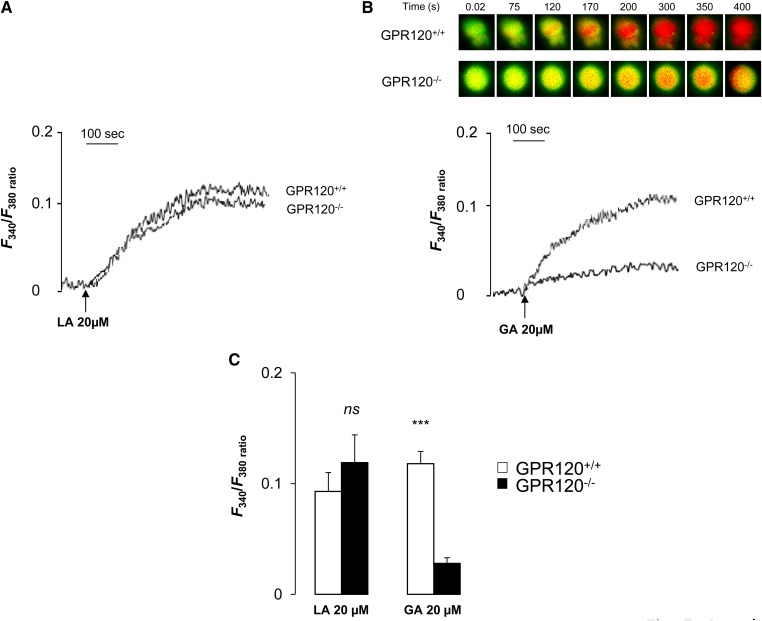
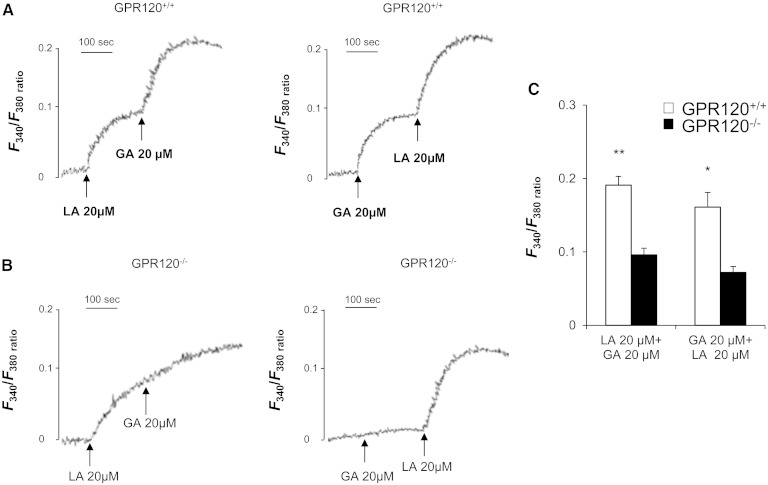
LCFAs are known to induce Ca2+ signaling in mouse circumvallate TBCs (8, 9, 19, 28). To explore the role played by the GPR120 receptor in this event, freshly isolated circumvallate TBCs from wild-type and GPR120−/− mice were subjected to 20 µmol/l LA or GA, a specific GPR120 partial agonist (29), and were analyzed using calcium imaging. LA triggered a similar induction of [Ca2+]i both in TBCs from control and GPR120−/− mice, indicating that GPR120 was not indispensable for the LA-mediated calcium response in taste buds (Fig. 5A, C). As expected, the rapid and huge rise in [Ca2+]i triggered by GA in control TBCs was dramatically curtailed when the GPR120 gene was lacking (Fig. 5B, C). To strengthen these observations, the effect of combinations of LA and GA on [Ca2+]i response in TBCs was studied. The addition of 20 µmol/l GA after that of 20 µmol/l LA, or inversely, triggered an additive response in TBCs from wild-type controls (Fig. 6A, C) while this effect was lacking in GPR120−/− mice (Fig. 6B, C).
Fig. 5.
Effects of LA or GA on Ca2+ signaling in mouse circumvallate TBCs. Ca2+ imaging studies were performed in calcium-containing (100% Ca2+) media. The changes in intracellular Ca2+ (F340/F380) were monitored under a Nikon microscope (TiU) by using S-fluor 40× oil immersion objectives, as described in the Materials and Methods section. Colored time-lapse changes show the kinetics of the rise in [Ca2+]i levels in a CD36-positive TBC freshly isolated from CVP from GPR120+/+ and GPR120−/− mice following addition of 20 µM LA or GA in the medium. The arrows indicate when LA or GA were added into the cuvette without interruptions in the recording. A: changes (F340/F380) in intracellular Ca2+ evoked by LA, 20 µM and (B) by GA, 20 µM. C: Means ± SEM (n = 7). ***, P < 0.001. ns, nonsignificant.
Fig. 6.
Differential intracellular Ca2+ signaling responses induced by LA and GA in TBCs. Ca2+ imaging studies were performed in calcium-containing (100% Ca2+) media. The changes in intracellular Ca2+ (F340/F380) were monitored under the Nikon microscope (TiU) by using S-fluor 40× oil immersion objectives, as described in the Materials and Methods section. Colored time-lapse changes show the kinetics of the rise in [Ca2+]i levels in a CD36-positive taste bud cell freshly isolated from CVP from GPR120+/+ and GPR120−/− mice following addition of 20 µM LA and GA one after another in the medium. The arrows indicate when LA or GA were added into the cuvette without interruptions in the recording. A: in GPR120+/+ mice and (B) in GPR120−/− mice. C: Means ± SEM (n = 7). *, P < 0.05; **, P < 0.01.
Discussion
A growing number of studies support the involvement of a taste component in the oro-sensory detection of dietary lipids. By detecting LCFAs, this sensing system might play a significant role in food choices. The plasma membrane glycoprotein CD36 was first identified as a candidate for the function of gustatory lipid sensor in mice (6, 10). More recently, the LCFA sensors GPR40 and GPR120 were shown to be possible alternative candidates (15). Mice lacking GPR40 or GPR120 were unable to detect and prefer a lipid source (OLA or LA) during behavioral tests. Moreover, direct recording of gustatory nerves reveals that GPR40 and GPR120 were required to generate nerve signals in response to oral lipid stimulation (15). This original finding correlated quite well with the gustatory function of other GPCRs, like Taste receptor type 1 and Taste receptor type 2 receptors involved in sweet, bitter, and umami tastes. Nevertheless, a direct implication of GPR40 in oral fat detection is unlikely because we did not retrieve any transcript of this receptor in mouse CVP; also, it was not found in mouse circumvallate, fungiform, nor foliate papillae by others, in contrast to what was found for GPR120. Similarly, GPR40 was not retrieved in gustatory papillae in rats (17) or humans (18).
Despite the expression of GPR120 in mouse type-II taste cells (17) and its activation by LCFAs (25), an implication of GPR120 in fat detection and preference remains a matter of debate. As aforementioned, it was recently reported that oral GPR120 is not essential for this function in mice (20, 30), contrary to prior findings (15). To further unravel the GPR120 role in the “taste of fat”, we chose to revisit the effects of a GPR120 gene invalidation on taste bud functionality and lipid preference in mice. Because the aforementioned contradictory data were generated in the same KO mouse model, present experiments were conducted using an alternative GPR120−/− mouse model. We report herein that GPR120−/− mice and controls displayed a similar appetence for oily solution or Intralipid emulsion during 12 h two-bottle preference tests. This behavior was reproduced when free LCFA (LA) solutions were checked by using long-term (12h) two-bottle preference tests, but also in conditions known to deeply minimize post-ingestive influences (i.e., 1 min licking tests with LA or OLA) suggesting that the absence of GPR120 in oral cavity did not alter the taste-guided attraction for fat. Moreover, GPR120−/− mice subjected to conditioned taste aversion tests learned to avoid 0.5% LA like wild-type mice, whereas their preference for sweet was maintained. Altogether, these results suggest that GPR120 is not indispensable for the oro-sensory detection of lipids in mice. Long-term two-bottle preference tests performed with LA or OLA allows a direct comparison with the previous study (15), but leads to an opposite conclusion. Origin of this discrepancy remains elusive. In the present study, LCFA concentrations tested were deliberately limited to values reported as leading to significant differences between controls and GPR120−/− mice during two-bottle preference tests in (15), but remaining within the limits that may be found in the feed. Moreover, an influence of the KO mouse model used is unlikely because we found an attraction for Intralipid similar to the one found by Sclafani et al. (20), whose experiments were conducted using the same GPR120−/− mice as Cartoni et al. (15).
Ca2+ signaling being an early event downstream of taste receptor activation, calcium imaging studies were performed in freshly isolated mouse TBCs to gain insight into the functional incidence of a GPR120 gene deletion. In line with our behavioral data, LA-mediated induction of [Ca2+]i was not altered in GPR120−/− mice. This result is consistent with a recent report showing that LA-mediated increase in [Ca2+]i is poorly or not affected by the siRNA knockdown of GPR120 in a human fungiform cell line (19). By contrast, the huge rise in [Ca2+]i triggered by GA, a GPR120 partial agonist, was greatly diminished when GPR120 was lacking. Therefore, in the gustatory cells from mouse CVP, GPR120 can be efficiently activated by GA, demonstrating the functionality of the receptor. However, the LCFA-mediated rise in [Ca2+]i does not absolutely require GPR120. These unexpected results suggest that activation of GPR120 and preference for lipids might not be connected. This conclusion is consistent with a recent report showing that wild-type mice did not display any preference for non-fatty acid GPR120 agonists over water in two-bottle preference tests, despite a GPR120-dependent activation of the glossopharyngeal nerve (30). Further experiments are needed to elucidate these paradoxical results. It is noteworthy that expression of GPR120 extends to surrounding non-gustatory epithelium in humans (18). Moreover, GPR120 has been identified in a lingual population of trigeminal neurons in rodents (31, 32). These observations raise the possibility of an implication of GPR120 in other oro-sensory cues. Interestingly, a tight relationship exists between taste and somatosensory signals with a convergence in the NTS (33). In some cases, this association inhibits taste response (34). Existence of a complex modulation of NTS neurons by GPR120-positive trigeminal afferents might explain the dissociation between taste nerve activation and attraction for fat reported by Godinot and coworkers (30).
GPR120 was first identified in the entero-endocrine L-cells in which it mediates the GLP-1 secretion induced by LCFAs (25). Several observations support a similar implication of GPR120 in mouse gustatory papillae. Indeed, GPR120 and GLP-1 are coexpressed in a subset of TBCs (22). Moreover, GLP-1 release is triggered by the specific GPR120 agonist GSK137647A in CVP explants (22) and by GA in freshly isolated TBCs (19). Finally, concomitant presence of the GLP-1 receptor (GLP-1R) in the gustatory mucosa (35) suggests that this hormone is locally active and, thus, might directly affect the basic functions in mouse taste buds. Consistent with this assumption, it was reported that the GLP-1 signaling modulates the sweet taste sensitivity (35).
We recently reported that CD36 protein levels in mouse CVP is subjected to a highly sensitive short-term downregulation during food intake, in contrast to GPR120 (11). This regulation is strictly dependent on the presence of lipids in the diet. It is a local control, because a direct oil deposition onto the tongue is sufficient to trigger the decrease of CD36 protein in CVP. As reported for numerous surface receptors, this negative feedback might constitute a desensitization system of CD36-positive TBCs during persistent exposure to dietary lipids during a meal (11). Interestingly, the absence of GLP-1R suppresses this lipid-mediated downregulation, suggesting that CD36 protein levels in CVP are dependent on the GLP-1 signaling pathway (22). Because TBCs coexpress GLP-1 and GPR120 (22), it is tempting to speculate that the lipid-mediated drop in CD36 in CVP is locally regulated by GLP-1 via GPR120. Therefore, the lower attraction for fat found in GLP-1R−/− mice (22) might be due to the functional impairment of CD36 in CVP. The molecular mechanism of such regulation remains to be determined. It is known that the invalidation of the CD36 gene elicits a dramatic decrease in the attraction for lipids despite the absence of changes in GPR120 expression in CVP (11). We reported herein that GPR120 gene disruption is not associated with a change in CD36 expression levels in CVP and in preference for fat. All these data strongly suggest that CD36 and GPR120 play different, but complementary, functions in the mediation of fat preference. CD36 should be critical for oral fat detection and preference, whereas GPR120, through the local GLP-1 signaling might modulate the lipid detection sensitivity. Further studies are needed to explore this hypothesis. Because taste-guided preference for highly palatable fatty foods contributes to obesity, the molecular deciphering of the mechanisms responsible for the detection of dietary lipids by the peripheral gustatory pathway constitutes a real public health challenge that might lead to new insights to reduce the obesity risk in a near future.
Footnotes
Abbreviations:
- [Ca2+]i
- intracellular calcium level
- CTA
- conditioned taste aversion
- CVP
- circumvallate papilla
- FFAR
- free fatty acid receptor
- GA
- grifolic acid
- GLP-1R
- GLP-1 receptor
- GPCR
- G-protein coupled receptor
- LA
- linoleic acid
- LCFA
- long-chain fatty acid
- OLA
- oleic acid
- TBC
- taste bud cell
This work was supported by a grant from the French National Research Agency (ANR-12-BSV1-0027-01, SensoFAT-2 project to P.B.).
REFERENCES
- 1.Kawai T., Fushiki T. 2003. Importance of lipolysis in oral cavity for orosensory detection of fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285: R447–R454. [DOI] [PubMed] [Google Scholar]
- 2.Pepino M. Y., Love-Gregory L., Klein S., Abumrad N. A. 2012. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 53: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voigt N., Stein J., Galindo M. M., Dunkel A., Raguse J. D., Meyerhof W., Hofmann T., Behrens M. 2014. The role of lipolysis in human orosensory fat perception. J. Lipid Res. 55: 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baillie A. G., Coburn C. T., Abumrad N. A. 1996. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J. Membr. Biol. 153: 75–81. [DOI] [PubMed] [Google Scholar]
- 5.Fukuwatari T., Kawada T., Tsuruta M., Hiraoka T., Iwanaga T., Sugimoto E., Fushiki T. 1997. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 414: 461–464. [DOI] [PubMed] [Google Scholar]
- 6.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., Besnard P. 2005. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115: 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simons P. J., Kummer J. A., Luiken J. J., Boon L. 2011. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 113: 839–843. [DOI] [PubMed] [Google Scholar]
- 8.Gaillard D., Laugerette F., Darcel N., El-Yassimi A., Passilly-Degrace P., Hichami A., Khan N. A., Montmayeur J. P., Besnard P. 2008. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 22: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 9.El-Yassimi A., Hichami A., Besnard P., Khan N. A. 2008. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283: 12949–12959. [DOI] [PubMed] [Google Scholar]
- 10.Sclafani A., Ackroff K., Abumrad N. A. 2007. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293: R1823–R1832. [DOI] [PubMed] [Google Scholar]
- 11.Martin C., Passilly-Degrace P., Gaillard D., Merlin J. F., Chevrot M., Besnard P. 2011. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE. 6: e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C. S., Bench E. M., Allerton T. D., Schreiber A. L., Arceneaux K. P., 3rd, Primeaux S. D. 2013. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305: R1346–R1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love-Gregory L., Sherva R., Schappe T., Qi J. S., McCrea J., Klein S., Connelly M. A., Abumrad N. A. 2011. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 20: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller K. L., Liang L. C., Sakimura J., May D., van Belle C., Breen C., Driggin E., Tepper B. J., Lanzano P. C., Deng L., et al. 2012. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring). 20: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., le Coutre J., Ninomiya Y., Damak S. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30: 8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montmayeur J. P., Fenech C., Kusumakshi S., Laugerette F., Liu Z. H., Wiencis A., Boehm U. 2011. Screening for G-protein-coupled receptors expressed in mouse taste papillae. Flavour Fragrance J. 26: 223–230. [Google Scholar]
- 17.Matsumura S., Mizushige T., Yoneda T., Iwanaga T., Tsuzuki S., Inoue K., Fushiki T. 2007. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed. Res. 28: 49–55. [DOI] [PubMed] [Google Scholar]
- 18.Galindo M. M., Voigt N., Stein J., van Lengerich J., Raguse J. D., Hofmann T., Meyerhof W., Behrens M. 2012. G protein-coupled receptors in human fat taste perception. Chem. Senses. 37: 123–139. [DOI] [PubMed] [Google Scholar]
- 19.Ozdener M. H., Subramaniam S., Sundaresan S., Sery O., Hashimoto T., Asakawa Y., Besnard P., Abumrad N. A., Khan N. A. 2014. CD36- and GPR120-mediated Ca signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclafani A., Zukerman S., Ackroff K. 2013. GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305: R1490–R1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., et al. 2012. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 483: 350–354. [DOI] [PubMed] [Google Scholar]
- 22.Martin C., Passilly-Degrace P., Chevrot M., Ancel D., Sparks S. M., Drucker D. J., Besnard P. 2012. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J. Lipid Res. 53: 2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevrot M., Bernard A., Ancel D., Buttet M., Martin C., Abdoul-Azize S., Merlin J. F., Poirier H., Niot I., Khan N. A., et al. 2013. Obesity alters the gustatory perception of lipids in the mouse: plausible involvement of lingual CD36. J. Lipid Res. 54: 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. 2005. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11: 90–94. [DOI] [PubMed] [Google Scholar]
- 26.Sclafani A., Ackroff K. 2012. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302: R1119–R1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin A. C., Townsend R. L., Patterson L. M., Berthoud H. R. 2011. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301: R1267–R1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dramane G., Abdoul-Azize S., Hichami A., Vogtle T., Akpona S., Chouabe C., Sadou H., Nieswandt B., Besnard P., Khan N. A. 2012. STIM1 regulates calcium signaling in taste bud cells and preference for fat in mice. J. Clin. Invest. 122: 2267–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara T., Hirasawa A., Sun Q., Sadakane K., Itsubo C., Iga T., Adachi T., Koshimizu T. A., Hashimoto T., Asakawa Y., et al. 2009. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch. Pharmacol. 380: 247–255. [DOI] [PubMed] [Google Scholar]
- 30.Godinot N., Yasumatsu K., Barcos M. E., Pineau N., Ledda M., Viton F., Ninomiya Y., le Coutre J., Damak S. 2013. Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. Neuroscience. 250: 20–30. [DOI] [PubMed] [Google Scholar]
- 31.Gilbertson T. A., Yu T., Shah B. P. 2010. Gustatory mechanisms for fat detection. In Fat Detection: Taste, Texture, and Post Ingestive Effects. J. P. Montmayeur and J. le Coutre, editors, Boca Raton (FL). [PubMed] [Google Scholar]
- 32.Yu T., Shah B. P., Hansen D. R., Park-York M., Gilbertson T. A. 2012. Activation of oral trigeminal neurons by fatty acids is dependent upon intracellular calcium. Pflugers Arch. 464: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faurion A. 2006. Sensory interactions through neural pathways. Physiol. Behav. 89: 44–46. [DOI] [PubMed] [Google Scholar]
- 34.Osada K., Komai M., Bryant B. P., Suzuki H., Goto A., Tsunoda K., Kimura S., Furukawa Y. 1997. Capsaicin modifies responses of rat chorda tympani nerve fibers to NaCl. Chem. Senses. 22: 249–255. [DOI] [PubMed] [Google Scholar]
- 35.Shin Y. K., Martin B., Golden E., Dotson C. D., Maudsley S., Kim W., Jang H. J., Mattson M. P., Drucker D. J., Egan J. M., et al. 2008. Modulation of taste sensitivity by GLP-1 signaling. J. Neurochem. 106: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]