Abstract
To determine how the level of dietary n-6 PUFA affects the rate of loss of arachidonic acid (ARA) and DHA in brain phospholipids, male rats were fed either a deprived or adequate n-6 PUFA diet for 15 weeks postweaning, and then subjected to an intracerebroventricular infusion of 3H-ARA or 3H-DHA. Brains were collected at fixed times over 128 days to determine half-lives and the rates of loss from brain phospholipids (Jout). Compared with the adequate n-6 PUFA rats, the deprived n-6-PUFA rats had a 15% lower concentration of ARA and an 18% higher concentration of DHA in their brain total phospholipids. Loss half-lives of ARA in brain total phospholipids and fractions (except phosphatidylserine) were longer in the deprived n-6 PUFA rats, whereas the Jout was decreased. In the deprived versus adequate n-6 PUFA rats, the Jout of DHA was higher. In conclusion, chronic n-6 PUFA deprivation decreases the rate of loss of ARA and increases the rate of loss of DHA in brain phospholipids. Thus, a low n-6 PUFA diet can be used to target brain ARA and DHA metabolism.
Keywords: polyunsaturated fatty acid, docosahexaenoic acid, kinetics, metabolism, eicosanoid
The brain is specifically enriched with the two PUFAs arachidonic acid (20:4n-6; ARA) and DHA (22:6n-3), which are considered important for normal brain function. Bioactive mediators from ARA and DHA, the eicosanoids and docosanoids, respectively, regulate many processes including neuroinflammation, pain perception, and blood flow (1–6). As a result, altered levels or metabolism of these PUFAs have been implicated in many neurological and psychiatric disorders, including Alzheimer’s disease, Parkinson’s disease, bipolar disorder, and major depression (7–9).
ARA and DHA can either be obtained directly from the diet or synthesized from their nutritionally essential precursor fatty acids linoleic acid (18:2n-6; LA) and α-linolenic acid (18:3n-3; ALA) respectively, which are the main dietary n-6 and n-3 PUFAs. Thus, it is of interest to determine whether changes in the level of dietary n-6 or n-3 PUFAs can alter the concentrations of ARA and/or DHA in the brain, and more importantly, whether these dietary changes affect their metabolism. In fact, clinical trials in humans are underway, with the assumption that lowering n-6 PUFAs may decrease brain ARA and its metabolism (1).
Several studies have investigated the effects of dietary n-3 PUFA deprivation on brain ARA and DHA concentrations and metabolism in rats. Feeding rats an n-3 PUFA-deprived diet that contains ALA at a concentration of 0.04% versus an n-3 PUFA-adequate diet containing ALA at 4.4% of all fatty acids decreased the concentration of DHA by 37% and increased docosapentaenoic acid (DPA) n-6 concentration by 95% in brain total phospholipids, but did not change the concentration of ARA (10). This study also reported that n-3 PUFA deprivation conserves DHA in rat brain phospholipids extending the half-life from 33 to 90 days. This conservation response was selective for DHA, as n-3 PUFA deprivation did not alter the half-life or rate of ARA metabolic consumption (11). Alterations in brain DHA concentration and metabolism appear to occur below a threshold of 0.8% ALA in the diet, where there is a decrease in the activity of the DHA-metabolizing enzymes, calcium-independent phospholipase A2 (iPLA2) and cyclooxygenase (COX) 1, and an increase in the activity of the ARA-selective enzymes calcium-dependent cytosolic phospholipase A2 (cPLA2) and secretory phospholipase A2 as well as COX-2 (12, 13). Lower concentrations of DHA in the brains of n-3 PUFA-deprived rats are likely due to a reduction in the incorporation of DHA into brain phospholipids, as well as a reduction in DHA recycling (14).
Compared with dietary n-3 PUFA manipulation, few studies have examined dietary n-6 PUFAs and the brain. Reducing the percentage of total fatty acid from 28% LA to 2% LA decreases brain ARA by 28% and increases DHA by 11% (15). Dietary n-6 PUFA deprivation also reduces expression of ARA-selective enzymes (cPLA2 and COX-2) and increases expression of DHA-selective enzymes [iPLA2 and 15-lipoxygenase (LOX)] (16). There was a corresponding decrease in the protein levels of activator protein (AP)-2α and nuclear factor κB p65 (transcription factors for cPLA2 and COX-2), as well as an increase in the levels of sterol-regulatory element binding protein 1 (an iPLA2 transcription factor) (16). Furthermore, the DHA uptake rate into brain phospholipids is increased by 45% in rats fed an n-6 PUFA-deprived diet, which could account for the increase in phospholipid DHA (17). In the same study, the turnover of DHA in brain total phospholipids was increased 30–84% in the choline glycerophospholipid (ChoGpl), phosphatidylinositol (PtdIns), and phosphatidylserine (PtdSer) fractions, but not the ethanolamine glycerophospholipid (EtnGpl) fraction.
As of yet, no study has investigated the effect of n-6 PUFA deprivation on the rate of loss of ARA and DHA in brain phospholipids. The objective of this study was to determine the rate of loss of both ARA and DHA from brain phospholipids in rats that are fed either the adequate or deprived n-6 PUFA diet as used in previous studies (15–17). We hypothesized that upon chronic low n-6 PUFA consumption, ARA would be conserved while DHA would be lost at a more rapid rate. To test this hypothesis, rats consumed either an adequate or a deprived n-6 PUFA diet for 15 weeks postweaning, after which they were infused with either 3H-ARA or 3H-DHA via an intracerebroventricular infusion. At set time points post intracerebroventricular infusion, rats were euthanized and the radioactivity of their brains was measured to plot a curve depicting the loss of radioactive ARA or DHA over time. After a logarithmic transformation of the curves, a linear regression analysis was done, and the regression slopes were used to calculate the ARA and DHA half-lives in brain phospholipids (t1/2), as well as the rate of loss of ARA and DHA on a molar basis (Jout). ARA was found to be conserved in the n-6 PUFA-deprived rats, while DHA appeared to be lost more rapidly from the brain phospholipids.
MATERIALS AND METHODS
Animals
All procedures were approved by the Animal Ethics Committee at the University of Toronto (Protocol # 20010100), in accordance with policy statements of the Canadian Council on Animal Care. One hundred and twelve male Fischer (CDF) rats were purchased from Charles River Laboratories (Saint-Constant, QC, Canada) and arrived at the Division of Comparative Medicine animal facility at 21 days of age. Rats were housed in a 22°C environment with a 12 h light-dark cycle, and they received ad libitum access to water and food throughout the study. Upon arrival, they were randomized to receive either the n-6 PUFA-deprived (n = 56) or n-6 PUFA-adequate diet (n = 56) (supplementary Fig. 1). Measurements of body weight and food intake were carried out on a weekly basis for 15 weeks. Following 15 weeks of feeding, 8 rats from each dietary group were euthanized by high-energy, head-focused microwave irradiation (13.5 kW for 1.6 s; Cober Electronics Inc., Norwalk, CT) as previously described (18–21). Their brains were removed, dissected sagittally, and stored at −80°C for measurements of eicosanoid levels, docosanoid levels, and baseline brain phospholipid fatty acid concentrations.
n-6 PUFA-adequate and n-6 PUFA-deprived diets
The n-6 PUFA-adequate and n-6 PUFA-deprived rodent diets were based on the AIN-93G formulation with a 10% fat composition (22, 23) as used by others (15–17). The diets were purchased from Dyets Inc. (Bethlehem, PA), under the following product names: Revised Modified n-6 PUFA Adequate Diet (Dyet# 180780) and Custom Modified n-6 PUFA Deficient Diet (Dyet #180784). In order to be consistent with the literature (15–17), we chose to use the terms “adequate” and “deprived” to describe the 24% and 2% of total fatty acids LA levels in the diets. However, we recognize that LA requirements are controversial and point the reader to the methods of Igarashi et al. (17) as well as other papers for more details (24, 25). The compositions of both diets are shown in (Table 1). As in previous studies (15–17), the n-6 PUFA-adequate diet contained safflower oil (32.3 g/kg), hydrogenated soybean oil (5 g/kg), and coconut oil (55 g/kg). The n-6 PUFA-deprived diet did not contain safflower oil, a significant source of LA. Instead, it contained hydrogenated coconut oil (87.3 g/kg) and olive oil (5 g/kg). Both diets had equal amounts of flaxseed oil (7.7 g/kg).
TABLE 1.
Composition of n-6 PUFA-adequate and n-6 PUFA-deprived diets
| Ingredient | n-6 PUFA-Adequate Diet (g/kg of Diet) | n-6 PUFA-Deprived Diet (g/kg of Diet) |
| Casein | 200 | 200 |
| l-Cystine | 3 | 3 |
| Sucrose | 99.98 | 99.98 |
| Cornstarch | 150 | 150 |
| Maltose dextrin | 150 | 150 |
| Dextrose | 200 | 200 |
| Hydrogenated coconut oil | 55 | 87.3 |
| Olive oil | 0 | 5 |
| Flaxseed oil | 7.7 | 7.7 |
| Safflower oil | 32.3 | 0 |
| Hydrogenated soybean oil | 5 | 0 |
| t-Butylhydroquinone | 0.02 | 0.02 |
| Cellulose | 49.5 | 49.5 |
| Mineral Mix #210025 | 35 | 35 |
| Vitamin Mix #310025 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 |
The only difference between the two diets is the amount of hydrogenated coconut oil, olive oil, safflower oil, and hydrogenated soybean oil. The safflower oil and soybean oil in the n-6 PUFA-adequate diet were replaced by hydrogenated coconut oil and olive oil.
Total lipids were extracted from ∼0.5 g of each diet (n = 3) and analyzed by gas chromatography with flame-ionization detection (GC-FID) as described below. Resultant fatty acid concentrations are shown in (Table 2). LA accounted for ∼24% of total fatty acids in the n-6 PUFA-adequate diet, and only 2% of total fatty acids in the n-6 PUFA-deprived diet. Approximately 4% of total fatty acids were ALA in both diets. There were negligible amounts of both ARA and DHA (<0.05%).
TABLE 2.
Fatty acid percent composition of adequate and deprived n-6 PUFA diets
| Fatty Acid | Adequate n-6 PUFA Diet | Deprived n-6 PUFA Diet |
| % Total Fatty Acid | % Total Fatty Acid | |
| 8:0 | 1.01 ± 0.159 | 1.08 ± 0.405 |
| 10:0 | 3.23 ± 0.054 | 4.79 ± 0.316 |
| 12:0 | 29.26 ± 0.237 | 46.14 ± 0.450 |
| 14:0 | 11.09 ± 0.089 | 17.62 ± 0.174 |
| 16:0 | 8.65 ± 0.042 | 9.79 ± 0.249 |
| 16:1n-7 | ND | 0.12 ± 0.003 |
| 18:0 | 10.76 ± 0.079 | 9.40 ± 0.338 |
| 18:1n-9 | 6.79 ± 0.056 | 4.56 ± 0.173 |
| 18:1n-7 | 0.26 ± 0.002 | 0.21 ± 0.009 |
| 18:2n-6 | 24.39 ± 0.170 | 2.08 ± 0.076 |
| 18:3n-3 | 4.05 ± 0.026 | 3.98 ± 0.148 |
| 20:0 | 0.20 ± 0.002 | 0.13 ± 0.006 |
| 20:1n-9 | 0.07 ± 0.002 | ND |
| ∑ Saturated | 64.21 ± 0.25 | 88.95 ± 0.41 |
| ∑ MUFA | 7.25 ± 0.06 | 4.93 ± 0.19 |
| ∑ n-3 PUFA | 4.10 ± 0.02 | 4.01 ± 0.15 |
| ∑ n-6 PUFA | 24.44 ± 0.17 | 2.10 ± 0.08 |
| n-6/n-3 | 5.9 | 0.5 |
Data are mean ± SEM. Fatty acid percent compositions were quantified by GC-FID. Levels ≤0.05% were considered nondeterminable (ND). The following fatty acids were measured but were ND in both dietary groups: 14:1n-7, 18:3n-6, 20:2n-6, 20:3n-3, 20:4n-6, 20:5n-3, 22:0, 22:1n-9, 22:6n-3, and 24:1n-9.
Radiotracers
5,6,8,9,11,12,14,15-3H arachidonic acid (3H-ARA) and 4,7,10,13,16,19-3H docosahexaenoic acid (3H-DHA), each dissolved in toluene, were purchased from Moravek Biochemicals (Brea, CA). The 3H-ARA had a specific activity of 207 Ci/mmol and a purity of 98.4%. The 3H-DHA had a specific activity of 1.0 Ci/mmol and a purity of 97.2%. To create each infusate, the tracer was dissolved in a 5 mM HEPES buffer solution (pH 7.4) with a ratio of tracer molecules to fatty acid-free BSA that was larger than 3:2. The mixture was sonicated and stored at −80°C (11). Tracer purity was confirmed by HPLC and liquid scintillation counting (LSC).
Intracerebroventricular infusion of 3H-ARA and 3H-DHA
After 15 weeks of feeding, the nonbaseline rats underwent an intracerebroventricular infusion of either 3H-ARA (n = 24 per dietary group) or 3H-DHA (n = 24 per dietary group) (supplementary Fig. 1). As previously described (11, 26), rats were anesthetized with isoflurane inhalation (3% induction, 1–2% maintenance). Their heads were shaved at the area of incision and they were positioned onto a stereotaxic frame (Stoelting, Wood Dale, IL). To prevent dehydration, they were injected subcutaneously with 1 ml of sterile saline, and for pain control, 5 mg/ml ketoprofen solution (5 mg/kg of rat weight). At the incision site, 100 μl of local analgesic (0.1% Marcaine solution) was injected subcutaneously. Five minutes after this injection, the skull was exposed with a small incision on the dorsal side of the rat’s head. A small hole was drilled in the skull (+1.5 mm lateral/medial and −1 mm anterior/posterior to bregma) with a micromotor drill (Stoelting). Using a 33-gauge beveled needle and syringe (World Precision Instruments, Sarasota, FL), 5 μl of 3H-ARA or 3H-DHA infusate solution, containing 10 uCi of labeled fatty acid was infused at a rate of 0.17 μl/min into the right lateral ventricle of the brain (+1.5 mm lateral/medial, −1 mm anterior/posterior, and −4 mm dorsal/ventral to bregma). After the infusion, the needle was slowly removed, and the hole in the skull was sealed with cranioplastic cement (Stoelting). The incision was closed with self-dissolving sutures. For recovery, rats were placed under a heating lamp for 20–30 min before being returned to their respective cages. Rats received a second dose of ketoprofen 1 day postsurgery. For the rest of the study, rats continued to consume their respective diets.
Collection of radioactive brains
Rats were euthanized by microwave irradiation (13 kW for 1.6 s) at the following time points: 4, 16, 32, 64, and 128 days post intracerebroventricular infusion (supplementary Fig. 1). Four n-6 PUFA-deprived rats and 4 n-6 PUFA-adequate rats were euthanized at each time point from both the 3H-ARA-infused and 3H-DHA-infused groups. Brains were removed and stored at −80°C.
Extraction and isolation of brain phospholipids
Brains were homogenized, and their total lipids were extracted by the chloroform-methanol-0.88% KCl (2:1:0.75) Folch, Lees, and Stanley method (27). TLC was used to isolate the total phospholipids, as well as the phospholipid classes from the total lipid extract as previously described (11, 26). TLC plates were washed in chlorofrom-methanol (2:1 by volume) and were activated for 1 h at 100°C. TLC G-plates (EMD Chemical, Gibbstown, NJ) in a heptane-diethyl ether-glacial acetic acid solution system (60:40:2 ml by volume) were used for neutral lipid separation. TLC H-plates (Analtech, Newark, DE) in a chloroform-methanol-2-propanol-0.25% KCl-triethylamine (30:9:25:6:18 by volume) system were used to separate the phospholipid classes. The plates were sprayed with 0.1% 8-anilo-1-naphthalene sulfonic acid for UV visualization of the fatty acid bands. The bands containing total phospholipids, and the phospholipids fractions (ChoGpl, EtnGpl, PtdSer, and PtdIns) were collected into tubes. For GC-FID analysis, a known amount of heptadecanoic acid (17:0) standard was added. In preparation for both GC-FID and HPLC analysis, the fatty acids were converted into fatty acid methyl esters (FAMEs) by treatment with 14% boron trifluoride-methanol at 100°C for 1 h.
Quantitation of baseline brain phospholipid fatty acids by GC-FID
FAMEs were analyzed by a Varian-430 gas chromatograph (Varian, Lake Forest, CA) with an FID and a Varian FactorFour capillary column (VF-23ms; 30 m × 0.25 mm inner diameter × 0.25 μm film thickness). The FAMEs were dissolved in hexane and injected in splitless mode. The injector and detector ports were set at 250°C. The FAMEs were eluted with increasing temperatures. The temperature program started at 50°C for 2 min, increased 20°C/min, held at 170°C for 1 min, increased 3°C/min, and finally, held at 212°C for 5 min. The helium carrier gas had a flow rate of 0.7 ml/min. Output peaks were identified using known retention times of authentic FAME standards (Nu-Chek Prep Inc., Elysian, MN). Fatty acid concentrations were calculated by comparison of the GC fatty acid peak areas to the internal 17:0 standard peak area (11, 26, 28).
Quantitation of baseline brain eicosanoids and docosanoids
As described previously (29), composite standards of lipid metabolites (natural or deuterated; Cayman Chemicals Co., Ann Arbor, MI) were diluted in ethanol from stock solutions to perform an eight-point calibration curve (0.05 to 5 ng). Internal standard mixtures in ethanol were added to both the composite standards and the samples prior to extraction. Extraction and sample preparations were performed in siliconized glassware. To minimize autooxidation, fatty acids were extracted on ice, in a reduced light condition, using solvents that contained 0.1% butylated hydroxyl-toluene. The frozen brain halves were homogenized in methanol. One nanogram of internal standard mixture was added to a 250 mg aliquot of each homogenized brain. External ARA, EPA (20:5n-3), and DHA standards were prepared in a similar way. The samples were mixed for 1 min, incubated on ice for 30 min, and centrifuged at 1,000 g for 10 min. The supernatants were collected. The pellet was resuspended in ethanol for 1 min and centrifuged again for a second extraction. The resultant ethanolic supernatants were combined with the methanolic supernatants, previously extracted. After evaporation with nitrogen gas, the supernatants were suspended in 10% ethanol, acidified to pH 3 with 1 N HCl, and triply extracted with ethyl acetate. The ethyl acetate layer was washed to neutrality with water and dried under nitrogen gas. The residues from the brain and external standard samples were reconstituted in acetonitrile-water (1:1 by volume) and transferred into the inserts of amber vials for immediate LC/MS/MS analysis. LC/MS/MS was performed using a 1290 UHPLC System (Agilent Technologies, Santa Clara, CA) and a QTRAP5500 Mass Spectrometer (ABSciex, Framingham, MA). The chromatography was done at a 600 μl/min flow rate on a Zorbax SB-Phenyl column (Agilent Technologies; 3.0 × 50 mm, 3.5 μm). The gradient started at 80% water and, over 9 min, ramped up to 100% acetonitrile. The mass spectrometer was operated in negative electrospray ionization mode with a source temperature setting of 600°C and a voltage setting of 4,500 V. The precursors to product ion mass transitions were obtained through scheduled multiple reaction monitoring. Quantitative analysis was performed by Analyst 1.5.2 Software (ABSciex). The area ratios of the integrated peaks (natural to deuterated standard) were plotted against the standard curves for quantification. The limit of quantification was 0.025 ng per sample, and values between 0.005 ng and 0.025 ng were considered semiquantitative.
Confirmation of radiotracer identity by HPLC
Total phospholipids were extracted from brain homogenate and methylated as described above. Samples were reconstituted in acetonitrile. As described in previous studies (11, 26, 28, 30), FAMEs were separated by HPLC (Waters 2690, Boston, MA) with a Luna C18 reverse column (4.6 × 250 mm, 100 Ǻ Phenomenex, Torrance, CA) and an in-line UV photodiode array detector (Waters 996) set at a 242 nm wavelength. The system was first stabilized at a 1 ml/min flow rate with a gradient system consisting of i) 100% water and ii) 100% acetonitrile. The gradient was then set to 85% (ii) for 30 min, and then increased to 100% (ii) over 10 min. It was held there for 20 min before returning back to 85% (ii) over a 5 min period. Fractions were collected at 1 min intervals for 55 min, and each of the 55 fractions was measured for radioactivity by LSC. Similar to what has previously been reported, ARA and DHA had elution times of 35 and 31 min, respectively (11, 31).
Quantification of radioactivity by LSC
LSC was used to measure the radioactivity of the total phospholipids, fractions, as well as 4-day brain phospholipid HPLC fractions. Samples were put into scintillation vials, and 5 ml of scintillation cocktail (GE Healthcare, Life Sciences, Baie d’Urfe, QC, Canada) was added. Radioactivity was quantified using a Packard TRI-CARB2900TR liquid scintillation analyzer (Packer, Meriden, CT) with a detector efficiency of 61.07% for tritium. The measurements were given in disintegrations per minute and were converted to nCi/brain (11, 26).
Calculations and statistics
The data were expressed as means ± SEM. Differences in body weights and food intake between the two dietary groups were assessed using repeated-measures ANOVA (SigmaPlot 12.5; SigmaPlot Software, San Jose, CA). Curves depicting the loss of radioactivity over time post intracerebroventricular infusion were logarithmically transformed and fit with linear regression. The slopes of these linear regressions were tested using an ANOVA to determine whether they were significantly different from zero as well as if they differed between the adequate and deprived n-6 PUFA groups (GraphPad Prism 5; GraphPad Software, La Jolla, CA). Statistical significance was taken at P < 0.05. Loss half-lives of 3H-ARA and 3H-DHA were calculated from the slopes of the linear regressions using equation 1, and the rate of loss (Jout) in nmol/g brain/day was calculated using equation 2 (10, 11, 32):
| (Eq. 1) |
| (Eq. 2) |
where CFA is the baseline brain phospholipid concentration of the fatty acid of interest (ARA or DHA). For the purpose of calculating Jout, the half-life that was calculated from the slope of the linear regression was treated as a constant and applied to all measurements of baseline brain phospholipid concentrations (n = 8 baseline measures per dietary group). This produced a distribution of Jout values, for which we calculated the mean and SEM. Differences in Jout between the deprived and adequate n-6 PUFA rats were assessed using the Student’s t-test.
RESULTS
Body weights and food intake
Body weights increased over time as expected (supplementary Fig. 2). Although there were some significant differences between body weights of adequate and n-6 PUFA-deprived rats at certain points in time (P < 0.05), the magnitude of these differences were small as shown previously by others (15). After 15 weeks of feeding, in the 3H-ARA infusion group, the n-6 PUFA-adequate and n-6 PUFA-deprived rats had a mean weight of 363 ± 2.9 g and 356 ± 2.2 g, respectively. In the 3H-DHA infusion group, the n-6 PUFA-adequate rats had a mean weight of 347 ± 3.7 g and the n-6 PUFA-deprived rats had a mean weight of 344 ± 3.1 g.
Food intake, like body weight, was largely the same between both dietary groups (supplementary Fig. 3). The few significant differences at specific time points were small, and the pattern of food intake differences did not match the pattern of body weight differences. The mean weights of the adequate and deprived n-6 PUFA rat brains after 15 weeks of feeding were 1.7 ± 0.006 g and 1.7 ± 0.02 g, respectively (P > 0.05).
Radiotracer identification
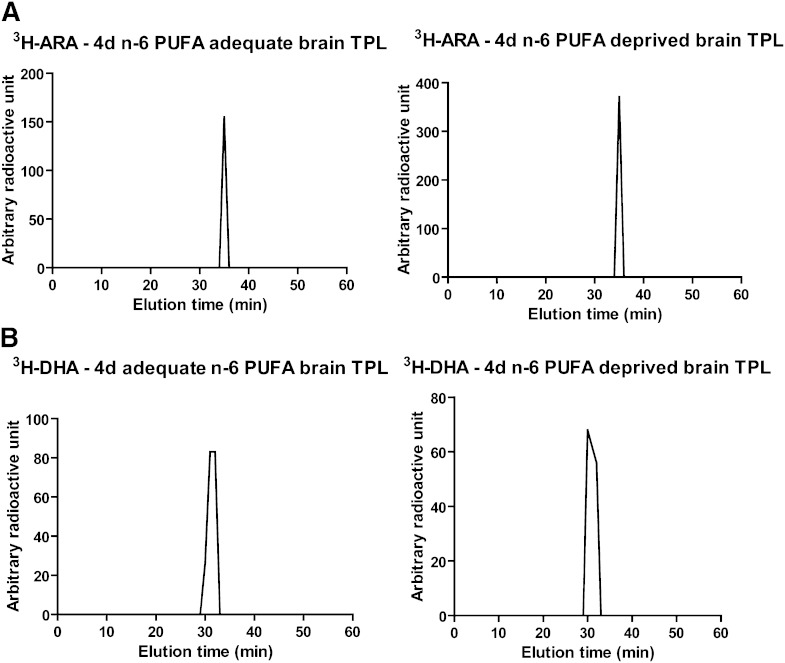
Brain samples (4 days postinfusion) were analyzed by HPLC and LSC to confirm radiotracer identity. ARA elutes at 35 min (11). All the radioactivity in the 3H-ARA-infused rat brain phospholipids eluted at 35 min and was identified as ARA in both the adequate and deprived n-6 PUFA rats (Fig. 1A). DHA elutes at 31 min. All of the radioactivity in the 3H-DHA-infused rat brain phospholipids eluted at 31 min and was identified as DHA in both dietary groups (33) (Fig. 1B).
Fig. 1.
HPLC separation of radioactivity in brain total phospholipids of 3H-ARA- or 3H-DHA-infused rats. HPLC separation of radioactivity in brain total phospholipids from 3H-ARA-infused (A) and 3H-DHA-infused (B) deprived and adequate n-6 PUFA rat brains, 4 days postinfusion (n = 4 in each dietary group). A: Radioactivity elutes at 35 min, the elution time of ARA. B: Radioactivity elutes at 31 min, the elution time of DHA.
Baseline brain eicosanoid and docosanoid concentrations
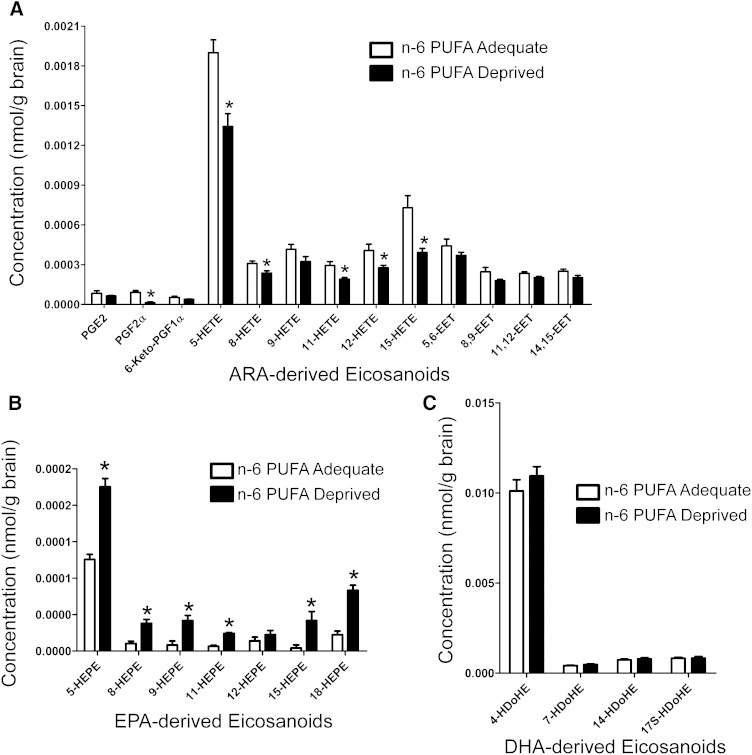
After 15 weeks of feeding, the n-6 PUFA-deprived rats had significantly lower levels (P < 0.05) of several ARA-derived eicosanoids (prostaglandin [PG]F2α, 5-hydroxyeicosatetraenoic acid [HETE], 8-HETE, 11-HETE, 12-HETE, and 15-HETE), but not PGE2, 6-keto-PGF1α, 9-HETE, 5,6-epoxyeicosatrienoic acid [EET], 8,9-EET, 11,12-EET, and 14,15-EET (Fig. 2A). The largest difference was seen in PGF2α, which was ∼86% lower in the n-6 PUFA-deprived rats than the n-6 PUFA-adequate rats. In contrast, nearly all detectable EPA-derived eicosanoids were found at higher concentrations in the deprived n-6 PUFA rats (5-hydroxyeicosapentaenoic acid [HEPE], 8-HEPE, 9-HEPE, 11-HEPE, 15-HEPE, and 18-HEPE, but not 12-HEPE) (Fig. 2B). The magnitude of these differences ranged from 1.8-fold (5-HEPE) to 9.9-fold (15-HEPE). Interestingly, there were no significant differences in docosanoid levels between the two dietary groups (Fig. 2C). The following eicosanoids and docosanoids were also assayed, but their levels were below the detection limit: Thromboxane (TX)B2, 8-isoprostane, PGD2, 11HDy-TXB2, LXA4, LTB4, PGJ2, TXA3, TXB3, D17-6keto-PGF1α, TXB3, 8-iso-PGF3α, PGF3α, PGE3, PGD3, lipoxin (LX)A-5, leukotriene (LT)B5, 8(9)-epoxy(Ep) eicosatetraenoic acid (ETE), 17(18)-EpETE, resolvin-D1, resolvin-D2, resolvin E1, 10(S),17(S)-DiHDoHE(Protectin DX), 7(R)Maresin1, and protectin (P)D1.
Fig. 2.
Eicosanoid and docosanoid concentrations of baseline uninfused rat brains after 15 weeks of the n-6 PUFA-adequate or n-6 PUFA-deprived diet. Data are means ± SEM (n = 8 n-6 PUFA-adequate rats, n = 7 n-6 PUFA-deprived rats). ARA-derived eicosanoids (A), DHA-derived eicosanoids (B), and DHA-derived docosanoids (C). Eicosanoids and docosanoids were measured by LC/MS/MS. Differences were assessed using the Student’s t-test. The asterisk indicates significant difference from n-6 PUFA-adequate group (P < 0.05).
Baseline brain phospholipid fatty acid concentrations
Table 3 depicts the concentrations of esterified fatty acids found in the total brain phospholipids of both adequate and n-6 PUFA-deprived rats after 15 weeks of feeding. There was a 15% lower concentration of ARA in the deprived versus n-6 PUFA adequate rats (5,191 ± 158 vs. 6,077 ± 103 nmol/g of brain, P < 0.05). This was accompanied by a 50% lower concentration of DPAn-6 (22:5n-6) (79 ± 2.7 vs. 158 ± 3.7 nmol/g of brain, P < 0.05). Conversely, there was an 18% higher concentration of DHA and a 164% higher concentration of DPAn-3 (22:5n-3) in the n-6 PUFA-deprived rats (7,323 ± 200 vs. 6,232 ± 97 and 267 ± 8.5 vs. 101 ± 2.4 nmol/g of brain, respectively, P < 0.05). Interestingly, the largest percentage difference between the dietary groups was seen in EPA. The n-6 PUFA-deprived rats had a >10-fold higher level of EPA (20:5n-3) than the adequate n-6 PUFA rats (9.2 ± 1.5 vs. 94 ± 3.4 nmol/g of brain).
TABLE 3.
Fatty acid concentrations in brain total phospholipids
| Fatty Acid | Total Phospholipid (nmol/g Brain) | |
| n-6 PUFA Adequate | n-6 PUFA Deprived | |
| 14:0 | 185.3 ± 2.6 | 226.5 ± 6.2a |
| 14:1n-7 | 5.8 ± 0.6 | 6.5 ± 0.7 |
| 16:0 | 18,361.7 ± 261.5 | 19,008.9 ± 513.5a |
| 16:1 n-7 | 347.3 ± 5.9 | 439.5 ± 10.4 |
| 18:0 | 15,290.7 ± 152.1 | 15,555.4 ± 407.8 |
| 18:1 n-9 | 14,442.9 ± 144.4 | 16,483.1 ± 375.6a |
| 18:1 n-7 | 2,995.2 ± 23.9 | 3,120.9 ± 66.4 |
| 18:2 n-6 | 414.1 ± 7.9 | 282.6 ± 8.3a |
| 18:3 n-6 | 36.2 ± 0.3 | 34.2 ± 1.4 |
| 18:3 n-3 | 5.6 ± 0.6 | 6.3 ± 0.3 |
| 20:0 | 414.4 ± 6.6 | 406.1 ± 9.6 |
| 20:1 n-9 | 1,446.7 ± 27.9 | 1,536.5 ± 35.9 |
| 20:3 n-3 | 247.9 ± 6.9 | 331.1 ± 11.5a |
| 20:4 n-6 (ARA) | 6,076.9 ± 103.2 | 5,190.9 ± 158.0a |
| 22:0 | 10.7 ± 0.9 | 14.7 ± 1.6 |
| 20:5 n-3 (EPA) | 9.2 ± 1.5 | 93.9 ± 3.4a |
| 22:1 n-9 | 176.8 ± 3.5 | 177.5 ± 2.1 |
| 22:4 n-6 | 1,949.3 ± 33.5 | 1,329.0 ± 36.4a |
| 22:5 n-6 | 158.2 ± 3.7 | 79.2 ± 2.7a |
| 22:5 n-3 | 101.4 ± 2.4 | 267.5 ± 8.5a |
| 22:6 n-3 (DHA) | 6,232.4 ± 96.7 | 7,324.9 ± 200.1a |
| 24:1 n-9 | 1,164.9 ± 32.7 | 1,252.8 ± 32.7 |
Brain fatty acid concentrations in phospholipid fractions of baseline uninfused rats after 15 weeks of feeding (n = 8 per dietary group). Data are means ± SEM.
Indicates significant difference from n-6 PUFA adequate group (P < 0.05).
The changes in total phospholipid concentrations were reflected by changes in the phospholipid fractions, although some fractions changed more than others for certain fatty acids. ARA was ∼20% lower (P < 0.05) in the ChoGpl, EtnGpl, and PtdSer fractions but not the PtdIns fraction of the n-6 PUFA-deprived rats (Table 4). DPAn-6 was 50–56% lower (P < 0.05) in all four fractions. A 9% and 11% higher concentration of DHA was found in the n-6 PUFA-deprived rat PtdSer and ChoGpl fractions, respectively (1,940 ± 38 vs. 1,776 ± 13 and 1,137 ± 2 vs. 1,017 ± 19 nmol/g of brain, P < 0.05). DPAn-3 was higher in the n-6 PUFA-deprived rats by 105, 159, 145, and 167% in the ChoGpl, EtnGpl, PtdSer, and PtdIns fractions, respectively (P < 0.05). EPA was >10-fold higher in the ChoGpl and EtnGpl fractions of the n-6 PUFA-deprived rats and was >1.5-fold higher in the PtdSer and PtdIns fractions compared with the n-6 PUFA-adequate rats.
TABLE 4.
Fatty acid concentrations in brain glycerophospholipid fractions
| Concentration (nmol/g Brain) | ||||||||
| ChoGpl | EtnGpl | PtdIns | PtdSer | |||||
| Fatty Acid | Adequate | Deprived | Adequate | Deprived | Adequate | Deprived | Adequate | Deprived |
| 14:0 | 103.9 ± 1.7 | 124.8 ± 2.5a | 9.4 ± 0.4 | 11.2 ± 0.6a | 2.3 ± 0.3 | 3.1 ± 0.4 | 4.5 ± 0.6 | 4.5 ± 0.2 |
| 14:1n-7 | ND ± ND | ND ± ND | ND ± ND | ND ± ND | ND ± ND | ND ± ND | ND ± ND | ND ± ND |
| 16:0 | 13,546.8 ± 113.2 | 13,764.7 ± 203.2 | 1,794.0 ± 21.2 | 1,806.2 ± 51.6 | 267.6 ± 19.8 | 275.8 ± 20.4 | 189.1 ± 5.4 | 177.6 ± 4.2 |
| 16:1 n-7 | 147.2 ± 1.9 | 182.1 ± 4.6a | 282.3 ± 118.8 | 218.7 ± 86.6 | 5.2 ± 0.3 | 6.7 ± 0.5a | 7.2 ± 0.1 | 8.8 ± 0.3a |
| 18:0 | 3,573.1 ± 22.4 | 3,639.3 ± 44.5 | 5,059.1 ± 148.8 | 4,869.2 ± 146.1 | 562.1 ± 62.2 | 559.9 ± 55.0 | 4,090.8 ± 85.9 | 4,138.7 ± 85.2 |
| 18:1 n-9 | 5,987.7 ± 71.3 | 6,617.8 ± 88.7a | 2,975.5 ± 144.9 | 3,307.7 ± 192.1 | 407.4 ± 13.9 | 455.9 ± 17.3 | 2,059.9 ± 43.0 | 2,260.8 ± 56.8a |
| 18:1 n-7 | 1,448.1 ± 8.2 | 1,394.1 ± 65.8 | 754.7 ± 9.8 | 838.3 ± 29.4a | 89.1 ± 4.3 | 90.9 ± 5.2 | 154.0 ± 3.1 | 156.4 ± 3.9 |
| 18:2 n-6 | 192.9 ± 2.3 | 126.8 ± 1.9a | 92.4 ± 3.6 | 66.0 ± 2.4a | 18.6 ± 0.8 | 14.0 ± 0.8a | 18.0 ± 0.7 | 14.6 ± 1.2a |
| 18:3 n-6 | 9.1 ± 0.3 | 9.2 ± 0.2 | 10.4 ± 0.6 | 9.3 ± 0.6 | 1.6 ± 0.1 | 1.5 ± 0.1 | 8.4 ± 0.2 | 8.2 ± 0.2 |
| 18:3 n-3 | 8.5 ± 0.4 | 9.4 ± 1.0 | 17.4 ± 1.7 | 17.2 ± 1.9 | 1.1 ± 0.1 | 1.1 ± 0.1 | 3.3 ± 0.1 | 2.9 ± 0.1a |
| 20:0 | 55.6 ± 1.0 | 54.4 ± 1.1 | 78.9 ± 1.1 | 61.6 ± 2.5a | 11.4 ± 0.3 | 10.9 ± 0.3 | 48.3 ± 1.2 | 50.1 ± 1.5 |
| 20:1 n-9 | 286.6 ± 5.8 | 289.6 ± 5.5 | 755.6 ± 104.8 | 902.5 ± 35.1 | 54.1 ± 1.7 | 48.1 ± 4.6 | 233.8 ± 7.4 | 225.1 ± 8.7 |
| 20:3 n-3 | 44.3 ± 0.7 | 72.0 ± 1.1a | 117.2 ± 4.6 | 123.4 ± 3.2 | 9.2 ± 0.4 | 11.2 ± 0.7a | 42.3 ± 0.7 | 50.4 ± 1.1a |
| 20:4 n-6 | 1,441.0 ± 20.7 | 1,147.5 ± 16.7a | 3,419.4 ± 21.6 | 2,693.5 ± 64.9a | 368.9 ± 57.8 | 337.6 ± 53.9 | 321.3 ± 3.0 | 248.5 ± 6.0a |
| 22:0 | 9.2 ± 1.0 | 8.5 ± 0.7 | 5.3 ± 0.3 | 5.5 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 20:5 n-3 | 2.1 ± 0.4 | 22.6 ± 0.6a | 3.3 ± 0.2 | 43.3 ± 1.3a | 1.6 ± 0.6 | 4.8 ± 0.7a | 1.5 ± 0.4 | 3.9 ± 0.3a |
| 22:1 n-9 | 36.7 ± 4.7 | 40.8 ± 1.4 | 53.4 ± 1.2 | 43.1 ± 2.0a | 10.8 ± 0.3 | 10.6 ± 0.3 | 48.2 ± 1.2 | 52.8 ± 1.8 |
| 22:4 n-6 | 171.7 ± 2.6 | 114.6 ± 1.6a | 1,472.7 ± 30.2 | 917.8 ± 23.0a | 35.0 ± 1.6 | 23.0 ± 1.3a | 296.8 ± 3.2 | 194.3 ± 5.2a |
| 22:5 n-6 | 19.7 ± 0.6 | 8.7 ± 0.2a | 94.0 ± 2.5 | 44.8 ± 0.7a | 2.1 ± 0.2 | 0.9 ± 0.0a | 48.1 ± 0.6 | 23.9 ± 0.5a |
| 22:5 n-3 | 18.5 ± 0.3 | 38.1 ± 1.0a | 68.4 ± 1.5 | 177.0 ± 5.5a | 1.5 ± 0.1 | 4.1 ± 0.3a | 16.6 ± 0.1 | 40.6 ± 1.3a |
| 22:6 n-3 | 1,017.0 ± 18.9 | 1,137.0 ± 22.6a | 4,575.3 ± 106.9 | 4,865.7 ± 92.1 | 92.4 ± 6.9 | 102.6 ± 7.6a | 1,776.1 ± 12.6 | 1,940.2 ± 37.6a |
| 24:1 n-9 | 41.6 ± 4.2 | 46.1 ± 2.1 | 38.7 ± 1.8 | 31.2 ± 2.4a | 11.9 ± 0.3 | 12.4 ± 0.4 | 34.2 ± 0.6 | 39.1 ± 1.4 |
Brain fatty acid concentrations in phospholipid fractions of baseline uninfused rats after 15 weeks of feeding (n = 8 per dietary group). Data are means ± SEM.
Indicates significant difference from the adequate n-6 PUFA group (P < 0.05).
Brain phospholipid ARA and DHA rate of loss
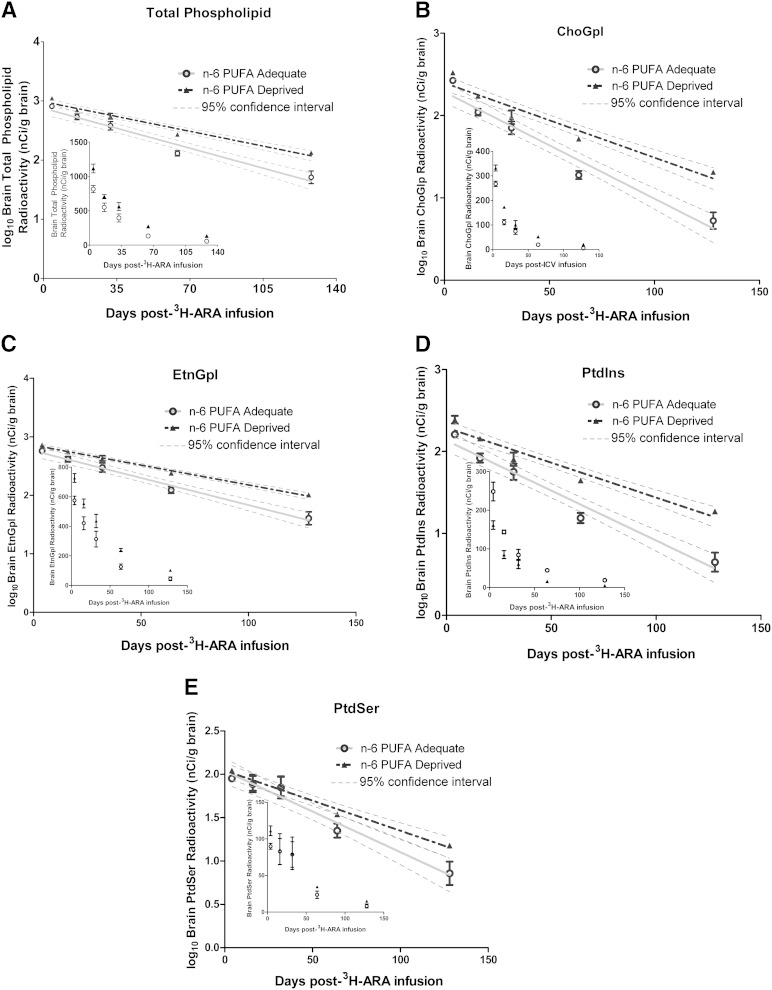
The loss of 3H-ARA and 3H-DHA from brain phospholipids was plotted from 4 to 128 days postinfusion and then logarithmically transformed for linear regression analysis (Figs. 3, 4). All slopes were negative and significantly different from zero (P < 0.0001) (Table 5). Linear regression analysis of the slopes for 3H-ARA showed significant differences between n-6 PUFA-adequate and n-6 PUFA-deprived rats in the total phospholipid pool as well as all phospholipid fractions, except for PtdSer (P < 0.05). The slopes were more negative in the adequate n-6 PUFA group than the n-6 PUFA-deprived group, reflective of a shorter loss half-life and a more rapid loss of 3H-ARA over time (P < 0.05). To take into account the differences in baseline concentrations of ARA between the two dietary groups, the number of ARA molecules lost per gram of brain each day (Jout) was calculated. The difference in Jout between dietary groups was generally even larger than the difference in the t1/2, except in the PtdIns fraction; the n-6 PUFA-deprived group had a significantly lower Jout than the n-6 PUFA-adequate group (P < 0.001) in the total phospholipid, ChoGpl, EtnGpl, and PtdSer fractions. Jout in the total phospholipid pool was 134.7 ± 2.3 nmol/g of brain/day in the n-6 PUFA-adequate group versus 85.4 ± 2.6 nmol/g of brain/day in the n-6 PUFA-deprived group (P < 0.05). This corresponds to a daily fractional loss of 2.2% versus 1.6% in the adequate versus deprived groups. The Jout (nmol/g of brain/day) in the phospholipid fractions ranged from 6.9 ± 0.06 (PtdSer) to 43 ± 0.6 (ChoGpl) in the n-6 PUFA-adequate group, and from 4.0 ± 0.1 (PtdSer) to 24 ± 0.3 (ChoGpl) in the n-6 PUFA-deprived group. Overall, ARA was lost from brain phospholipids at a slower rate in the n-6 PUFA-deprived group.
Fig. 3.
Loss of 3H-ARA from brain total phospholipids and fractions over time. Logarithmically transformed curves of the loss of 3H-ARA from brain total phospholipids and fractions over time with linear regression analysis (untransformed curves are inset). A: Total phospholipid. B: ChoGpl. C: EtnGpl. D: PtdIns. E: PtdSer. Data are mean ± SEM (n = 4 independent samples per dietary group per time point, except for the 4 day time point of the n-6 PUFA-adequate rats where n = 3). All slopes are significantly different from zero (P < 0.0001).
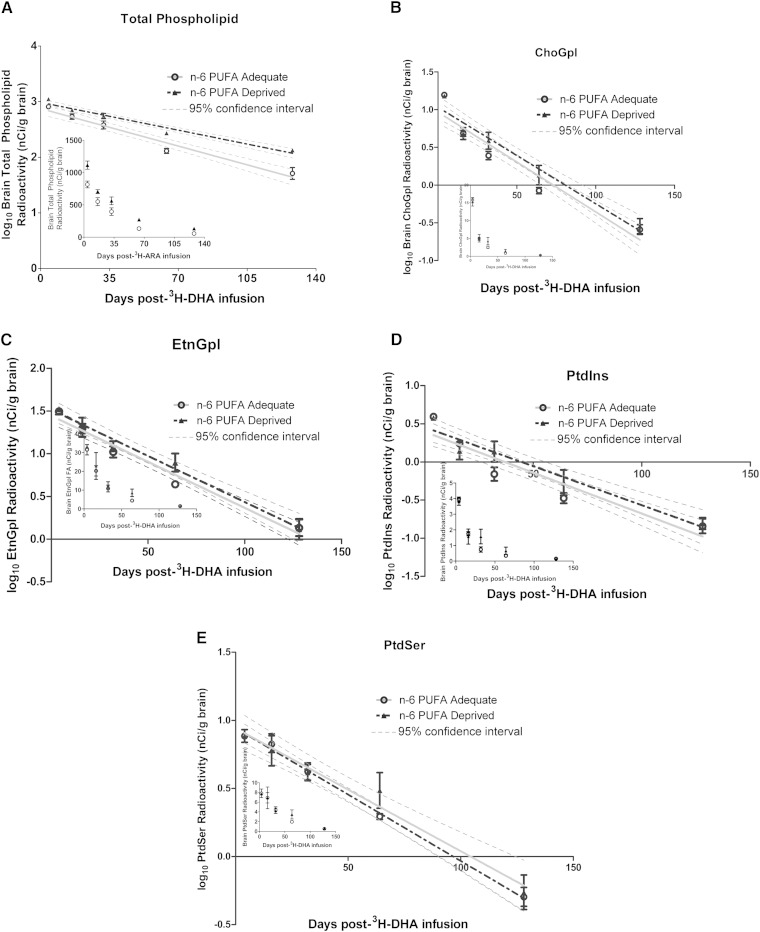
Fig. 4.
Loss of 3H-DHA from brain total phospholipids and fractions over time. Logarithmically transformed curves of the loss of 3H-DHA from brain total phospholipids and fractions over time with linear regression analysis (untransformed curves are inset). A: Total phospholipids. B: ChoGpl. C: EtnGpl. D: PtdIns. E: PtdSer. Data are mean ± SEM (n = 4 independent samples per dietary group per time point). All slopes are significantly different from zero (P < 0.0001).
TABLE 5.
Kinetic parameters for ARA and DHA in total phospholipids and phospholipid fractions of n-6 PUFA-adequate and n-6 PUFA-deprived rats
| Infusate | Lipid Class | Diet | Slope (per Day) | Slope SEM | Loss t1/2 (Day) | Jout(nmol/g/Day) | Fractional Loss (% per Day) |
| ARA | Total | Adequate | −0.0096 | 0.00076 | 31 ± 2.5 | 134.7 ± 2.3 | 2.2 |
| Deprived | −0.0071a | 0.00044 | 42 ± 2.6a | 85.4 ± 2.6a | 1.6 | ||
| ChoGpl | Adequate | −0.0130 | 0.00092 | 23 ± 1.6 | 43.0 ± 0.6 | 3.0 | |
| Deprived | −0.0091a | 0.00069 | 33 ± 2.5a | 24.0 ± 0.3a | 2.1 | ||
| EtnGpl | Adequate | −0.0093 | 0.00071 | 32 ± 2.5 | 73.0 ± 0.5 | 2.1 | |
| Deprived | −0.0068a | 0.00029 | 44 ± 1.9a | 42.2 ± 1.0a | 1.6 | ||
| PtdIns | Adequate | −0.0122 | 0.00093 | 25 ± 1.9 | 10.4 ± 1.6 | 2.8 | |
| Deprived | −0.0085a | 0.00065 | 35 ± 2.7a | 6.6 ± 1.1 | 2.0 | ||
| PtdSer | Adequate | −0.0094 | 0.00104 | 32 ± 3.6 | 6.9 ± 0.06 | 2.2 | |
| Deprived | −0.0069 | 0.00065 | 43 ± 4.0 | 4.0 ± 0.1a | 1.6 | ||
| DHA | Total | Adequate | −0.0090 | 0.00079 | 33 ± 2.9 | 129.1 ± 11.4 | 2.1 |
| Deprived | −0.0086 | 0.00089 | 35 ± 3.6 | 145.7 ± 15.5a | 2.0 | ||
| ChoGpl | Adequate | −0.0132 | 0.00105 | 23 ± 1.8 | 31.0 ± 2.5 | 3.0 | |
| Deprived | −0.0128 | 0.00109 | 24 ± 2.0 | 33.5 ± 2.9a | 2.9 | ||
| EtnGpl | Adequate | −0.0108 | 0.00070 | 28 ± 1.8 | 113.5 ± 7.8 | 2.5 | |
| Deprived | −0.0108 | 0.00089 | 28 ± 2.3 | 121.0 ± 10.2 | 2.5 | ||
| PtdIns | Adequate | −0.0107 | 0.00112 | 28 ± 2.9 | 2.3 ± 0.3 | 2.5 | |
| Deprived | −0.0102 | 0.00119 | 29 ± 3.4 | 2.4 ± 0.3 | 2.4 | ||
| PtdSer | Adequate | −0.0098 | 0.00052 | 31 ± 1.6 | 39.9 ± 2.1 | 2.2 | |
| Deprived | −0.0090 | 0.00098 | 33 ± 3.6 | 40.3 ± 4.4 | 2.1 |
Loss half-lives were calculated from the slopes of the linear regressions using the equation t1/2 = log102/(slope of the regression line). Baseline fatty acid concentrations were used to calculate the rates of loss (Jout) from the different fatty acid pools using the equation Jout = 0.693CFA/t1/2, where CFA is the baseline brain total phospholipids and fractions fatty acid concentration after 15 weeks of feeding. Data are mean ± SEM. Differences in slope and Joutwere assessed with an ANOVA and Student’s t-test, respectively.
P < 0.05 versus adequate n-6 PUFA.
There were no differences in the slopes and thus, no difference in the loss half-lives for 3H-DHA between the adequate and deprived n-6 PUFA rats. However, due to the higher baseline DHA concentrations in the deprived rats, the n-6 PUFA-deprived group appeared to have a higher Jout for DHA than the adequate n-6 PUFA group. This difference was only significant in the total phospholipid pool and the ChoGpl fraction. The Jout for total phospholipids in the adequate and n-6 PUFA-deprived groups was 129.1 ± 11.4 nmol/g of brain/day and 145.7 ± 15.5 nmol/g of brain/day, respectively (P < 0.05). The Jout (nmol/g of brain/day) for the phospholipid fractions ranged from 2.3 ± 0.3 (PtdIns) to 113.5 ± 7.8 (EtnGpl) in the n-6 PUFA-adequate group, and from 2.4 ± 0.3 (PtdIns) to 121 ± 10.2 (EtnGpl) in the n-6 PUFA-deprived group. Overall, DHA seemed to be lost, when measured in terms of Jout, from brain phospholipids at a more rapid rate in the n-6 PUFA-deprived rats.
DISCUSSION
This study investigated the effect of an adequate (24% LA) versus deprived (2% LA) n-6 PUFA diet on the rate of loss of ARA and DHA from rat brain phospholipids. The diets used in this study were similar to those used in previous studies examining body and organ weights, enzyme expression, and brain DHA uptake and turnover (15–17). In brain total phospholipids, there was a 15% reduction in ARA concentration and an 18% increase in DHA concentration with the n-6 PUFA-deprived diet. These changes are comparable to the 28% reduction in ARA and the 11% increase in DHA found previously in brain total lipids (15). For the first time, we show that lowering the amount of n-6 PUFA in the diet leads to longer ARA loss half-lives in brain total phospholipids, ChoGpl, EtnGpl, and PtdIns pools. After factoring in the concentration of ARA in the baseline, uninfused rats, the n-6 PUFA-deprived rats had a slower net rate of loss (a lower Jout) of ARA in the total phospholipids, ChoGpl, EtnGpl, and PtdSer pools. This may have been caused by a decrease in cPLA2 and COX-2 activity, which has been reported in rats upon 15 weeks consumption of a low n-6 PUFA diet (16). It would have also been interesting to calculate the half-lives of ARA and DHA within brain neutral lipids as fatty acid turnover within these lipids is PLA2 independent, and future studies should consider this. Decreased cPLA2 activity likely reflects a decreased ARA turnover and less opportunity for ARA to be lost through eicosanoid production or β-oxidation. The decrease in eicosanoid production shown here may not be enough to account for the decreased loss of ARA, unless the eicosanoids have very short half-lives. Thus, there is also likely a decreased amount of β-oxidation or other catabolic processes, which is an area requiring further research.
In contrast to our observations with ARA, there was a more rapid net loss (a higher Jout) of DHA from the brain total phospholipids and the ChoGpl pool in the n-6 PUFA-deprived rats, but not from the other phospholipid fractions. This difference in the rate of loss of DHA was a function of the higher brain DHA concentrations in the deprived versus adequate n-6 PUFA rats because the fractional losses and the loss half-lives for DHA were not significantly different between the two dietary groups in any of the phospholipid pools. In the n-6 PUFA-deprived rats, the Joutof DHA for total phospholipids was smaller than the Jin (net rate of incorporation) of DHA found previously, whereas the Jin approximately matched the Jout in the n-6 PUFA-adequate rats (17). A more rapid daily uptake rate compared with loss rate of DHA may account for the higher concentration of DHA in the n-6 PUFA-deprived rats than in the n-6 PUFA-adequate rats upon 15 weeks of feeding. However, caution should be taken when comparing and combining results for kinetic analyses from two different studies, and future experiments should be completed under similar conditions. One important distinction between our intracerebroventricular method to calculate phospholipid half-lives and the use of a pulse intravenous infusion can be seen in glycerophospholipid species. While the half-life or the net rate of entry (Jin), as calculated upon an intravenous pulse infusion, approximates the half-life or Jout for brain total phospholipids, this does not hold true for measured glycerophospholipid species. One reason for the discrepancy in glycerophospholipid species half-lives is likely due to remodeling/exchange of radiolabeled fatty acids between phospholipid species that occurs over time, which does not occur upon acute pulse labeling (34). However, it is also possible that fatty acids other than the plasma unesterified pool enter the brain, which would be captured in our study.
Similar to previous studies, one limitation of this study was that measuring the radioactivity of the rat brains required euthanization of the rats and removal of their brains. Thus, different rats had to be used at each time point postinfusion, and the actual loss of radioactivity in the each individual rat brain could not be tracked over 128 days. This posed a problem when trying to calculate the SEM of Jout because the Jout calculation was the quotient of two measurements: the baseline ARA or DHA concentration and the loss half-life of ARA or DHA. Only the baseline ARA or DHA concentration measurements had discernable sample sizes. Thus, consistent with past literature, the calculated loss half-lives were treated as constants and were applied to all baseline concentration values in order to create a distribution of Jout values from which the Jout SEM could be calculated (10, 11). It is noteworthy, however, that incorporation of the slope SEM would result in more conservative analyses of the difference between Jout values of the adequate and deprived n-6 PUFA rats. For ARA, this assumption does not change the overall conclusion, as the differences in ARA Jout between the dietary groups are significant up to a very high SEM. For DHA, however, an incorporation of larger slope error would likely result in no significant differences between the DHA Jout values of the n-6 PUFA-adequate and n-6 PUFA-deprived rats in any of the phospholipids. Thus, the conclusions made about DHA metabolism must be taken with this in mind. Nonetheless, the finding is consistent with the significantly higher DHA levels in the brain phospholipids of the n-6 PUFA-deprived rats. It should also be noted that intracerebroventricular administration of fatty acids can lead to neuroinflammation and damage to the blood-brain barrier, which could alter our results. To minimize the effect of intracerebroventricular administration, similar to our previous experiments (11, 26), we used a 33-gauge needle and allowed the rats to recover for 4 days before beginning our analyses.
One of the fates of unesterified ARA and DHA is metabolism into eicosanoids and docosanoids. We reported decreases in the levels of certain ARA-derived eicosanoids in rats fed the deprived versus adequate n-6 PUFA diet. The largest reduction was seen in the enzymatically derived PGF2α. As seen in previous studies, an n-6 PUFA-deprived diet causes a reduction in the activity of cPLA2 and COX-2, which are both enzymes required for the synthesis of PGF2α (16). However, this effect was selective for PGF2α because there were no significant reductions in the levels of the other nonautooxidative eicosanoids that were detected (PGE2 and PGI2). This suggests that the reductions in enzyme activity can selectively reduce certain eicosanoids but not others. The mechanism behind this is unknown. Furthermore, it could be that the differences in the concentration of other eicosanoids may only appear when the brain is responding to stress. For example, in a neuroinflammatory state, there is an increased production of PGE2 (5). Perhaps, in such a state, the n-6 PUFA-adequate rats would have a larger increase in eicosanoid production than the n-6 PUFA-deprived rats. The remaining eicosanoids that changed are thought to be autooxidative, meaning that they are capable of being nonenzymatically derived (29). This suggests that these eicosanoid reductions could be due to the lower ARA concentration in the brain phospholipids of n-6 PUFA-deprived rats.
One thing to note is that in the brain, eicosanoids are present in the fentomole to picomole per gram range, whereas concentrations of ARA in brain phospholipids are just over a micromole per gram. The most concentrated eicosanoid detected was 5-HETE, which had a concentration of 0.0019 ± 0.00001 nmol/g of brain in the n-6 PUFA-adequate rats and 0.0013 ± 0.000007 nmol/g of brain in the n-6 PUFA-deprived rats. Brain phospholipid ARA concentrations were 6,077 ± 103 nmol/g of brain and 5,191 ± 158 nmol/g of brain, respectively. This raises an interesting question: why does a reduction to 5,191 nmol/g of brain lead to an ∼0.0006 nmol/g of brain reduction in 5-HETE when the amount of ARA available is still >2 million times the amount needed to supply the 0.0019 nmol of 5-HETE in the n-6 PUFA-adequate rats? Perhaps this highlights how important phospholipid ARA is in its other roles aside from eicosanoid production or that ARA is regulating the enzymatic synthesis of the eicosanoids. It may also be that there is a set rate at which ARA autooxidation occurs, which cannot be altered to compensate for alterations in brain phospholipid ARA concentration.
There was a significantly higher level of all detectable EPA-derived eicosanoids in the n-6 PUFA-deprived rats, except for 12-HEPE. These EPA-derived eicosanoids were all autooxidation products, and the increase we observed may be due to the 10-fold increase in brain total phospholipid EPA concentration in the n-6 PUFA-deprived rats. In our study, and as reported by others, the brain maintains very low levels of EPA (26, 36–38). Even supplementation of EPA in the diet does not create such large increases in EPA. For example, in fish a 4-fold increase in the EPA content of the diet only results in a 70% increase in brain EPA concentration (39). Recent meta-analyses suggest that EPA may be protective in major depression (40, 41). Thus, being able to raise EPA levels 10-fold by lowering n-6 PUFA may be therapeutic in certain mood disorders, including major depression, and should be further investigated.
There were no changes in the levels of docosanoids, though the only four docosanoids detected were also considered autooxidative (29). It is interesting here how even though the concentration of brain phospholipid DHA increased by ∼1,000 nmol/g of brain in the n-6 PUFA-deprived rats, there was no change in the level of these autooxidative docosanoids, unlike what was seen with the eicosanoids. Perhaps the extra DHA is largely being shunted toward increasing the levels of enzymatically derived docosanoids, which, similar to others, we were unable to detect in microwave-fixed rat brains (29). Previous studies using similar diets have reported that an n-6 PUFA-deprived diet increases the activity of iPLA2 and expression of 15-LOX, which are enzymes that metabolize DHA into resolvins, protectins, and maresins (16).
In rats, the level of LA at which tissue ARA concentrations plateau is 1,200 mg of LA per 100 g of diet, and this intake is considered the recommended minimum LA intake (25). This level is comparable to the recommended level of LA for humans: 1,000–1,500 mg of LA per 100 g of food, which equates to ∼2–3% of energy (42–44). However, more recent studies suggest that these are overestimations of the actual LA requirement, and there is a concern that the amount of LA in the average human diet is too high (45). For rats, the level at which tissue ARA levels plateau cannot necessarily be substituted for the LA requirement level, as there is no evidence that ARA at this peak level is critical for health. For instance, the n-6 PUFA-deprived diet used in our study and in previous studies had a LA level that was 10% of the suggested requirement, and rats did not show significant signs of LA deficiency (15). One of the problems with earlier experiments that formed the basis of LA requirements is that many of them did not ensure an adequate level of ALA (45–49). The effects of an inadequate level of ALA were attributed to having an inadequate level of LA, and thus may have led to an overestimation of LA requirement. The n-6 PUFA-deprived diet in our study contained an adequate amount of ALA, allowing for the effects here to be attributed to changes in dietary n-6 PUFAs, without the influence of dietary n-3 PUFAs.
CONCLUSION
In summary, rats fed an n-6 PUFA-deprived diet (2% LA) or an n-6 PUFA-adequate diet (24% LA) for 15 weeks were infused with 3H-ARA or 3H-DHA, which allowed for the determination of the rate of loss of ARA and DHA from their brain phospholipids. ARA had a longer half-life and was lost at a slower rate in the total phospholipids, ChoGpl, EtnGpl, and PtdSer pools of the n-6 PUFA-deprived rats, illustrating the brain’s ability to conserve ARA in response to lower dietary LA. In contrast, DHA was lost more rapidly in the total phospholipids and ChoGpl pools of the n-6 PUFA-deprived rats, but not the other phospholipids fractions. These effects are approximately opposite of what was observed with the low versus high n-3 PUFA diets in previous studies where low n-3 PUFA leads to conservation of DHA but not ARA (10, 11). Recently, the Nurses’ Health Study reported that an elevated intake of LA was associated with an increased risk of developing major depression (50), and preclinical studies suggest that drugs used to manage bipolar disorder decrease brain arachidonic acid metabolism (51, 52). While much interest in dietary LA levels have focused around coronary heart disease risk, understanding how dietary n-6 PUFAs regulate the metabolism of ARA and DHA in the brain may also be important for determining dietary requirements.
Supplementary Material
Acknowledgments
The authors thank Michael Leadley and Dr. Denis Reynaud of the Analytical Facility for Bioactive Molecules at The Centre for the Study of Complex Childhood Diseases, The Hospital for Sick Children, Toronto, Canada, for assistance with the LC/MS/MS.
Footnotes
Abbreviations:
- ALA
- α-linolenic acid
- ARA
- arachidonic acid
- ChoGpl
- choline glycerophospholipid
- COX
- cyclooxygenase
- cPLA2
- cytosolic phospholipase A2
- DPA
- docosapentaenoic acid
- EtnGpl
- ethanolamine glycerophospholipid
- FAME
- fatty acid methyl esters
- GC-FID
- gas chromatography with flame-ionization detection
- 3H-ARA
- 5,6,8,9,11,12,14,15-3H arachidonic acid
- 3H-DHA
- 4,7,10,13,16,19-3H docosahexaenoic acid
- iPLA2
- calcium-independent phospholipase A2
- LA
- linoleic acid
- LSC
- liquid scintillation counting
- PtdIns
- phosphatidylinositol
- PtdSer
- phosphatidylserine
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (R.P.B.) and studentship (L.E.L.). R.P.B. also holds a Canada Research Chair in Brain Lipid Metabolism.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Ramsden C. E., Faurot K. R., Zamora D., Suchindran C. M., Macintosh B. A., Gaylord S., Ringel A., Hibbeln J. R., Feldstein A. E., Mori T. A., et al. 2013. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 154: 2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attwell D., Buchan A. M., Charpak S., Lauritzen M., Macvicar B. A., Newman E. A. 2010. Glial and neuronal control of brain blood flow. Nature. 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazan N. G., Molina M. F., Gordon W. C. 2011. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 31: 321–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazan N. G. 2003. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res. 44: 2221–2233. [DOI] [PubMed] [Google Scholar]
- 5.Serhan C. N., Petasis N. A. 2011. Resolvins and protectins in inflammation resolution. Chem. Rev. 111: 5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr S. K., Bazinet R. P. 2008. The emerging role of docosahexaenoic acid in neuroinflammation. Curr. Opin. Investig. Drugs. 9: 735–743. [PubMed] [Google Scholar]
- 7.Rapoport S. I. 2008. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot. Essent. Fatty Acids. 79: 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakunin E., Loeb V., Kisos H., Biala Y., Yehuda S., Yaari Y., Selkoe D. J., Sharon R. 2012. Alpha-synuclein neuropathology is controlled by nuclear hormone receptors and enhanced by docosahexaenoic acid in a mouse model for Parkinson’s disease. Brain Pathol. 22: 280–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazinet R. P., Laye S. 2014. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 15: 771–785. [DOI] [PubMed] [Google Scholar]
- 10.DeMar J. C., Jr, Ma K., Bell J. M., Rapoport S. I. 2004. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J. Neurochem. 91: 1125–1137. [DOI] [PubMed] [Google Scholar]
- 11.Green J. T., Liu Z., Bazinet R. P. 2010. Brain phospholipid arachidonic acid half-lives are not altered following 15 weeks of N-3 polyunsaturated fatty acid adequate or deprived diet. J. Lipid Res. 51: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H. W., Rao J. S., Rapoport S. I., Igarashi M. 2011. Regulation of rat brain polyunsaturated fatty acid (PUFA) metabolism during graded dietary n-3 PUFA deprivation. Prostaglandins Leukot. Essent. Fatty Acids. 85: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao J. S., Ertley R. N., DeMar J. C., Jr, Rapoport S. I., Bazinet R. P., Lee H. J. 2007. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry. 12: 151–157. [DOI] [PubMed] [Google Scholar]
- 14.Contreras M. A., Greiner R. S., Chang M. C., Myers C. S., Salem N., Jr, Rapoport S. I. 2000. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J. Neurochem. 75: 2392–2400. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi M., Gao F., Kim H. W., Ma K., Bell J. M., Rapoport S. I. 2009. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim. Biophys. Acta. 1791: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H. W., Rao J. S., Rapoport S. I., Igarashi M. 2011. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim. Biophys. Acta. 1811: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igarashi M., Kim H. W., Chang L., Ma K., Rapoport S. I. 2012. Dietary n-6 polyunsaturated fatty acid deprivation increases docosahexaenoic acid metabolism in rat brain. J. Neurochem. 120: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C. T., Trepanier M. O., Hopperton K. E., Domenichiello A. F., Masoodi M., Bazinet R. P. 2014. Inhibiting mitochondrial beta-oxidation selectively reduces levels of nonenzymatic oxidative polyunsaturated fatty acid metabolites in the brain. J. Cereb. Blood Flow Metab. 34: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domenichiello A. F., Chen C. T., Trepanier M. O., Stavro P. M., Bazinet R. P. 2014. Whole body synthesis rates of DHA from alpha-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J. Lipid Res. 55: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trépanier M. O., Lim J., Lai T. K., Cho H. J., Domenichiello A. F., Chen C. T., Taha A. Y., Bazinet R. P., Burnham W. M. 2014. Intraperitoneal administration of docosahexaenoic acid for 14days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model. Epilepsy Behav. 33: 138–143. [DOI] [PubMed] [Google Scholar]
- 21.Trépanier M. O., Taha A. Y., Mantha R. L., Ciobanu F. A., Zeng Q. H., Tchkhartichvili G. M., Domenichiello A. F., Bazinet R. P., Burnham W. M. 2012. Increases in seizure latencies induced by subcutaneous docosahexaenoic acid are lost at higher doses. Epilepsy Res. 99: 225–232. [DOI] [PubMed] [Google Scholar]
- 22.Reeves P. G., Rossow K. L., Lindlauf J. 1993. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J. Nutr. 123: 1923–1931. [DOI] [PubMed] [Google Scholar]
- 23.Reeves P. G., Nielsen F. H., Fahey G. C., Jr 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123: 1939–1951. [DOI] [PubMed] [Google Scholar]
- 24.Cunnane S. C., Guesnet P. 2011. Linoleic acid recommendations—a house of cards. Prostaglandins Leukot. Essent. Fatty Acids. 85: 399–402. [DOI] [PubMed] [Google Scholar]
- 25.Bourre J. M., Piciotti M., Dumont O., Pascal G., Durand G. 1990. Dietary linoleic acid and polyunsaturated fatty acids in rat brain and other organs. Minimal requirements of linoleic acid. Lipids. 25: 465–472. [DOI] [PubMed] [Google Scholar]
- 26.Chen C. T., Liu Z., Bazinet R. P. 2011. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. J. Neurochem. 116: 363–373. [DOI] [PubMed] [Google Scholar]
- 27.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 28.Chen C. T., Liu Z., Ouellet M., Calon F., Bazinet R. P. 2009. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot. Essent. Fatty Acids. 80: 157–163. [DOI] [PubMed] [Google Scholar]
- 29.Chen C. T., Trepanier M. O., Hopperton K. E., Domenichiello A. F., Masoodi M., Bazinet R. P. 2014. Inhibiting mitochondrial beta-oxidation selectively reduces levels of nonenzymatic oxidative polyunsaturated fatty acid metabolites in the brain. J. Cereb. Blood Flow Metab. 34: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aveldano M. I., VanRollins M., Horrocks L. A. 1983. Separation and quantitation of free fatty acids and fatty acid methyl esters by reverse phase high pressure liquid chromatography. J. Lipid Res. 24: 83–93. [PubMed] [Google Scholar]
- 31.Igarashi M., Ma K., Chang L., Bell J. M., Rapoport S. I., DeMar J. C., Jr 2006. Low liver conversion rate of alpha-linolenic to docosahexaenoic acid in awake rats on a high-docosahexaenoate-containing diet. J. Lipid Res. 47: 1812–1822. [DOI] [PubMed] [Google Scholar]
- 32.Stinson A. M., Wiegand R. D., Anderson R. E. 1991. Recycling of docosahexaenoic acid in rat retinas during n-3 fatty acid deficiency. J. Lipid Res. 32: 2009–2017. [PubMed] [Google Scholar]
- 33.Chen C. T., Domenichiello A. F., Trepanier M. O., Liu Z., Masoodi M., Bazinet R. P. 2013. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J. Lipid Res. 54: 2410–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson P. J., Noronha J., DeGeorge J. J., Freed L. M., Nariai T., Rapoport S. I. 1992. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res. Brain Res. Rev. 17: 187–214. [DOI] [PubMed] [Google Scholar]
- 35.Chen C. T., Bazinet R. P. 2014. Beta-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot. Essent. Fatty Acids. Epub ahead of print June 5, 2014. pii S0952-3278(14)00083-0. 10.1016/j.plefa.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Svennerholm L. 1968. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 9: 570–579. [PubMed] [Google Scholar]
- 37.Igarashi M., Ma K., Gao F., Kim H. W., Greenstein D., Rapoport S. I., Rao J. S. 2010. Brain lipid concentrations in bipolar disorder. J. Psychiatr. Res. 44: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C. T., Ma D. W., Kim J. H., Mount H. T., Bazinet R. P. 2008. The low density lipoprotein receptor is not necessary for maintaining mouse brain polyunsaturated fatty acid concentrations. J. Lipid Res. 49: 147–152. [DOI] [PubMed] [Google Scholar]
- 39.Trushenski J., Schwarz M., Bergman A., Rombenso A., Delbos B. 2012. DHA is essential, EPA appears largely expendable, in meeting the n−3 long-chain polyunsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture. 326–329: 81–89. [Google Scholar]
- 40.Martins J. G., Bentsen H., Puri B. K. 2012. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry 17: 1144–1149; discussion 1163–1167. [DOI] [PubMed] [Google Scholar]
- 41.Martins J. G. 2009. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 28: 525–542. [DOI] [PubMed] [Google Scholar]
- 42.Goodgame J. T., Lowry S. F., Brennan M. F. 1978. Essential fatty acid deficiency in total parenteral nutrition: time course of development and suggestions for therapy. Surgery. 84: 271–277. [PubMed] [Google Scholar]
- 43.Wene J. D., Connor W. E., DenBesten L. 1975. The development of essential fatty acid deficiency in healthy men fed fat-free diets intravenously and orally. J. Clin. Invest. 56: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins F. D., Sinclair A. J., Royle J. P., Coats D. A., Maynard A. T., Leonard R. F. 1971. Plasma lipids in human linoleic acid deficiency. Nutr. Metab. 13: 150–167. [DOI] [PubMed] [Google Scholar]
- 45.Cunnane S. C. 2003. Problems with essential fatty acids: time for a new paradigm? Prog. Lipid Res. 42: 544–568. [DOI] [PubMed] [Google Scholar]
- 46.Burr G. O., Burr M. M. 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 82: 345–367. [DOI] [PubMed] [Google Scholar]
- 47.Burr G. O., Burr M. M. 1930. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 86: 587–621. [Google Scholar]
- 48.Holman R. T. 1958. Essential fatty acids. Nutr. Rev. 16: 33–35. [DOI] [PubMed] [Google Scholar]
- 49.Guesnet P., Lallemand S. M., Alessandri J. M., Jouin M., Cunnane S. C. 2011. alpha-Linolenate reduces the dietary requirement for linoleate in the growing rat. Prostaglandins Leukot. Essent. Fatty Acids. 85: 353–360. [DOI] [PubMed] [Google Scholar]
- 50.Lucas M., Mirzaei F., O’Reilly E. J., Pan A., Willett W. C., Kawachi I., Koenen K., Ascherio A. 2011. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am. J. Clin. Nutr. 93: 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao J. S., Lee H. J., Rapoport S. I., Bazinet R. P. 2008. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol. Psychiatry. 13: 585–596. [DOI] [PubMed] [Google Scholar]
- 52.Rapoport S. I. 2014. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem. Neurosci. 5: 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.