Abstract
Steroid sulfatase (STS) deficiency is the underlying cause of the skin condition known as recessive X-linked ichthyosis (RXLI). RXLI patients show scales on their skin caused by high concentrations of cholesterol sulfate (CS), as they are not capable of releasing the sulfate group from its structure to obtain free cholesterol. CS has been reported, so far, as the sole sulfated steroid with increased concentrations in the blood of RXLI patients. A non-targeted LC-MS approach in negative mode detection (LC-MS precursor ion scan mode) was applied to serum samples of 12 RXLI patients and 19 healthy males. We found that CS was not the only sulfated compound consistently elevated in RXLI patients, because a group of compounds with a m/z of 481 was found in high concentrations too. Further LC-MS/MS demonstrated that the main contributor to the m/z 481 signal in RXLI serum is 27-hydroxycholesterol-3-sulfate (27OHC3S). Accordingly, a new method for 27OHC3S quantification in the context of RXLI has been developed and validated. Other hydroxycholesterol sulfate compounds were elevated as well in RXLI patients.
Keywords: sulfated steroids, steroid metabolism, liquid chromatography/mass spectrometry, recessive X-linked ichthyosis
Steroids play critical physiological roles in a wide number of organisms, including animals, plants, and fungi. In humans, most of the steroids present in blood and in urine are conjugated steroids rather than free forms. This conjugation is mainly due to the addition of a sulfate or glucuronide group. An example is the high concentration of dehydroepiandrosterone sulfate (DHEAS) in blood when compared with its unconjugated form, DHEA. The addition of a very polar group to the structure of steroids increases their solubility in water and facilitates their urinary excretion. Moreover, the physiological activity of some steroidal hormones is blocked by this sulfation mechanism. Therefore, action of steroid sulfatase (STS) processes active free steroids.
For decades, sulfated steroids have been regarded as inactive precursors only. More recently, new roles have been assigned to some of these molecules. For instance, pregnenolone sulfate (PregS) and DHEAS have been found to be neuroactive compounds (1). Furthermore, a specific membrane transporter channel, the sodium-dependent organic anion transporter (SOAT), has been identified for the cellular uptake of sulfated steroids (2).
The formation of sulfated steroids has been mostly attributed to the activity of sulfotransferases, which sulfonate a hydroxyl group present in the steroid, usually the one in position 3β. However, an alternative steroidogenic pathway, which starts from the conversion of cholesterol sulfate (CS) into PregS, was proved to be a physiological process (3, 4). Further research has been done recently to characterize this pathway biochemically (5). Additionally, a recent study has shown evidence pointing to the existence of a sulfated steroidogenic pathway in porcine Leydig cells (6).
Recessive X-linked ichthyosis (RXLI) is a common inherited disorder of cornification almost exclusively found in males, with a prevalence of about 1:2,000–4,000. The ichthyosis is characterized by moderate hyperkeratosis (thickening of the stratum corneum of the epidermis) and scaling of the integument. It is regarded as a nonsyndromic disease in most patients. However, an association with autism (up to 20%), cryptorchidism (up to 20%), or attention-deficit hyperactivity syndrome (up to 40%) has been reported (7). RXLI is caused by mutations of the STS gene, often small deletions, leading to deficiency of the STS enzyme, which impairs the conversion of sulfated steroids into their corresponding unconjugated compounds. Various studies have found that CS is the only sulfated steroid elevated in RXLI patients (8–10).
Other sulfated steroids have been investigated in RXLI patients, mainly DHEAS, which contribute in a high percentage to the sulfated steroidal fraction. In blood, most studies have reported that DHEAS concentration is similar in RXLI patients and in healthy people (8–12). The fact that DHEAS is not as elevated in RXLI patients as would be expected, points to the existence of an auxiliary STS activity different than STS. Some authors have proposed that gut bacterial STS could serve as an explanation for this fact (8, 13).
CS is one of the most abundant steroid sulfates found in the blood of healthy people (14). Different physiological roles have been ascribed to CS, including membrane stabilization, support of platelet adhesion, or regulation of serine proteases (14). It has been shown that elevated CS levels inhibit serine proteases and thus inhibit normal desquamation, resulting in a retention hyperkeratosis (15).
CS analysis is challenging and it has been mostly carried out using GC-MS (9, 10, 16–18). The amphipathic character of this relatively simple molecule, which is due to the conjugation of the lipophilic cholesterol with the polar sulfate group, makes it particularly susceptible to suffer from matrix effects in LC-MS (19). To the best of our knowledge, only two papers have been published making use of LC-MS to quantify CS in blood (19, 20). Only one study focused on the CS concentration in patients with RXLI (20).
So far, no research has been conducted to study in detail whether other compounds different than CS are increased in RXLI. This is of importance because it has been hypothesized that an additional STS activity is present in humans complementing the activity of STS (8). In this context, LC-MS is the technique of choice, as it offers powerful tools to study the human sulfated steroidome. The sulfated steroids can be analyzed as intact molecules (21) without any need for derivatization, providing important structural information, e.g., position of the sulfate in the structure. Of note, sulfated steroids have very specific transitions in MS/MS experiments (21–23).
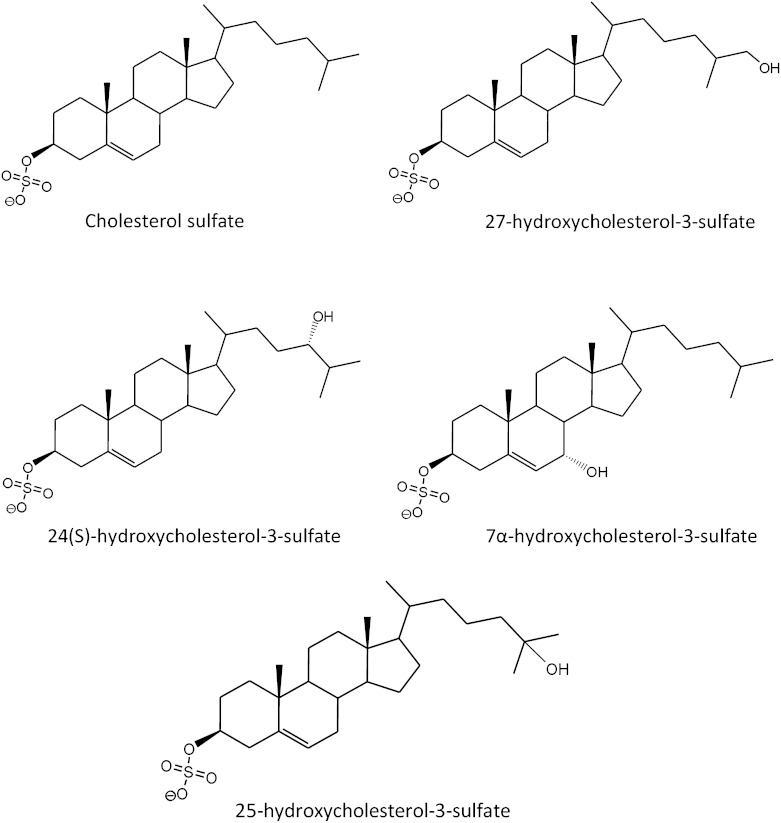
For this study, we collected serum samples from 12 patients with a clinically and enzymatically confirmed diagnosis of RXLI and made use of LC-MS to search for differences in the sulfated steroidome. By means of non-targeted MS tools, the comparison of serum from RXLI patients with serum from healthy volunteers demonstrated an increase in the signal for sulfated oxysterols (sulfated hydroxycholesterols). Hydroxycholesterol sulfates are molecules structurally related to CS, with an additional hydroxyl group (Fig. 1). Free hydroxycholesterols include 24(S)-hydroxycholesterol (24OHC), 25-hydroxycholesterol (25OHC), 27-hydroxycholesterol (27OHC), better named as (25R)-26-hydroxycholesterol (24) or 7α-hydroxycholesterol. The latest ones are the starting molecules for the acidic and neutral pathways for bile acid synthesis, respectively.
Fig. 1.
Structures of CS and some important hydroxysterol sulfates.
Some of the most relevant monosulfated and disulfated hydroxysterols have been previously identified, synthesized, and characterized (25–28). Among these, 25-hydroxycholesterol-3-sulfate (25OHC3S) has been studied with more detail, and its physiological activities and regulatory capacities have been described (28).
Our findings will help to understand the characteristics of the global STS activity in humans. A sample from a patient with cholestasis was compared with the profiles obtained in RXLI patients, showing remarkable differences. Finally, an assay for the determination of 27-hydroxycholesterol-3-sulfate (27OHC3S) has been developed and validated. This method can facilitate the diagnosis of RXLI.
MATERIALS AND METHODS
Study design and selection of patients
The study, being part of the Network for Ichthyosis and Related Keratinization disorders, was approved by the institutional review board of the University Hospital of Münster (2013-573-f-S). Written informed consent was obtained from all study participants. A detailed medical and dermatological history was obtained from all affected persons. Clinical diagnosis was made by experienced dermatologists (V.O. and H.T.). Serum blood was collected for STS activity testing. Punch biopsies (4 mm) for histology or ultrastructure were taken in some patients.
Materials
CS was obtained from Sigma-Aldrich (Taufkirchen, Germany) and stable isotope labeled 25,26,26,26,27,27,27-d7-CS (d7CS) was from C/D/N Isotopes Inc. (Quebec, Canada). The standards 22β-hydroxycholesterol, 4β-hydroxycholesterol, 7α-hydroxycholesterol, 20α-hydroxycholesterol, 25OHC, and 27OHC were purchased from Steraloids Inc. (Newport, RI), whereas 25OHC3S, 25,26,26,26,27,27-d6-27-hydroxycholesterol (d627OHC) and 24OHC were from Avanti Polar Lipids (Alabaster, AL).
Ammonium acetate, sodium hydroxide, sulfur trioxide triethylamine, and sulfatase from Helix pomatia type H-1 were obtained from Sigma-Aldrich.
Water (LC-MS grade), ammonium hydroxide, and formic acid were acquired from Fluka (Taufkirchen, Germany). Methanol (MeOH), acetonitrile (ACN), chloroform, acetone, hydrochloric acid, pyridine, and n-hexane were purchased from Merck (Darmstadt, Germany) and SepPak C18 cartridges (360 mg) were from Waters Corporation (Milford, MA). Zinc sulfate heptahydrate was from Roth (Karlsruhe, Germany).
Experimental procedures for the identification of the steroids elevated in RXLI patients
Sample preparation for isolation and identification of sulfated steroids.
Sample workup was designed to permit effective isolation of sulfated steroids from free steroids. The method required 250 μl of serum. Protein content was precipitated with 1 ml of ACN-ZnSO4 (89 g/l) 4:1 (v/v). The supernatant, obtained after centrifugation of the samples, was taken into a glass tube and then mixed with 3 ml of water. The mixture was then transferred to a Seppak C18 cartridge which was conditioned with 2 ml of MeOH and 2 ml of water. Once the sample was loaded onto the cartridge, it was washed in four steps: 3 ml of water first, followed by 3 ml of hexane, 4 ml of chloroform, and finally 4 ml of MeOH. The methanolic fraction containing the sulfated steroids was then evaporated under a flow of nitrogen, heating the samples at 40°C, and redissolved in a specific solution according to the final purpose of the analysis.
Non-targeted sulfated steroidome analysis.
Two hundred and fifty microliters of each sample was concentrated two times to a final volume of 125 μl following the aforementioned sample preparation, and redissolved in methanolic solution (90% MeOH, 9.95% water, and 0.05% NH3). The preparation was later diluted two times (sample without dilution) and four times (2-fold diluted). The samples were analyzed by LC-MS with electrospray ionization, using a gradient of MeOH with water, with a continuous increase of the percentage of MeOH at a flow rate of 0.32 ml/min. The gradient started with 20% of MeOH (80% water), reaching 99% of MeOH after 15 min. This final percentage was maintained for 1.5 min more and then a gradient was applied to return to initial conditions.
The mass spectrometer was operated first in negative parent ion scan mode (precursor ion scan mode) with a product mass of 96.9, a scan range of m/z 30–650, a scan time of 1 s, and collision energy of 45 V. The same parameters were applied to a product mass of 79.9. These product masses were selected taking into consideration previous studies, which showed that 96.9 and 79.9 are the most common fragmentation products of sulfated steroids, with the exception of estrogen sulfates (21–23).
Confirmatory experiments were performed thereafter, with the specific transitions from compounds which were increased or only found in RXLI samples after dilution. The mass spectrometer was operated in multiple reaction monitoring mode with two transitions for each parent mass: 96.9 and 80 (collision energy, 45 eV; tube lenses, 100 V).
MS conditions were always as already published for the analysis of sulfated steroids (21).
Characterization of sulfated steroids with increased concentrations in RXLI.
After sample preparation of RXLI serum samples, methanolic extracts were solvolyzed. Methanolic extract (50 μl) was mixed with 450 μl acetone and 10 μl HCl 1 M at room temperature during 18 h (29). The reaction was stopped with 20 μl of NaOH 0.5 M (in MeOH) and then evaporated under a nitrogen flow with a temperature of 40°C. Afterwards, the sample was redissolved in a methanolic solution (91.0% MeOH, 8.9% water, and 0.1% formic acid) and detected by LC-MS with atmospheric pressure chemical ionization (APCI). MS conditions were as described in Galuska et al. (21), with detection in positive full scan mode. LC method was isocratic with 85% MeOH and 15% water, using an Accucore C30 column (50 × 4.6 mm, 2.6 μm) with a defender guard Accucore C8 (10 × 4.6 mm, 2.6 μm) (Thermo Fisher Scientific, Dreieich, Germany). The flow was 0.70 ml/min during 7 min. The proportion of MeOH was then increased to 99% MeOH to clean the column with a flow of 0.80 ml/min. After 1.5 min, both flow and percent of MeOH were restored to initial conditions.
Experimental procedures for the quantification of the identified compound elevated in RXLI patients
Synthesis of standards and purity assessment.
To obtain the standard for 27OHC3S, 8 mg of pure 27OHC (20 μmol) were mixed with 3.6 mg of sulfur trioxide triethylamine and dissolved in 500 μl of pyridine. The mixture was kept at 37°C overnight. The reaction was stopped with 40 μl of NaOH (0.5 M in MeOH) and evaporated at 50°C under a nitrogen flow. The white solid obtained (sodium salt) was redissolved in a mixture of ACN:water [1:1 (v/v)] for HPLC separation. The resulting fraction was then evaporated at 50°C and weighed. Sulfonation of d627OHC was performed in the same fashion as was done for 27OHC, but without addition of NaOH to avoid deuterium exchange. Sulfonation of the remaining oxysterols for qualitative identification purposes (24OHC, 20α-hydroxycholesterol, 22β-hydroxycholesterol, 7α-hydroxycholesterol, and 4β-hydroxycholesterol) was carried out by mixing 0.34 mg of each standard with 0.17 mg of sulfur trioxide triethylamine in 100 μl of pyridine, following the same protocol as for 27OHC.
Solvolysis was applied to study the purity of the standard 27OHC3S. The solvolysis was performed as for the identification of the compound with increased concentrations in RXLI, with some modifications. Solvolysis took place overnight at 37°C, and the evaporation temperature for the solvolysis product was 50°C instead of 40°C. The standard was redissolved in the same methanolic solution (91.0% MeOH, 8.9% water, and 0.1% formic acid) and detected by the same LC-MS method used for the characterization of sulfated oxysterols after solvolysis of RXLI samples (LC-APCI-MS).
Sample preparation for analysis of intact oxysterol sulfates.
Sample workup was as described in the section “Sample preparation for isolation and identification of sulfated steroids.” For each serum sample, 15 μl of d627OHC3S, the internal standard (IS) of 27OHC3S, were mixed with 250 μl of serum to a final concentration of 12 ng/ml. After 1 h equilibration under continuous shaking, protein content was precipitated following the aforementioned protocol. After evaporation of the methanolic fraction at 40°C, samples were reconstituted in a 250 μl solution containing 54.75% water, 45% ACN, and 0.25% ammonium hydroxide. If needed, the solution was diluted two times for quantification of 27OHC3S in RXLI samples. Centrifugation was applied to those samples which were not clean after reconstitution, analyzing the supernatant.
MS and LC for analysis of intact oxysterol sulfates.
MS analyses of sulfated compounds were performed on a triple quadrupole mass spectrometer with negative electrospray ionization (TSQ, Quantum Ultra; Thermo Fisher Scientific). Mass spectrometer conditions were as described before for sulfated steroids (21). The specific multiple reaction monitoring transitions for the sulfate oxysterols were: quantifier,: 481.2 → 96.9 (collision energy, 47 eV, tube lense voltage, 141 V); qualifier, 481.2 → 80 (collision energy, 61 eV; tube lense voltage 167 V); and IS d627OHC3S, 487.2 → 96.9 (collision energy, 36 eV; tube lense voltage, 167 V).
LC was performed with a reversed phase C18 column (Hypersil Gold column 50 × 2.1 mm, 5 μm), with a defender guard Hypersil Gold C18 (10 × 2.1 mm, 5 μm) (Thermo Fisher Scientific) coupled to an HPLC system (Agilent 1200SL; Waldbronn, Germany). Solvents used for separation of oxysterol sulfate were water with ammonium acetate [10 mM and pH 7 (A) and ACN (B)]. The flows and gradients are shown in Table 1. The method had a total duration of 7.5 min.
TABLE 1.
Flows and gradients for the LC method
| Step | Start | Seconds | Flow (ml/min) | Gradient | Percent A | Percent B |
| 1 | 0.00 | 10 | 0.40 | Step | 70 | 30 |
| 2 | 0.17 | 10 | 0.40 | Step | 70 | 30 |
| 3 | 0.33 | 80 | 0.40 | Step | 50 | 50 |
| 4 | 1.67 | 180 | 0.40 | Ramp | 45 | 55 |
| 5 | 4.67 | 30 | 0.40 | Ramp | 1 | 99 |
| 6 | 5.17 | 90 | 0.45 | Ramp | 1 | 99 |
| 7 | 6.67 | 50 | 0.40 | Ramp | 70 | 30 |
Controls
Different sample controls were analyzed to address whether oxysterol sulfates from RXLI patients could have been originated by sample workup or sample manipulation. CS standard (100 μl) in MeOH (250 μg/l) were evaporated in a glass vial at 40°C under a nitrogen flow and redissolved in a serum pool from healthy people. The same experiment was repeated with d7CS.
Method validation
Several experiments were performed to validate the method for the determination of 27OHC3S in human serum. Charcoal-treated serum was chosen for method validation. Real serum from a pool of healthy patients with no detectable amounts of 27OHC3S was used as well, in order to study possible differences. Charcoal-treated serum was preferred, as it is free of other sulfated oxysterols, which may interfere with the analysis.
Linearity, calibration, detection limit, and quantification limit
The area ratio of 27OHC3S to d627OHC3S was plotted against the specific concentration in nanograms per milliliter. Linearity data were calculated using the program Xcalibur 2.1 with a 1/x weighting regression. Preparation of the calibration curves consisted of spiking increasing concentrations of 27OHC3S into 250 μl of charcoal-treated serum, plus a constant concentration of d627OHC3S. After sample preparation, including solid phase extraction, the samples were reconstituted and concentrations of the calibrators were 1, 2.5, 10, 25, 100, 200, 300, and 500 ng/ml. The final concentration for IS was 12 ng/ml. The limits of detection (LODs) and the limits of quantification (LOQs) were determined at a signal-to-noise ratio higher than 3 and higher than 10, respectively.
Selection of quality control concentration levels.
Based on preliminary results, two quality controls were chosen to study the validation process: low quality control (5 ng/ml) and high quality control (50 ng/ml). These were prepared by spiking the standards to charcoal-treated serum.
Recovery.
The recovery of the method, which evaluates the effect on analyte loss by C18 cartridges, was calculated by dividing the area ratios for the quality controls (with addition of 27OHC3S and IS pre-extraction to charcoal-treated serum, incubation, precipitation and solid phase extraction, evaporation of the methanolic extract, and reconstitution) by the area ratio obtained after spiking the same amounts of the analyte and IS postextraction (spiked onto a methanolic extract from charcoal-treated serum which was then evaporated and reconstituted).
Matrix effects.
The evaluation of possible matrix effects associated to the method was performed by plotting response ratios of controls spiked in charcoal-treated serum after sample workup (y axis) against the same concentrations of analyte and IS in the reconstitution solution (x axis). The same experiment was repeated with a serum pool with no signal for 27OHC3S instead of charcoal-treated serum. A line slope of one means no matrix effects, as the responses are the same with or without the serum matrix components. Slopes above one correspond to an ion enhancement, and slopes below one correspond to ion suppression.
Carryover.
Carryover was studied by injecting blank samples (ACN:water, 1/1) after injection of a 27OHC3S calibration sample with a concentration of 500 ng/ml.
Intra-assay and inter-assay precision and accuracy.
Each quality control was injected four times during the same day to calculate intra-assay precision and accuracy. Analysis of the quality controls injected three times each day during three consecutive days were used for the determination of inter-assay precision and accuracy.
Analysis of samples
Twelve serum samples from RXLI male patients of different ages, previously diagnosed by genetic analysis, were studied and compared with 19 samples from healthy males of different ages. A sample from a baby (3.5 months) diagnosed with cholestasis was studied too.
RESULTS
Synthesis of sulfated standards and sulfonation of d627OHC
The method described rendered a higher yield of 27OHC3S than the sulfated 27OHC in position 27 (27OHC27S) or the disulfate. HPLC separation was needed to isolate the compound. In a similar way, sulfonation of the deuterated compound d627OHC produced three peaks: disulfated 27OHC and the monosulfates 27OHC27S and 27OHC3S.
The identification of the retention times for each monosulfate was achieved by studying the specific fragmentation patterns of the sulfonation products obtained from d627OHC. Two deuterium atoms are present in carbon 27 of d627OHC and hence fragmentation of the two peaks with an m/z of 487.2 rendered different main products (product ion scanning): 96.9 (27OHC3S, retention time 2.75 min) and 97.9 (27OHC27S, retention time 3.25 min).
Sulfonation of 24OHC produced three compounds, but for the rest of oxysterol standards a predominant single peak appeared. Retention times for each of the sulfated oxysterols with respect to the IS d627OHC3S are shown in the supplementary information, together with some mixtures of those standards.
Sample workup
Sample preparation was implemented for specific isolation of the sulfated steroids from their unconjugated forms. Consequently, sulfated steroids, as well as steroid glucuronides and some bile acids, were isolated on the last step of the sample preparation (methanolic extract), whereas unconjugated steroids were collected in the chloroform fraction.
Non-targeted approach results
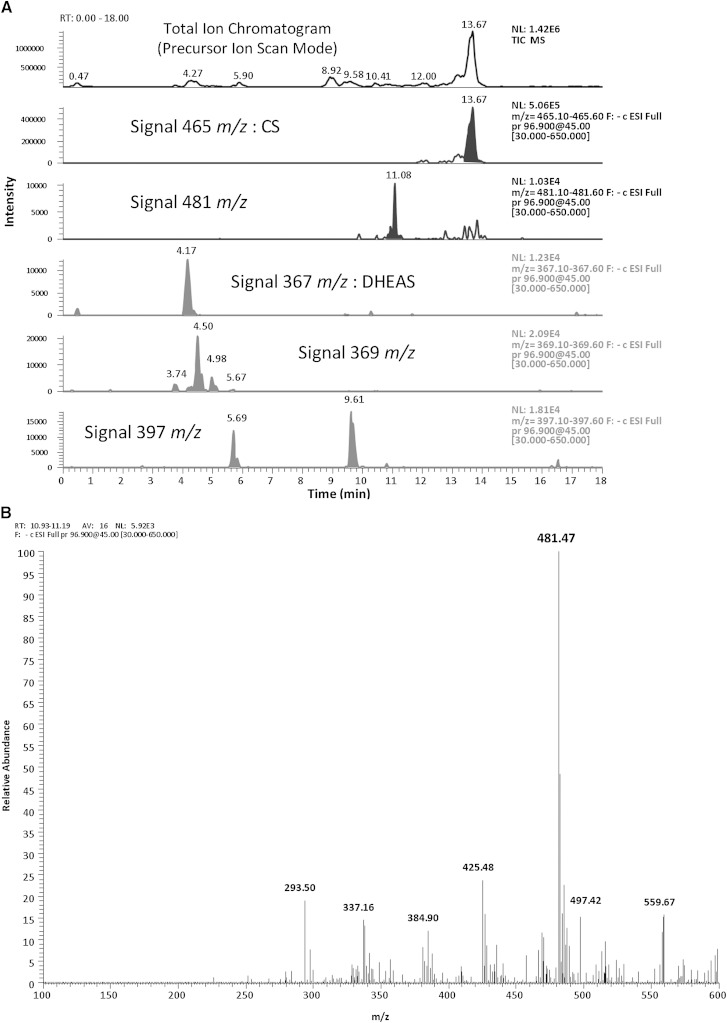
Two signals were consistently increased with independence of the age of the patient. These were m/z 465.2 from CS and m/z 481.2 coming from oxysterol sulfates (Fig. 2).
Fig. 2.
A: Total ion chromatogram obtained from a serum sample of a RXLI patient using precursor ion scan mode, as described in the “Non-targeted sulfated steoidome analysis” section. The sulfated compounds were extracted in the methanolic fraction, and were reconstituted without dilution. The base peak parent ion corresponds to CS. Other sulfated steroids were identified too, and their signal is represented. The signal m/z 481 is present in all RXLI samples. B: Detail of the m/z values found for the signal at 11 min from the same sample. The spectrum results from 16 averaged scans, for the retention time between 10.93 and 11.19 min.
Chromatography
ACN was chosen, as it allowed for a better separation of oxysterol sulfates when compared with MeOH. The method permitted a clear distinction of 27OHC3S with respect to the main oxysterol sulfates, confirmed by the mixture of different standards: 25OHC3S 24OHC monosulfates, and 27OHC27S (supplementary information).
Characterization of compounds with increased concentrations in RXLI after solvolysis
Samples from RXLI patients were solvolyzed and analyzed by LC-MS, showing a signal with a [M+H]+ of m/z 385 and retention time of 6.75 min. The injection of a mixture of the most common oxysterols in human serum (30) proved that the peak was due to 27OHC. This was confirmed by the addition of d627OHC to the samples after solvolysis.
An additional final confirmation was made by the addition of the IS, d627OHC3S, to real RXLI serum samples and controls prior to the sample preparation for the analysis of intact oxysterol sulfates. The fragmentation pattern obtained from the chromatographic peak of the chemically synthesized 27OHC3S standard (product ion mode), was compared with the one acquired from a serum RXLI sample. This comparison is depicted in supplementary Fig. 1.
A sample from a baby diagnosed with cholestasis was prepared as well. The main peak found in RXLI patients was due to 27OHC3S, whereas one of the most abundant peaks in the cholestasis sample profile was 27OHC27S.
Controls
The addition of high concentrations of CS or d7CS did not produce oxysterol sulfates, as they could not be detected by LC-MS/MS.
Validation
Results for the validation process of the 27OHC3S determination assay are shown in Table 2.
TABLE 2.
Assay validation parameters for 27OHC3S
| Nanograms per Milliliter (Spiked Concentration) | Mean (Concentration Measured) | SD | Precision (% CV) | Accuracy (% RE) | |
| Intra-assay (n = 5) | 5 | 4.9 | 0.6 | 11.3 | −1.4 |
| 50 | 52.8 | 2.2 | 4.2 | 5.6 | |
| Inter-assay (n = 12) | 5 | 4.9 | 0.7 | 14.8 | −2.4 |
| 50 | 52.3 | 3.4 | 6.4 | 4.5 | |
| Percent Recovery | 5 | 94.2 | |||
| 50 | 100.6 | ||||
| Linearity, LOQs, LOD (ng/ml) | Linearity | LOD | LLOQ | ULOQ | |
| 2.5–500 | 1 | 2.5 | 500 | ||
| Matrix effect study (regression line) | y = 0.975x + 0.018 (R2 = 0.999) (2.5–500 ng/ml) | ||||
CV, Coefficient of variation; RE,: relative error; LLOQ, lower limit of quantification; ULOQ, upper limit of quantification.
Sample analysis
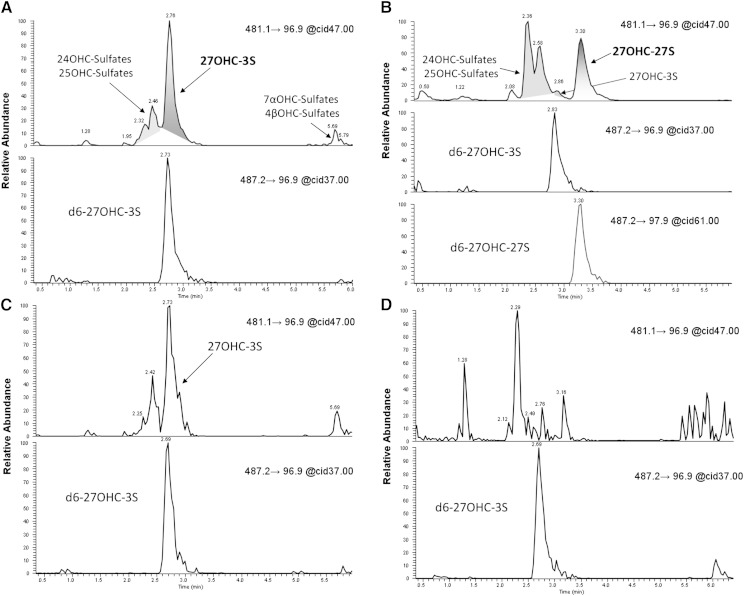
All RXLI samples showed a similar profile for the transition m/z 481→97 in our LC method for the analysis of intact sulfated steroids. The main peak found is due to 27OHC3S, as it coelutes with d627OHC3S and has the same retention time as the synthesized standard. Other important contributions to the m/z 481→97 transition are from 24OHC monosulfates (Fig. 3A). The sample from the patient with cholestasis revealed not only elevated levels of 27OHC3S, but much higher concentrations of 27OHC27S (co-elution with d627OHC27S) and of other sulfates present in RXLI (mostly 24OHC sulfates), as can be seen in Fig. 3B. The signal for 27OHC3S in samples from healthy volunteers was not measurable, as its concentration was below the LOQ. A peak which might be due to 25OHC3S or 24OHC sulfates was present (Fig. 3D). The calculated mean concentration of 27OHC3S in RXLI patients and healthy controls are shown in Table 3. The table listing the measured concentrations of 27OHC3S for all RXLI patients and controls can be found in the supplementary information. Clinical information from all RXLI patients is summarized in supplementary Table 1.
Fig. 3.
Chromatograms obtained after analysis of different samples (MS and LC methods for analysis of intact oxysterol sulfates). A: Profile obtained from a representative sample of a RXLI patient (sample concentrated three times). B: Chromatograms corresponding to a sample from a cholestatic baby patient (sample concentrated three times). C: Sample represented in Fig. 3A after dilution (four times) to achieve baseline separation of 27OHC3S with respect to the rest of oxysterol sulfates. D: Chromatograms from a representative healthy control sample.
TABLE 3.
27OHC3S mean concentration in RXLI patients and healthy controls
| RXLI Patients | Healthy Controls | |||
| Mean (% CV) | Range | Mean (% CV) | Range | |
| 27OHC3S concentration (ng/ml) | 32.3 (20.9) | 22.5–46.0 | NQ | — |
| Age (years) | 19.2 (80.0) | 3.0–51.0 | 26.1 (57.5) | 0.8–50 |
NQ, not quantifiable. The 27OHC3S could not be quantified in these samples. It could not be detected or its concentration was below LOQ.
DISCUSSION
Method development
The goal of this work was to discover whether any other sulfated steroid, different from CS, was elevated in the serum of patients suffering from RXLI, independent of age. Several approaches were needed to identify and quantify those compounds. A decisive step was to perform solvolysis of the serum extract under room or lower temperatures than usual, as it was verified that at higher temperatures some oxidized species of CS were formed (data not shown). Therefore, controls were essential in this case. Another important aspect was the separation of the sulfated fraction from the different unconjugated forms of steroids, in order to avoid matrix effects. Our final sample preparation protocol shares similarities with the one published by Raeside, Renaud, and Christie (31), in which their aim was to separate free and steroid sulfates. Likewise, the sample workup reported by Fong, Tam, and Leung (19) for CS is based on an equivalent principle.
The LC-MS/MS method presented here is, to our knowledge, the first one which permits the direct analysis of 27OHC3S in serum samples. Previously, Javitt et al. (25) studied the capacity of some sulfotransferases to produce sulfated oxysterols by HPLC. In this first attempt, intact monosulfates of 27OHC could not be resolved by LC. Our method achieves baseline separation of 27OHC3S and 27OHC27S, allowing for identification of the two related isobaric compounds in a single run time of 7.5 min. GC-MS cannot provide such information, because after cleavage of the sulfate(s) group(s) from the intact compound for derivatization, the resulting molecule is always 27OHC.
With our method, and after dilution of RXLI samples, 27OHC3S can be easily separated from other isobaric molecules too (25OHC and 24OHC monosulfates). For example, our method also enabled the identification of the specific compounds present in the serum sample from a cholestatic baby. Our patient with cholestasis showed a different pattern of oxysterol sulfates.
Most importantly, this assay represents a novel laboratory tool for the specific diagnosis and confirmation of RXLI.
Measurements in cholestasis
Recent research reported an elevation of 24OHC and 27OHC sulfates in cholestatic patients (32), but the method used in this work could not specify which positions were sulfated. The authors faced reproducibility problems using sulfatase from Helix pomatia H1. Therefore, solvolysis was chosen, so sulfated positions in the oxysterols could not be studied. Other publications studying cholestasis made use of GC-MS analysis too, and solvolysis was used again to cleave the sulfate from their structure (33, 34).
In the sample from our cholestatic patient, 27OHC3S is present. Quantification was not possible due to the presence of higher concentrations of other sulfated steroids. In this sample, 27OHC27S was significantly elevated in comparison to 27OHC3S.
The origin of oxysterol sulfates
CS is highly elevated in RXLI. This was previously reported by other authors (8, 9). Shackleton and Reid (20) measured CS in RXLI patients and healthy volunteers by LC-MS. The mean value for RXLI patients was 93.9 ± 31.2 μmol/l, with a range of 35–185 μmol/l, while normal volunteers showed a much lower mean CS concentration of 2.6 ± 0.6 μmol/l.
We found that CS is not the only sulfated steroid elevated in RXLI because other compounds, oxysterol sulfates, were confirmed to be elevated in RXLI too. This was independent of the age of the patients. We proved that hydroxysterol sulfates are not a consequence of sample handling or sample preparation, and therefore their origin is more likely enzymatic. There are two main probable routes by which oxysterol sulfates might be formed. Once the oxysterols are synthesized from cholesterol, they can undergo sulfonation by a sulfotransferase (25). But additionally, they could be enzymatically produced from CS, as was described by Norlin et al. (35) in 2003. In their studies, they found that in vitro, CS is a better substrate for CYP27A1 than cholesterol itself (35).
Cook et al. (26) discovered that human STS can cleave the sulfate in position 3 of 24OHC3S, but not the sulfate in position 24. Therefore, it is probable that the increased levels of 24OHC3S associated to RXLI are due to the lack of STS activity, and the impossibility of cleaving the sulfate group from 24OHC. A similar mechanism could contribute to the high concentrations of the rest of hydroxycholesterol sulfates, including 27OHC3S, but no experiments have been done so far to clarify whether human STS can desulfate 27OHC3S.
Sulfatase activity in RXLI
The experiments and controls performed in this work proved that all the samples from patients with STS deficiency presented high levels of oxysterol sulfates, particularly 27OHC3S.
The presence of these compounds is independent of the age of the patient. Previous studies found that CS is around 30 times higher in RXLI than in healthy people (20). If STS was the sole factor responsible for sulfatase activity in humans, then the rest of sulfated steroids (i.e., DHEAS) should be grossly elevated as CS and 27OHC3S are. Delfino et al. (16) studied the concentration of DHEAS in RXLI related to the age of the patient. They found DHEAS was increased about 1.8 times in RXLI with respect to controls, whereas CS was about 12.7 times higher. Therefore, based on our data and previously published results, we can conclude that there must be more than one STS activity in healthy humans.
This auxiliary STS activity, which is the only one active in RXLI, seems to be incapable of cleaving the sulfate from CS, 27OHC3S or any other sulfated steroid with 27 carbons.
As explained in the introduction, some authors proposed that gut microorganisms could be responsible for such auxiliary mechanism. Sulfated steroids would reach the gut flora through enterohepatic circulation (13). This mechanism seems quite possible because it has been proved that the ingestion of antibiotics increases the concentration of sulfated steroids in feces (36, 37).
During our experimental work, different approaches were used to try to release the sulfate from the structure of the steroids with 27 carbons. The use of a commercial sulfatase, obtained from Helix pomatia H1, did not provide reproducible data, in consonance with Acimovic et al. (32). CS cannot be desulfated in a reproducible way either (38).
Similarly, gut flora with steroid sulfatase activity seems unable to desulfate CS, whereas human STS can cleave the sulfate group from CS (39). Peptococcus niger H4 is an example of a human gut bacterium isolated from feces with STS activity, which fails to desulfate CS (40). The enzymatic substrate specificity of the sulfatases studied in Peptococcus niger H4 matches with the results found in this work and in previous ones studying RXLI.
Assuming a gut microorganism is partially responsible for the STS activity in humans, a disruption in this activity would have a stronger effect in RXLI patients than in healthy ones, as a healthy person has different sources of STS activity. Some sulfated steroids have been shown to be neuroactive molecules (DHEAS, PregS), and therefore a modification on the balance of sulfated steroids in RXLI in early childhood could affect their neuronal development. Following this hypothesis, a prolonged lack of auxiliary STS activity in children with RXLI (lack of gut bacterial STS activity) would elevate the levels of all their sulfated steroids. This more pronounced vulnerability could be the reason for the high percentage of RXLI patients suffering of neurodevelopmental disorders, because about 20% of them develop autistic spectrum disorders and 40% have attention-deficit hyperactivity syndrome.
In consequence with the findings described in this work, further research should be done to determine whether there is a correlation between sudden alterations in sulfated steroid concentrations and the development of some neurodevelopmental disorders in children. Additionally, the effects of the continuous presence of high concentrations of certain hydroxycholesterol sulfates, which have been proved to have regulatory capacities in some cases (26, 28), should be studied in detail.
Supplementary Material
Acknowledgments
The authors would like to thank all patients. The expert clinical assistance of Kira Süßmuth and the technical assistance of Birgit Wardega and Carmen Gregor are gratefully acknowledged.
Footnotes
Abbreviations:
- ACN
- acetonitrile
- APCI
- atmospheric pressure chemical ionization
- CS
- cholesterol sulfate
- DHEAS
- dehydroepiandrosterone sulfate
- d627OHC
- 25,26,26,26,27,27-d6-27-hydroxycholesterol
- d627OHC3S
- 25,26,26,26,27,27-d6-27-hydroxycholesterol-3-sulfate
- d7CS
- 25,26,26,26,27,27,27-d7-cholesterol sulfate
- IS
- internal standard
- LOD
- limit of detection
- LOQ
- limit of quantification
- LQC
- Low quality control
- MeOH
- methanol
- 24OHC
- 24(S)-hydroxycholesterol
- 24OHC3S
- 24(S)-hydroxycholesterol-3-sulfate
- 25OHC
- 25-hydroxycholesterol
- 25OHC3S
- 25-hydroxycholesterol-3-sulfate
- 27OHC
- 27-hydroxycholesterol
- 27OHC3S
- 27-hydroxycholesterol-3-sulfate
- PregS
- pregnenolone sulfate
- RXLI
- recessive X-linked ichthyosis
- STS
- steroid sulfatase
This work was supported by the Selbsthilfe Ichthyose e. V., the Medical Faculty (OJ111409) of the University of Münster, and by the German Research Foundation (DFG) within DFG Research Group 1369 “Sulfated Steroids in Reproduction” to subproject 7 (S.A.W., principal investigator).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of nine figures and one table.
REFERENCES
- 1.Reddy D. S. 2010. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 186: 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fietz D., Bakhaus K., Wapelhorst B., Grosser G., Günther S., Alber J., Döring B., Kliesch S., Weidner W., Galuska C. E., et al. 2013. Membrane transporters for sulfated steroids in the human testis–cellular localization, expression pattern and functional analysis. PLoS ONE. 8: e62638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts K. D., Bandi L., Calvin H. I., Drucker W. D., Lieberman S. 1964. Evidence that steroid sulfates serve as biosynthetic intermediates. IV. Conversion of cholesterol sulfate in vivo to urinary C19 and C21 steroidal sulfates. Biochemistry. 3: 1983–1988. [DOI] [PubMed] [Google Scholar]
- 4.Roberts K. D., Bandy L., Lieberman S. 1967. The conversion of cholesterol-3H-sulfate-35-S into pregnenolone-3H-sulfate-35S by sonicated bovine adrenal mitochondria. Biochem. Biophys. Res. Commun. 29: 741–746. [Google Scholar]
- 5.Neunzig J., Sánchez-Guijo A., Mosa A., Hartmann M. F., Geyer J., Wudy S. A., Bernhardt R. 2014. A steroidogenic pathway for sulfonated steroids: the metabolism of pregnenolone sulfate. J. Steroid Biochem. Mol. Biol. 144: 324–333. [DOI] [PubMed] [Google Scholar]
- 6.Schuler G., Dezhkam Y., Bingsohn L., Hoffmann B., Failing K., Galuska C. E., Hartmann M. F., Sánchez-Guijo A., Wudy S. A. 2014. Free and sulfated steroids secretion in postpubertal boars (Sus scrofa domestica). Reproduction. 148: 303–314. [DOI] [PubMed] [Google Scholar]
- 7.Traupe H., Fischer J., Oji V. 2014. Nonsyndromic types of ichthyoses - an update. J. Dtsch. Dermatol. Ges. 12: 109–121. [DOI] [PubMed] [Google Scholar]
- 8.Epstein E. H., Jr, Krauss R. M., Shackleton C. H. 1981. X-linked ichthyosis: increased blood cholesterol sulfate and electrophoretic mobility of low-density lipoprotein. Science. 214: 659–660. [DOI] [PubMed] [Google Scholar]
- 9.Bergner E. A., Shapiro L. J. 1981. Increased cholesterol sulfate in plasma and red blood cell membranes of steroid sulfatase deficient patients. J. Clin. Endocrinol. Metab. 53: 221–223. [DOI] [PubMed] [Google Scholar]
- 10.Serizawa S., Nagai T., Ito M., Sato Y. 1989. Simplified determination of serum cholesterol sulfate by gas-liquid chromatography combined with cyclohexylsilane-bonded phase column purification. Arch. Dermatol. Res. 281: 411–416. [DOI] [PubMed] [Google Scholar]
- 11.Ruokonen A., Oikarinen A., Palatsi R., Huhtaniemi I. 1980. Serum steroid sulphates in ichthyosis. Br. J. Dermatol. 103: 245–248. [DOI] [PubMed] [Google Scholar]
- 12.Serizawa S., Nagai T., Sato Y. 1987. Simplified method of determination of serum cholesterol sulfate by reverse phase thin-layer chromatography. J. Invest. Dermatol. 89: 580–587. [DOI] [PubMed] [Google Scholar]
- 13.Bergner E. A., Shapiro L. J. 1988. Metabolism of 3H-dehydroepiandrosterone sulphate by subjects with steroid sulphatase deficiency. J. Inherit. Metab. Dis. 11: 403–415. [DOI] [PubMed] [Google Scholar]
- 14.Strott C. A., Higashi Y. 2003. Cholesterol sulfate in human physiology: what’s it all about? J. Lipid Res. 44: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 15.Elias P. M., Crumrine D., Rassner U., Hachem J. P., Menon G. K., Man W., Choy M. H., Leypoldt L., Feingold K. R., Williams ML. 2004. Basis for abnormal desquamation and permeability barrier dysfunction in RXLI. J. Invest. Dermatol. 122: 314–319. [DOI] [PubMed] [Google Scholar]
- 16.Delfino M., Procaccini E. M., Illiano G. M., Milone A. 1998. X-linked ichthyosis: relation between cholesterol sulphate, dehydroepiandrosterone sulphate and patient’s age. Br. J. Dermatol. 138: 655–657. [DOI] [PubMed] [Google Scholar]
- 17.Björkhem I., Enocksson E., Harper P. 1985. Determination of cholesteryl sulphate by isotope dilution-mass spectrometry for diagnosis of steroid sulphatase deficiency. Scand. J. Clin. Lab. Invest. 45: 83–86. [DOI] [PubMed] [Google Scholar]
- 18.Muskiet F. A. J., Jansen G., Wolthers B. G., Marinkovic-Ilsen A., van Voorst Vader P. C. 1983. Gas-chromatographic determination of cholesterol sulfate in plasma and erythrocytes, for the diagnosis of recessive X-linked ichthyosis. Clin. Chem. 29: 1404–1407. [PubMed] [Google Scholar]
- 19.Fong B. M., Tam S., Leung K. S. 2013. Determination of plasma cholesterol sulfate by LC-APCI-MS/MS in the context of pediatric autism. Talanta. 116: 115–121. [DOI] [PubMed] [Google Scholar]
- 20.Shackleton C. H., Reid S. 1989. Diagnosis of recessive X-linked ichthyosis: quantitative HPLC/mass spectrometric analysis of plasma for cholesterol sulfate. Clin. Chem. 35: 1906–1910. [PubMed] [Google Scholar]
- 21.Galuska C. E., Hartmann M. F., Sánchez-Guijo A., Bakhaus K., Geyer J., Schuler G., Zimmer K. P., Wudy S. A. 2013. Profiling intact steroid sulfates and unconjugated steroids in biological fluids by liquid chromatography-tandem mass spectrometry (LC-MS-MS). Analyst. 138: 3792–3801. [DOI] [PubMed] [Google Scholar]
- 22.Yan Y., Rempel D. L., Holy T. E., Gross M. L. 2014. Mass spectrometry combinations for structural characterization of sulfated-steroid metabolites. J. Am. Soc. Mass Spectrom. 25: 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maekawa M., Shimada M., Iida T., Goto J., Mano N. 2014. Tandem mass spectrometric characterization of bile acids and steroid conjugates based on low-energy collision-induced dissociation. Steroids. 80: 80–91. [DOI] [PubMed] [Google Scholar]
- 24.Fakheri R. J., Javitt N. B. 2012. 27-Hydroxycholesterol, does it exist? On the nomenclature and stereochemistry of 26-hydroxylated sterols. Steroids. 77: 575–577. [DOI] [PubMed] [Google Scholar]
- 25.Javitt N. B., Lee Y. C., Shimizu C., Fuda H., Strott C. A. 2001. Cholesterol and hydroxycholesterol sulfotransferases: identification, distinction from dehydroepiandrosterone sulfotransferase, and differential tissue expression. Endocrinology. 142: 2978–2984. [DOI] [PubMed] [Google Scholar]
- 26.Cook I. T., Duniec-Dmuchowski Z., Kocarek T. A., Runge-Morris M., Falany C. N. 2009. 24-hydroxycholesterol sulfation by human cytosolic sulfotransferases: formation of monosulfates and disulfates, molecular modeling, sulfatase sensitivity, and inhibition of liver x receptor activation. Drug Metab. Dispos. 37: 2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren S., Kim J. K., Kakiyama G., Rodriguez-Agudo D., Pandak W. M., Min H. K., Ning Y. 2014. Identification of novel regulatory cholesterol metabolite, 5-cholesten, 3β,25-diol, disulfate. PLoS ONE. 9: e103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren S., Ning Y. 2014. Sulfation of 25-hydroxycholesterol regulates lipid metabolism, inflammatory responses, and cell proliferation. Am. J. Physiol. Endocrinol. Metab. 306: E123–E130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmentier G., Eyssen H. 1975. Synthesis of the specific monosulfates of cholic acid. Steroids. 26: 721–729. [DOI] [PubMed] [Google Scholar]
- 30.Honda A., Yamashita K., Hara T., Ikegami T., Miyazaki T., Shirai M., Xu G., Numazawa M., Matsuzaki Y. 2009. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 50: 350–357. [DOI] [PubMed] [Google Scholar]
- 31.Raeside J. I., Renaud R. L., Christie H. L. 1997. Postnatal decline in gonadal secretion of dehydroepiandrosterone and 3 beta-hydroxyandrosta-5,7-dien-17-one in the newborn foal. J. Endocrinol. 155: 277–282. [DOI] [PubMed] [Google Scholar]
- 32.Acimovic J., Lövgren-Sandblom A., Olin M., Ali Z., Heverin M., Schüle R., Schöls L., Fischler B., Fickert P., Trauner M., et al. 2013. Sulphatation does not appear to be a protective mechanism to prevent oxysterol accumulation in humans and mice. PLoS ONE. 8: e68031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng L. J., Griffiths W. J., Nazer H., Yang Y., Sjövall J. 1997. High levels of (24S)-24-hydroxycholesterol 3-sulfate, 24-glucuronide in the serum and urine of children with severe cholestatic liver disease. J. Lipid Res. 38: 926–934. [PubMed] [Google Scholar]
- 34.Summerfield J. A., Billing B. H., Shackleton C. H. 1976. Identification of bile acids in the serum and urine in cholestasis. Evidence for 6alpha-hydroxylation of bile acids in man. Biochem. J. 154: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norlin M., von Bahr S., Bjorkhem I., Wikvall K. 2003. On the substrate specificity of human CYP27A1: implications for bile acid and cholestanol formation. J. Lipid Res. 44: 1515–1522. [DOI] [PubMed] [Google Scholar]
- 36.Adlercreutz H., Martin F., Järvenpää P., Fotsis T. 1979. Steroid absorption and enterohepatic recycling. Contraception. 20: 201–223. [DOI] [PubMed] [Google Scholar]
- 37.Groh H., Schade K., Hörhold-Schubert C. 1993. Steroid metabolism with intestinal microorganisms. J. Basic Microbiol. 33: 59–72. [DOI] [PubMed] [Google Scholar]
- 38.Bleau G., Chapdelaine A., Roberts K. D. 1971. Studies on mammalian and molluscan steroid sulfatase. Solubilization and properties. Can. J. Biochem. 49: 234–242. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro L. J., Weiss R., Buxman M. M., Vidgoff J., Dimond R. L., Al Roller J., Wells R. S. 1978. Enzymatic basis of typical X-linked icthyosis. Lancet. 2: 756–757. [DOI] [PubMed] [Google Scholar]
- 40.Van Eldere J., Parmentier G., Asselberghs S., Eyssen H. 1991. Partial characterization of the steroidsulfatases in Peptococcus niger H4. Appl. Environ. Microbiol. 57: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.