Abstract
Adipocyte fatty acid binding protein 4, aP2, contributes to the pathogenesis of several common diseases including type 2 diabetes, atherosclerosis, fatty liver disease, asthma, and cancer. Although the biological functions of aP2 have classically been attributed to its intracellular action, recent studies demonstrated that aP2 acts as an adipokine to regulate systemic metabolism. However, the mechanism and regulation of aP2 secretion remain unknown. Here, we demonstrate a specific role for lipase activity in aP2 secretion from adipocytes in vitro and ex vivo. Our results show that chemical inhibition of lipase activity, genetic deficiency of adipose triglyceride lipase and, to a lesser extent, hormone-sensitive lipase blocked aP2 secretion from adipocytes. Increased lipolysis and lipid availability also contributed to aP2 release as determined in perilipin1-deficient adipose tissue explants ex vivo and upon treatment with lipids in vivo and in vitro. In addition, we identify a nonclassical route for aP2 secretion in exosome-like vesicles and show that aP2 is recruited to this pathway upon stimulation of lipolysis. Given the effect of circulating aP2 on glucose metabolism, these data support that targeting aP2 or the lipolysis-dependent secretory pathway may present novel mechanistic and translational opportunities in metabolic disease.
Keywords: adipokine, adipose triglyceride lipase, obesity, hormone-sensitive lipase, lipolysis, hormone
Adipose tissue is an endocrine organ whose products orchestrate the metabolic functions of various tissues, including brain, pancreas, and liver, to maintain systemic homeostasis. Adipocytes respond to metabolic and immune cues by mobilizing their fat stores through lipolysis and by secreting a variety of hormones and cytokines (1, 2). Such signals converge on target tissues, for example on liver to regulate glucose production, and on β cells to modulate insulin production. A critical molecule for the integration of adipocyte biology with systemic metabolic regulation is aP2 [fatty acid binding protein (FABP) 4], a lipid binding protein that is upregulated during differentiation of adipocytes and upon macrophage activation (3, 4). Since its identification, aP2 has been studied primarily for its intracellular functions in lipid metabolism and inflammation (3, 4). Genetic deletion models demonstrated that this FABP plays a critical role in the pathogenesis of several chronic metabolic diseases, including diabetes, atherosclerosis, and fatty liver. Mice deficient in aP2 or aP2 and the related protein FABP5/mal1 together have improved adipose and liver function, increased insulin sensitivity, and reduced fatty liver and cardiovascular disease in the context of high-fat diet and genetic mouse models of obesity and atherosclerosis (5–12). The link between aP2 and metabolic disease is also supported by genetic association studies in multiple populations demonstrating metabolic and cardiovascular benefits in individuals carrying a rare haploinsufficiency mutation in the aP2 locus, validating the relevance of this pathway in human disease (13, 14).
Traditionally, the metabolic improvements observed upon aP2 deficiency were attributed to changes in adipocytes or immune cells that in turn regulate secondary mediators to impact distant tissues and whole body homeostasis (10, 11). However, we recently demonstrated that aP2 is secreted from adipocytes in a regulated manner and directly increases hepatic glucose output in mice (15). These studies also demonstrated that circulating aP2 levels are significantly increased in dietary and genetic models of obesity, and that neutralizing circulating aP2 with a polyclonal antibody improved metabolic parameters and diabetes in mice (15). A growing body of association studies in humans also shows that circulating aP2 levels are positively correlated with obesity, metabolic pathologies, and cardiovascular disease (16–26). In addition, recent studies have revealed multiple other potential roles for extracellular aP2 (27, 28). These observations strengthen the emerging view that aP2 is a hormone linking adipose tissue to systemic metabolism and suggest that targeting circulating aP2 may have potential therapeutic value for a wide array of complex diseases. Hence, understanding the mechanisms that underlie and regulate its secretion is of critical importance.
As is the case for other glucoregulatory hormones such as insulin and glucagon, aP2 secretion from adipose tissue is regulated by feeding and fasting, but it also occurs simultaneously with lipolysis (15). It is well established that lipolysis is increased in the context of long-term fasting, as well as obesity, insulin resistance, and diabetes (29, 30). Lipolysis has mostly been explored in the context of signals that drive the response, rather than the hormonal signals that may be coupled to lipid breakdown and that integrate the status of adipose tissue depots with other metabolic responses of fasting or nutrient excess. We postulate that aP2 may be a key hormonal candidate coupled to lipolysis in adipocytes that supports systemic metabolic responses and adaptation to energy fluctuations. However, neither the direct role of the lipolytic pathway in regulating aP2 nor the mode of secretion is currently known. Sequence analysis using SignalP 4.1 Server (31) reveals that aP2 lacks a signal peptide required for endoplasmic reticulum/Golgi-mediated classical secretion, and it is predicted by SecretomeP 2.0 Server (32) to be a nonclassically secreted protein. While this is consistent with the earlier observations that aP2 secretion is resistant to inhibitors of classical secretion, brefeldin A and monensin (15), the identity of the nonclassical route involved in aP2 secretion is unknown.
In this study, we utilized chemical and genetic approaches to demonstrate that aP2 secretion is highly dependent on the specific activity of lipases and is driven by elevated lipids. Furthermore, we found that lipolytic stimulation leads to recruitment of aP2 into multivesicular bodies (MVBs), a nonclassical secretory compartment, and externalization in exosome-like vesicles. These findings support the concept that aP2 is an adipokine that responds to lipolytic activity in adipocytes to regulate metabolic status as controlled by liver and indicate a new pathway that is utilized by adipocytes to carry out, at least in part, their secretory and hormonal functions.
MATERIALS AND METHODS
Animals
Plin1−/− mice were obtained from the Jackson Laboratories (Bar Harbor, ME; stock number: 021887). Atglflox/flox mice (C57/BL6 background) were a kind gift from Dr. Erin Kershaw (University of Pittsburgh, Pittsburgh, PA), and Hslflox/flox and Maglflox/flox mice (mixed background) were a kind gift from Dr. Rudolf Zechner (University of Graz, Graz, Austria). Adiponectin-Cre (Adipoq-Cre) mice (mixed background) were a kind gift from Dr. Evan Rosen (Beth Israel Deaconess Medical Center, Harvard Medical School) and were backcrossed onto the C57Bl/6J genetic background. In order to obtain adipose tissue-specific lipase-deficient mice, homozygous floxed mice were crossed with Adipoq-Cre mice. Cre-positive progeny were then crossed with homozygous floxed mice to obtain flox/flox or flox/flox;Cre progeny for use in experiments. Adipose tissue explants were prepared from 10- to 16-week-old mice. Intralipid infusion experiments were performed using 24-week-old male mice. All mice were maintained on a 12 h light and dark cycle. Mice were maintained on regular chow diet (RD, PicoLab 5058 Lab Diet, 9% fat). The Harvard Medical Area Standing Committee on Animals approved all studies.
Antibodies, Western blotting, and data quantitation
Conditioned media (CM), cell lysates (CLs), tissue lysates (TLs), and vesicles were run on 15% SDS-PAGE gels. For immunoblotting, rabbit polyclonal anti-aP2 antibody was produced in-house against recombinant, full-length mouse aP2. Other antibodies were obtained from the following commercial sources: β-tubulin (Santa Cruz, sc-9104), anti-aP2 (for confocal and electron microscopy, Cell Signaling, 3544), anti-adipose triglyceride lipase (ATGL) (Cell Signaling Technology, 2138), anti-hormone-sensitive lipase (HSL) (Cell Signaling Technology, 4107S), anti-perilipin (Santa Cruz, sc-47322), anti-milk fat globule-EGF Factor 8 protein (MFG-E8; Santa Cruz, sc-33546), anti-protein disulfide isomerase (PDI; Stressgen, SPA-901), anti-cluster of differentiation 63 (CD63; Santa Cruz, sc-15363), anti-Programmed cell death 6 interacting protein (ALIX; BioLegend, 634501), anti-tumor susceptibility 101 (TSG101; Abcam, ab83). FLAG-tagged aP2 was immunoprecipitated from conditioned media using anti-FLAG M2 affinity agarose gel (Sigma) overnight at 4°C. Proteins were eluted with 2× SDS loading buffer and directly analyzed with Western blots. All antibodies were used in 1:1,000 dilution, and secondary antibody binding was detected using BM chemiluminescence blotting substrate (Roche) or SuperSignal West Femto (Pierce). aP2 or FLAG signal in Western blots of conditioned media was quantified using ImageJ software (The National Institutes of Health, Bethesda, MD).
Intralipid infusion and quantification of plasma aP2
Intralipid infusion was performed as previously described (33). Briefly, WT male mice were fasted overnight before the experiments and were infused with Intralipid at 5 ml/kg/h (Baxter Healthcare Corporation) for 5 h. Blood was collected before and after Intralipid infusion. Plasma was separated by microcentrifugation of whole blood at 13,000 rpm for 30 min. Plasma aP2 was determined with an ELISA system specific to mouse aP2 (Biovendor Inc.).
Cell culture
3T3-L1 or in-house derived WT, aP2-deficient, or FABP (aP2−/−; mal1−/−)-deficient preadipocytes were maintained in DMEM with 10% bovine calf serum. For differentiation, cells were seeded, and medium was changed after 2 days (day −2). On day 0, adipocyte differentiation was induced in DMEM with 10% Cosmic Calf Serum (CCS) using 500 µM 3-isobutyl-1-methylxanthine (IBMX), 5 µg/ml insulin, 10 µM dexamethasone, and 10 µM rosiglitazone. On day 4, medium was switched to DMEM with 10% CCS insulin and rosiglitazone. On day 6 and thereafter, medium was supplemented only with insulin. Lipolytic stimulation with or without inhibitors was done on days 8–12 of induction. For induction of aP2 secretion, adipocytes were treated with 1 mM IBMX or 20 µM forskolin (FSK) in DMEM with 10% CCS for 1 h, and medium was refreshed for another hour. Medium was collected and microcentrifuged at 5,000 rpm for 10 min. Supernatants were used for further analysis. For experiments involving inhibitors, differentiated adipocytes were pretreated with H89 (50 µM), Atglistatin (kind gift from Dr. Rudolf Zechner, University of Graz, Graz, Austria) (10 µM), 76-0079 (kind gift from Dr. Peter Kurtzhals and Dr. Christian Fledelius, Novo Nordisk, Denmark) (2.5 µM), CAY10499 (0.1 μM), or DMSO for 2 h. Then cells were cotreated with IBMX and inhibitors as explained above. For lipid treatment experiments, stocks of free fatty acids prepared with fatty acid-free, low-endotoxin BSA were diluted in DMEM and 10% CCS. Adipocytes were incubated with vehicle or lipids (500 µM) for 24 h, and conditioned medium was processed as explained above. For extracellular vesicle (EV) isolation experiments after lipolytic stimulation, adipocytes were treated with indicated stimulants for 1 h, and medium was refreshed for another 3 h. Conditioned medium was processed further as explained below to isolate vesicles.
Adipose tissue explants and biochemical measurements
Atglflox/flox or Hslflox/flox mice were crossed with Adipoq-Cre mice to obtain adipose-specific lipase-deficient models. Plin1+/− mice were intercrossed to obtain Plin1−/− and WT littermates. Perigonadal adipose depots were removed for preparation of explants. Adipose tissue samples were washed in PBS and DMEM containing 10% CCS consecutively and minced into roughly 2 mm-size pieces with scissors. Explants were washed with DMEM with 10% CCS and incubated for 1 h in the same medium. In lipase-deficiency models, lipolysis and aP2 secretion were induced with 1 mM IBMX, 20 µM FSK, 1 mM dibutyryl-cAMP (N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt), 10 µM isoproterenol, or 10 µM CL 316,243, and secreted aP2 levels in culture medium were evaluated using the protocol described above. In the perilipin1-deficiency model, explants were incubated for 16 h in DMEM with 10% CCS to assess the extent of aP2 secretion under basal conditions. Glycerol was measured using a commercial free glycerol determination system (Sigma-Aldrich), and NEFA levels were determined using HR Series NEFA-HR (2) (Wako Diagnostics) and normalized to total protein from cell or tissue lysates.
Vectors and expression
FLAG-tagged aP2 was cloned into pcDNA3 plasmid (Invitrogen). The lipid binding mutant (LBM) version of aP2 was produced using GeneTailorTM Site-directed mutagenesis system (Invitrogen). Adipocytes deficient in endogenous aP2 (aP2−/−) were electroporated on day 10 of differentiation using Cell Line L Nucleofector system (Lonza) and AMAXA electroporator. Medium was changed the next day, and experiments were performed on day 13 of differentiation. FLAG-tagged green fluorescent protein (GFP)-aP2 was produced by cloning aP2 into the lentiviral vector pRRLCMVGFP. 3T3-L1 adipocytes were infected with lentivirus for confocal and electron microscopy analysis.
Immunofluorescence and confocal microscopy
3T3-L1 cells were differentiated for 7 days and infected with lentivirus to express FLAG-tagged-GFP-aP2. On day 8, cells were kept in F12 medium for 1 h. They were then treated with water or isoproterenol (10 μM) in F12 medium for 1 h. Cells were fixed with 4% formaldehyde for 15 min and stained with LipidTOX Red and 4,6-diamidino-2-phenylindole (DAPI) for visualizing the lipid droplets and nuclei, respectively.
Electron microscopy
Adipocytes.
3T3-L1 cells were differentiated for 7 days and infected with lentivirus to express FLAG-tagged-GFP-aP2. On day 8, cells were kept in F12 medium for 1 h. They were then treated with isoproterenol (10 μM) or water in F12 medium for 2 h. Cells were fixed with formaldehyde (4% w/v in 200 mM HEPES pH 7.4 buffer) overnight at room temperature. Cells were washed two times using the same HEPES buffer and infiltrated with a solution of 2.3 M sucrose for 48 h at 4°C. Subsequently, the adipocytes were washed two times in HEPES buffer containing 0.5% BSA and centrifuged to obtain a pellet. This was in turn embedded in 10% gelatin and cryosectioned (34). Sections were picked up using a 1:1 solution of 2% methyl cellulose and 2.3 M sucrose. Cells were then labeled with aP2 or GFP antibody followed by protein A gold (10 nm) for their final visualization in transmission electron microscopy.
EVs.
Isolated vesicles were adsorbed to nickel mesh grids that had been coated with a formvar film and subjected to glow-discharge to increase hydrophilicity. The adsorbed suspensions were fixed for 30 min at 4°C in 4% formaldehyde in 0.1 M sodium phosphate buffer, pH 7.3. The following sequential washes and incubations for immunolabeling were performed at room temperature: four washes with PBS; permeabilization and blocking with 0.075% saponin in PBS, 10% normal goat serum, and 1% BSA for 30 min; rabbit anti-aP2 (1:100) in PBS with 1% BSA (PBS-BSA) for 1 h; four washes with PBS-BSA for 30 min; 1.4 nm gold-conjugated Fab′ goat anti-rabbit IgG (Nanoprobes, 1:100) in PBS-BSA for 1 h; four washes with PBS for 30 min; three washes with PBS; and refixation with 2% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.3 for 10 min. After washing with water, the immunogold-labeled samples were silver-enhanced for 1 min using the HQ silver enhancement kit (Nanoprobes) and washed again with water, followed by positive staining with uranyl acetate. Samples were stabilized with a carbon coating prior to imaging with a JEM 1200EX electron microscope (JEOL USA) equipped with an AMT XR-60 digital camera (Advanced Microscopy Techniques Corp.).
Microsome isolation
Differentiated 3T3-L1 adipocytes were treated with IBMX to induce aP2 secretion as explained above, and cells were collected at the time points indicated. Microsomes were fractionated as described previously (35). Briefly, cells were homogenized with a Teflon pestle, nuclei were cleared at 800 g, and mitochondria were cleared at 6,000 g. The supernatant was ultracentrifuged at 100,000 g for 1 h to pellet microsomes. The pellet was resuspended in lysis buffer and analyzed by Western blot.
EV isolation and continuous sucrose gradients
EV-depleted medium for use in conditioning was prepared by ultracentrifugation of DMEM with 20% CCS for 18 h at 28,500 rpm (∼100,000 g) using SW32 rotor (Beckman Coulter). Media were then filtered (0.2 µm) and diluted to 10% serum with DMEM. Differentiated 3T3-L1 or in-house adipocytes were incubated for 24 h in this vesicle-depleted media for conditioning. Processing of the media from this point on was performed at 4°C. Conditioned media from differentiated adipocytes were collected and centrifuged at 200 g for 10 min, 500 g for 10 min twice, and then 2,000 g for 15 min to eliminate floating cells. Media were then filtered through 0.45 µm pore filters and ultracentrifuged at 9,000 rpm for 30 min with SW32 rotor to clear debris. Supernatant was collected and ultracentrifuged at 28,500 rpm with SW32 rotor for 90 min to pellet vesicles. The pellet was resuspended in PBS and repelleted. The pellet was resuspended in 2× SDS loading buffer for Western blot analysis or in PBS for sucrose gradient experiments. EVs resuspended in PBS were layered on top of a continuous sucrose gradient (0.25–2 M in 200 mM Tris) and ultracentrifuged at 28,500 rpm using SW41 rotor for 20 h at 4°C. Fractions (1 ml) were collected, and density was determined with a refractometer. Each fraction was then diluted in 10 ml PBS and ultracentrifuged at 28,500 rpm for 90 min using SW41 rotor. Pellets were resuspended in 2× SDS loading buffer for analysis by Western blotting.
RESULTS
Chemical and genetic manipulation of the lipolytic pathway inhibits aP2 secretion
During prolonged fasting, adipose tissue liberates its stored lipid depot via increasing lipolysis. This critical function is achieved by hormonal activation of the β3-adrenergic receptor and a consequent increase in the secondary mediator cAMP. In turn, the cAMP-activated kinase, protein kinase A (PKA), indirectly activates ATGL and phosphorylates HSL, each of which liberate one molecule of free fatty acid from triacylglycerols (TAGs) and diacylglycerols (DAGs), consecutively. This cascade is finalized by the activity of monoacylglycerol lipase (MAGL), a constitutively active enzyme that hydrolyzes monoacylglycerols (MAGs) and liberates one free fatty acid and one glycerol molecule (36) (Fig. 1A).
Fig. 1.
Chemical manipulation of the lipolytic pathway inhibits aP2 secretion. A: Canonical lipolytic pathway and inhibitors used to block steps in this pathway. B–D: Differentiated 3T3-L1 adipocytes were pretreated with the PKA inhibitor H89 (B), ATGL inhibitor Atglistatin (C), HSL inhibitor 76-0079 (D), or HSL-MAGL dual inhibitor CAY10499 (E), followed by IBMX stimulation. Secreted aP2 was measured by Western blot analysis of CM. Glycerol release into CM was measured to assess lipolysis and normalized to total protein content (shown below or at right in each panel). Atgli, Atglistatin; CAY, CAY10499; CL, cell lysate; CM, conditioned media; Cont, control; I, IBMX; 76, 76-0079. Statistical analysis was done using Student’s t-test. * P < 0.05, *** P < 0.001. Bars indicate SEM. Western blots are representative of at least three independent experiments.
We have previously shown that lipolytic stimuli that modulate this pathway at the level of the β3-adrenergic receptor or those that increase cAMP induce aP2 secretion robustly from adipocytes in vitro, ex vivo, and in vivo (15). In order to test whether lipolysis and aP2 secretion occur independently, in parallel, or consecutively, and to characterize the molecular steps proximal to stimulated aP2 secretion, we first evaluated aP2 secretion in the presence of chemical lipolytic inhibitors acting on different stages of the lipolytic pathway (Fig. 1A). We found that H89, an inhibitor of PKA, completely blocked IBMX-stimulated aP2 secretion from differentiated 3T3-L1 adipocytes along with lipolysis, as indicated by a decrease in glycerol release in inhibitor-treated cells (Fig. 1B). Having shown that blocking PKA activation upstream of the lipolytic pathway can diminish aP2 secretion, we next asked whether the downstream lipases themselves play a role in regulating this phenomenon. Indeed, treatment of cultured 3T3-L1 adipocytes with a synthetic ATGL inhibitor, Atglistatin (37); an HSL inhibitor, 76-0079 (NNC 0076-0000-0079, Novo Nordisk, Denmark) (38); and an HSL-MAGL dual inhibitor, CAY10499 (39), blocked or reduced IBMX-induced aP2 secretion and also inhibited lipolysis as indicated by glycerol release (Fig. 1C–E). These data suggest that aP2 release relies specifically on increased lipase activity in adipocytes.
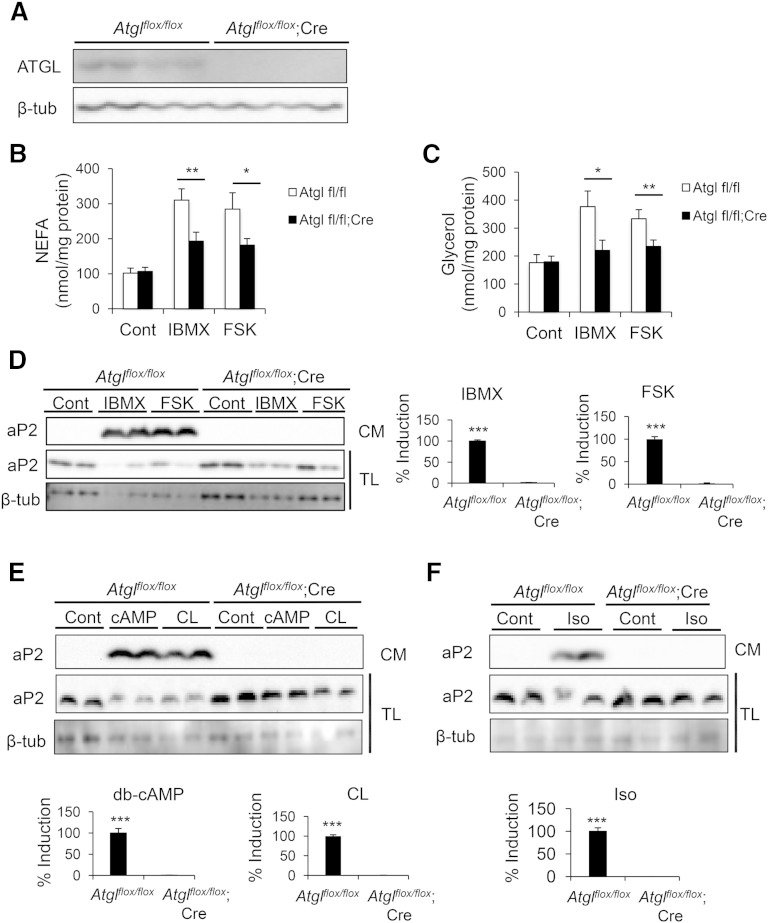
To map this pathway in further detail and considering the potential nonspecific activities of chemical inhibitors, we used complementary genetic approaches to elucidate the role of lipases in aP2 secretion. We first generated adipose tissue-specific ATGL-deficient mice by crossing Atglflox/flox mice with the Adipoq-Cre transgenic mice. After confirmation of successful deletion of Atgl in the adipose tissue (Fig. 2A), we examined aP2 secretion. Perigonadal adipose explants from these mice exhibited diminished IBMX- and FSK-induced NEFA and glycerol release (Fig. 2B, C), validating the loss of ATGL activity. In control adipose tissue explants, a robust secretion of aP2 was detectable upon stimulation of lipolysis with multiple agents (Fig. 2D–F). Despite a similar intracellular aP2 protein level, aP2 secretion was undetectable from ATGL-deficient explants upon stimulation of lipolysis with IBMX or FSK (Fig. 2D). We observed similar results with other stimulants of lipolysis such as dibutyryl-cAMP, CL 316,243, or isoproterenol (Fig. 2E, F). Considering that ATGL is the primary enzyme for TAG hydrolysis in adipocytes (40), the dramatic effect we observed with ATGL deficiency suggests that ATGL and products of TAG hydrolysis may be major regulators of aP2 secretion.
Fig. 2.
Genetic deficiency of ATGL blocks aP2 secretion ex vivo. A: Confirmation of Atgl deletion in adipose tissue explants from Atglflox/flox;Cre mice compared with Atglflox/flox mice. Cre, Adipoq-Cre. B–F: Atglflox/flox;Cre and control adipose explants were prepared and treated with various lipolytic stimulants. NEFA (B) and glycerol (C) levels in CM were measured and normalized to protein content to assess lipolysis. Secreted aP2 was measured by Western blot analysis of CM upon lipolysis stimulation with IBMX (1 mM) or FSK (20 μM) (D), dibutyryl-cAMP (db-cAMP, 1 mM) or CL 316,243 (CL, 10 μM) (E), or isoproterenol (iso, 10 μM) (F). CM, conditioned medium; TL, tissue lysate. Quantification of % induction for aP2 secretion in CM (presented on the right or bottom of each panel) was calculated based on two to five independent experiments, and representative Western blots are shown. Statistical analysis was done with Student’s t-test. * P < 0.05, ** P < 0.01, *** P < 0.001. Bars indicate SEM.
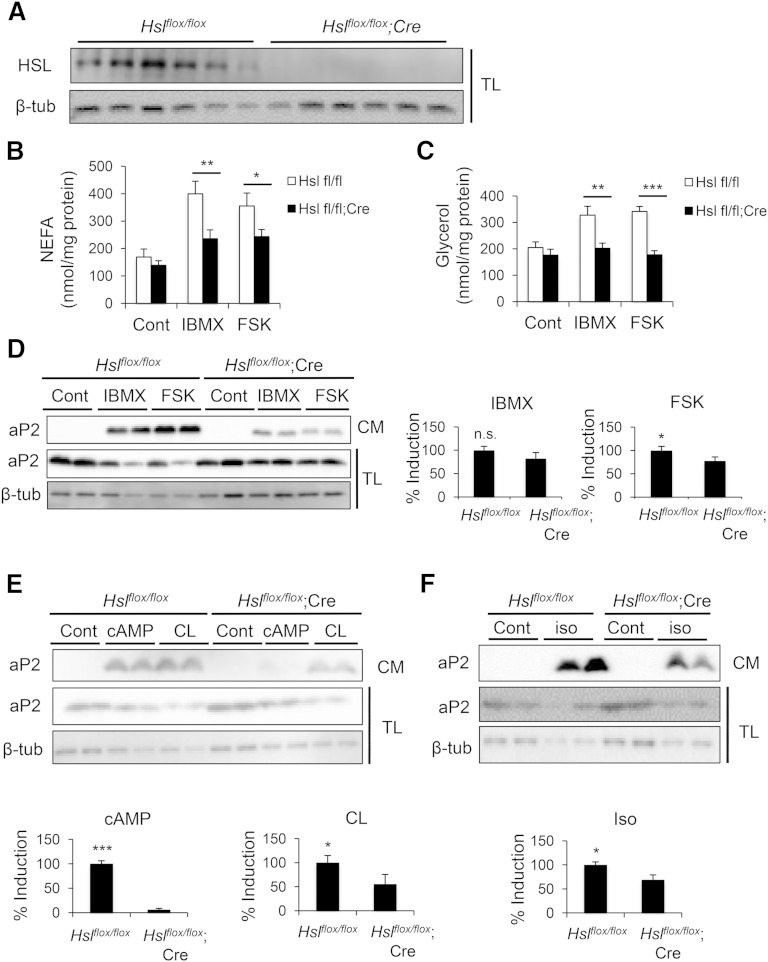
Next, we assessed aP2 secretion in adipose explants in which Hsl had been deleted (Hslflox/flox;Adipoq-Cre). In this system, we also confirmed the effective loss of HSL protein by Western blot analysis (Fig. 3A) and validated loss of stimulated lipolytic activity by decreased NEFA and glycerol release in HSL-deficient samples (Fig. 3B, C). In these experiments, there was also a robust induction of aP2 secretion in control explants upon stimulation of lipolysis (Fig. 3D–F). Although not as dramatic as the effect of ATGL deficiency, we observed a partial but significant reduction in aP2 secretion from adipose tissue explants of Hslflox/flox;Adipoq-Cre mice upon stimulation with FSK, dibutyryl-cAMP, CL 316,243, and isoproterenol, but not with IBMX (Fig. 3D–F). In addition, we have not observed a significant impact of MAGL-deficiency on aP2 secretion from adipocytes in preliminary studies (data not shown). Collectively, our chemical and genetic data demonstrate that aP2 secretion is regulated directly by the activity of the adipocyte lipases ATGL and HSL, although in this experimental paradigm the impact of HSL deficiency on aP2 secretion was smaller than that of ATGL deficiency. Differential effects of ATGL and HSL deficiency might suggest that there are lipase-specific properties regulating aP2 secretion.
Fig. 3.
Genetic deficiency of HSL decreases aP2 secretion ex vivo. A: Confirmation of Hsl deletion in adipose tissue explants from Hslflox/flox;Cre mice compared with Hslflox/flox mice. Cre, Adipoq-Cre. B–F: Hslflox/flox;Cre and control adipose explants were prepared and treated with various lipolytic stimulants. NEFA (B) and glycerol (C) levels in CM were measured and normalized to protein content to assess lipolysis. Secreted aP2 was measured via Western blot analysis of CM upon lipolysis stimulation with IBMX (1 mM) or FSK (20 μM) (D), dibutyryl-cAMP (db-cAMP, 1 mM) or CL 316,243 (CL, 10 μM) (E), or isoproterenol (iso, 10 μM) (F). CM, conditioned medium; TL, tissue lysate. Quantification of % induction for aP2 secretion in CM (presented on the right or bottom of each panel) was calculated based on three independent experiments, and representative blots are shown. n.s., not significant. Statistical analysis was done with Student’s t-test. * P < 0.05, ** P < 0.01, *** P < 0.001. Bars indicate SEM.
Free fatty acid availability increases aP2 secretion
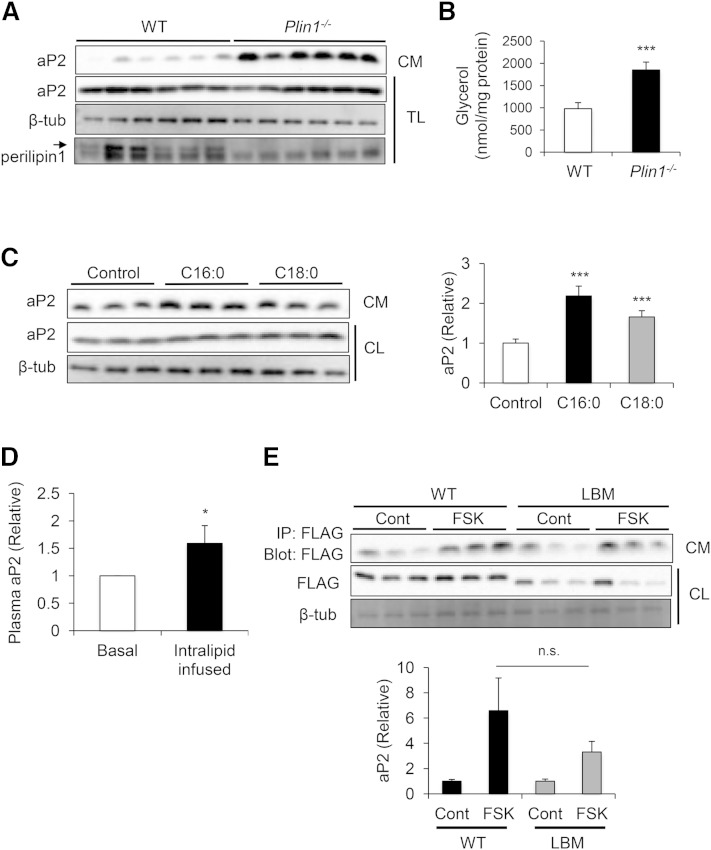
Numerous biochemical and structural studies have demonstrated that aP2 has a ligand binding domain formed by an antiparallel β-barrel structure, and that this pocket can bind to several hydrophobic ligands, including free fatty acids (4). Because lipase activity is strongly tied to aP2 secretion and the end products of lipolysis can potentially bind to aP2, we next examined the potential role of lipid mobilization in regulating aP2 secretion from adipocytes and whether direct lipid binding to aP2 is a requirement for its secretion. To begin to address this question, we utilized conditions in which lipid breakdown or availability in adipocytes could be stimulated without an additional signal to induce lipolysis. We first examined aP2 secretion in adipose tissue explants from perilipin1-deficient mice, in which adipose triglycerides are continuously metabolized due to lack of the protective function of perilipin on lipid droplets (41–44). In these explants, aP2 secretion was markedly increased compared with controls even in the absence of stimuli (Fig. 4A), accompanied by increased lipolysis, as measured by glycerol release (Fig. 4B).
Fig. 4.
Free fatty acid availability increases aP2 secretion. A: Perilipin1-deficient (Plin1−/−) and WT adipose explants were prepared and incubated for 16 h under basal conditions. Secreted aP2 was measured via Western blot analysis of CM. Perilipin1 deficiency was confirmed by Western blot analysis of TL. Arrow indicates the perilipin1 band. B: Higher basal lipolysis in Plin1−/− explants was confirmed by measurement of glycerol levels in CM and normalization with protein content. C: Differentiated 3T3-L1 adipocytes were treated with vehicle, 500 µM palmitic acid (C16:0), or stearic acid (C18:0) for 24 h. Secreted aP2 was measured by Western blot analysis of CM. Quantification of aP2 secretion in CM (right panel) was calculated based on at least four independent experiments, and a representative Western blot is shown. D: Plasma was collected from WT mice at the basal state, and after intralipid infusion (5 ml/kg/h) for 5 h, aP2 levels were measured using ELISA (n = 8). E: In-house aP2−/− differentiated adipocytes were electroporated with cytomegalovirus (CMV) promoter-driven FLAG-aP2 expression constructs that are either WT or lipid binding mutant (LBM). The extent of aP2 secretion was tested after lipolysis stimulation with FSK. FLAG-aP2 was immunoprecipitated from CM, and levels were determined by immunoblotting for FLAG. Quantification of aP2 secretion in CM (bottom panel) was calculated based on three independent experiments, and a representative Western blot is shown. CL, cell lysate; CM, conditioned medium; n.s., not significant; TL, tissue lysate. Statistical analysis was done with Student’s t-test. * P < 0.05, *** P < 0.001. Bars indicate SEM.
These results raise the possibility that increasing lipid availability may increase aP2 secretion from adipocytes. To test this possibility in vitro, we treated cultured 3T3-L1 adipocytes with two saturated fatty acids, palmitic acid (C16:0) and stearic acid (C18:0), and found that this resulted in significantly increased aP2 secretion (Fig. 4C). Next, we administered a complex lipid mixture, Intralipid, into mice, and observed significantly increased circulating aP2 levels (Fig. 4D). These data demonstrate that increasing lipids in vitro and in vivo was indeed sufficient to increase aP2 release from adipocytes.
Finally, we utilized a lipid binding mutant (LBM) form of aP2 to further investigate whether direct binding to lipids was required for aP2 secretion. This mutant version of aP2 harbors two point mutations in the ligand binding pocket (R126L, Y128F) that disrupt hydrogen bonding with the carboxyl group of lipids (45). To prevent potential confounding effects of endogenous aP2, we reconstituted in vitro differentiated aP2-deficient adipocytes with exogenous FLAG-tagged WT or LBM aP2 and compared their secretion in the presence of FSK (Fig. 4E). Interestingly, FSK stimulation induced the secretion of both WT and LBM aP2, suggesting that aP2 secretion is not entirely dependent on direct interactions between lipids and the aP2 ligand binding pocket. However, induction of LBM aP2 secretion tended to be lower, raising the possibility that this functionality contributes to stimulated aP2 secretion. Therefore, the induction of aP2 secretion may involve secondary effects of released free fatty acids on cellular processes as well as lipid binding activity for full capacity.
A nonclassical vesicular pathway mediates aP2 secretion upon lipolytic stimulation
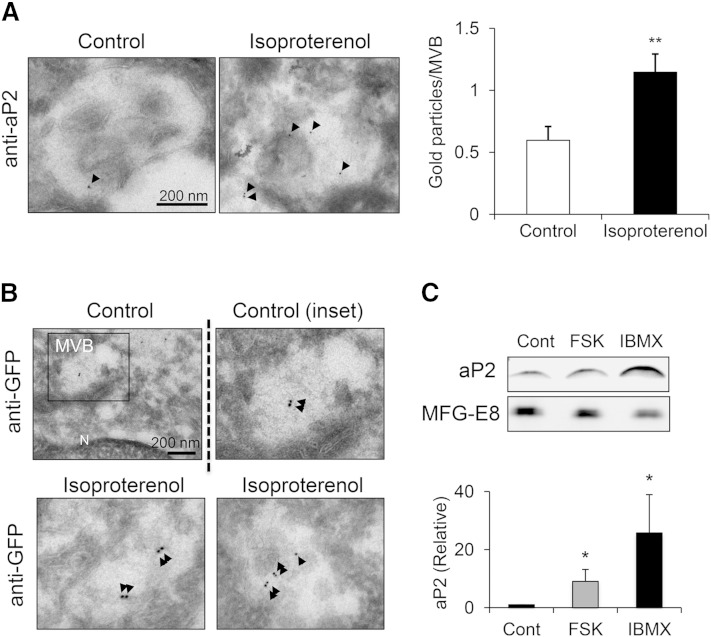
We have previously shown that aP2 release is resistant to inhibitors of classical secretion (15), consistent with its lack of a canonical signal peptide. To explore the nonclassical secretory pathway involved in aP2 secretion, we examined subcellular localization of aP2 upon lipolytic stimulation. First, to visualize localization in intact cells, we infected differentiated 3T3-L1 adipocytes with lentivirus to express GFP-fused aP2 and analyzed subcellular distribution by confocal microscopy with or without treatment with the β-adrenergic receptor agonist isoproterenol. In resting cells, aP2 was diffusely distributed throughout the cytosol (Fig. 5A). However, upon induction of lipolysis, GFP signal became less diffuse and assumed a more peripheral and punctate pattern, suggesting that aP2 may be compartmentalized into structures that mediate its secretion. Nonclassical secretion can occur through several pathways, many of which include intracellular membranous structures and vesicles (46). Therefore, we asked whether aP2 localizes to a membranous compartment during lipolysis by conducting subcellular fractionation experiments. Differentiated 3T3-L1 adipocytes were treated with IBMX and harvested at several time points after induction. The cells were then fractionated to isolate total microsomes, which should contain a mixture of membranous structures within the cytosol. We observed a time-dependent increase in aP2 localization to the total microsome fraction upon lipolytic stimulation (Fig. 5B), suggesting lipolysis-induced recruitment of aP2 to a membrane compartment. To further define the membrane compartment to which aP2 is localized within the adipocyte, we also analyzed intact 3T3-L1 adipocytes using electron microscopy to reach the necessary resolution. Immunogold labeling against GFP in adipocytes expressing GFP-fused aP2 protein revealed that aP2 was present in compartments resembling MVBs, while labeling was absent in cells that did not express the GFP-aP2 fusion construct (Fig. 5C).
Fig. 5.
aP2 secretion is mediated by an exosome-like pathway. A: Differentiated 3T3-L1 adipocytes were infected with lentivirus to express GFP-fused aP2. Cells were treated with isoproterenol (10 μM) for 1 h and stained with lipidTOX and DAPI, and confocal images were taken. Arrows indicate peripheral localization of aP2 (green: FLAG-tagged-GFP-aP2; red: lipidTOX, lipid droplet; blue, DAPI, nuclear staining). B: Differentiated 3T3-L1 adipocytes were treated with IBMX or vehicle, and a microsomal fraction was prepared at indicated time points. aP2 levels were assessed by Western blotting. PDI is used as microsomal marker. C, control; I, IBMX. C: 3T3-L1 adipocytes infected with lentivirus to express FLAG-tagged-GFP-aP2 were immunogold labeled against GFP and examined by electron microscopy. Negative control is uninfected cells. A lower magnification image is shown in top panel. G, Golgi; M, mitochondria. GFP labeling is indicated with arrows. D and E: EVs isolated from 3T3-L1 adipocyte CM were layered on a continuous sucrose gradient (0.25–2 M), ultracentrifuged for 20 h at 100,000 g for density-based separation. Fractions (1 ml) were collected, and densities were measured with a refractometer (shown on top, g/ml). Fractions were diluted with PBS and ultracentrifuged at 100,000 g to pellet vesicles in the fractions followed by immunoblotting for aP2 or exosomal markers CD63, ALIX, and TSG101 (D) or MFG-E8 and ALIX (E). F: Isolated EVs from WT or FABP−/− (aP2−/−; mal1−/−) differentiated adipocyte CM were mounted on a grid for immunogold labeling against aP2. Arrows indicate some of the aP2 labeling.
MVBs are large cytosolic vesicles derived from late endosomes that invaginate to form smaller vesicles called intraluminal vesicles (ILVs) (47). Once MVBs fuse with the plasma membrane, ILVs are released into the extracellular space as exosomes. Exosomes, as well as other secretory vesicles, have been reported to have specific size and marker profiles and float to specific fractions in sucrose gradients (47, 48). In order to further characterize the structures that contain aP2 and to test whether exosomes are associated with nonclassical aP2 secretion, we layered isolated EVs onto a continuous sucrose gradient and blotted for aP2 protein together with established exosomal markers. In these experiments, aP2-containing vesicles floated at a density of ∼1.20 g/ml and also contained established exosome markers CD63, TSG101, and ALIX (Fig. 5D) (49). We also confirmed in a separate sucrose gradient experiment that aP2-containing vesicles are positive for MFG-E8, another EV marker (Fig. 5E). These lines of evidence suggest that aP2 physically associates with secretory vesicles that have characteristics consistent with exosomes.
We then performed immunogold labeling against aP2 on EVs from the conditioned media of in-house-generated differentiated adipocytes by electron microscopy, using EVs isolated from FABP-deficient (aP2−/−; mal1−/−) cells as controls (Fig. 5F). In these experiments, aP2 was detected in ∼100 nm diameter vesicles isolated from WT but not FABP-deficient adipocyte conditioned media. The size and homogeneity of the EVs containing aP2 were also consistent with the exosome-like properties of these vesicular structures.
To test whether lipolysis increases aP2 secretion through this vesicular pathway, we immunogold labeled aP2 in differentiated 3T3-L1 adipocytes treated with either vehicle or isoproterenol and quantified immunogold labeling on MVBs. These experiments demonstrated that the presence of aP2 in these vesicular structures was significantly increased upon lipolytic stimulation (Fig. 6A). Similar results were obtained by expressing GFP-fused aP2 in 3T3-L1 adipocytes and immunogold labeling against GFP (Fig. 6B). To confirm that lipolysis increases aP2 secretion through this vesicular pathway biochemically, we also isolated exosome-like vesicles from conditioned media of differentiated 3T3-L1 adipocytes using gradual ultracentrifugation with or without induction of lipolysis. We found that lipolysis induction with FSK or IBMX significantly increased the level of vesicular aP2 (Fig. 6C). Taken together, our results demonstrated that lipolysis recruits aP2 to MVBs for nonclassical secretion partially via exosome-like vesicles.
Fig. 6.
aP2 is recruited to the nonclassical exosome-like pathway upon lipolysis. A: 3T3-L1 adipocytes were treated with isoproterenol (10 μM), fixed, and immunogold labeled for aP2. Right panel: Gold particles per MVB were quantified from at least 62 randomly selected MVBs in each treatment. aP2 labeling is indicated with arrows. B: 3T3-L1 adipocytes expressing FLAG-tagged-GFP-aP2 were treated with isoproterenol (10 μM) or vehicle, immunogold labeled against GFP, and examined by electron microscopy. Lower magnification of control is shown at top left panel. N, nucleus. GFP labeling is indicated with arrows. C: EVs were isolated from 3T3-L1 adipocyte CM treated with vehicle or lipolytic stimuli, and aP2 was measured by Western blot. MFG-E8 was used as vesicular marker. Western blots were quantified (lower panel) using seven independent experiments. * P < 0.05, ** P < 0.01. Bars indicate SEM.
DISCUSSION
aP2 (FABP4) has been implicated in the pathogenesis of many chronic metabolic diseases including type 2 diabetes, atherosclerosis, fatty liver disease, asthma, and cancer. Until recently, the biological functions of aP2 in the pathogenesis of chronic diseases have been attributed exclusively to its intracellular action. However, newer studies have demonstrated that aP2 is released from adipocytes; its levels are significantly increased in experimental and human obesity and strongly correlate with cardiometabolic diseases in numerous human studies (15–26). We have also shown that the secreted form of aP2 is a bona fide adipokine that regulates systemic metabolism, and that antibody-mediated neutralization exhibits potent antidiabetic activity in preclinical models (15). Other groups have also demonstrated that the secreted form of aP2 plays other multiple potential roles (27, 28). Despite this emerging paradigm of aP2 function as a hormone, mechanisms involved in aP2 secretion and the regulation of this process remained largely unknown.
Here, we describe a novel mechanism and route of aP2 hormone secretion from adipocytes through a nonclassical secretory pathway responsive to lipolytic activity and the availability of free fatty acids. We demonstrate that adipocyte lipase activities, specifically ATGL and, to a lesser extent, HSL, are involved in the regulated secretion of aP2, and genetic deletion or chemical inhibition of these enzymes dramatically diminishes aP2 secretion from adipocytes. It has been reported in various systems that cAMP signaling positively regulates classical and nonclassical hormone secretion (50–53). However, induction of hormone secretion has not previously been associated with specific lipase activity or free fatty acid availability in adipocytes and has not been linked to aP2 secretion.
We have shown that increasing free fatty acid availability to mimic the state of increased lipolysis is sufficient to elevate aP2 secretion in vitro and to increase circulating aP2 levels in vivo. It is possible that activation of lipases liberates fatty acids that may serve as ligands for aP2 and promote its secretion by this mechanism. While this possibility is consistent with stimulation of aP2 secretion in response to lipids, an experimental mutation that prevents lipid binding to aP2 was not sufficient to completely block its secretion from cultured adipocytes. Hence, there are likely to be other cellular events that signal and support aP2 secretion. For example, it is possible that free fatty acids drive aP2 secretion indirectly by activating signaling cascades after they are further metabolized into alternative products or that they play a role as metabolites or signaling molecules for activation or synthesis of the machinery that supports aP2 secretion through vesicular pathways (54). In addition, lipids and lipid-derived species such as phospholipids and ceramides may affect aP2 secretion via their incorporation into membranes. For instance, ceramide can be produced by condensation of palmitic acid with serine into sphingolipids; this particular lipid species is enriched in exosomal membranes and is potentially involved in budding of exosomes into MVBs (55).
Based on our studies in the genetic deletion models, we suggest that such lipid signals would be preferentially produced by ATGL, and less so by HSL activity, as deletion of ATGL essentially abolished aP2 secretion from adipocytes, whereas the impact of adipocyte-HSL deficiency was only partial. This differential activity may guide the efforts to identify specific lipid molecules involved in regulating aP2 secretion in future studies. Recent studies on stereo/regioselectivity of ATGL and HSL revealed that ATGL prefers sn-2 and sn-1 positions of TAGs and generates sn-1,3 and sn-2,3 DAGs. HSL thereafter prefers sn-3 position on DAGs (56). The differential impact of ATGL and HSL deficiency on aP2 secretion might suggest that fatty acids mostly found in the sn-2 or sn-1 but not sn-3 positions of TAGs may be important factors in aP2 secretion. Alternatively, if DAGs generated by ATGL activity as well as lipids in the sn-3 position liberated by HSL are important, the accumulation of DAGs in HSL deficiency might undermine our ability to interpret the role of HSL activity and lipids in the sn-3 position on aP2 secretion. Another possible explanation is that the mild effect of HSL-deficiency model reflects the contribution of HSL activity to TAG hydrolysis, which is 10% of its activity on DAG hydrolysis (56).
Our biochemical and microscopic analysis revealed that within adipocytes, lipolysis induces relocalization of aP2 to a nonclassical secretory pathway that involves the MVB compartment. Once secreted, the majority of aP2 is detectable in the free form in circulation, but a small fraction persists in vesicles (data not shown). The vesicles bearing aP2 resemble exosomes in terms of size and marker expression, in agreement with previous reports (57). Our analysis of the density of the aP2-containing particles showed that they are at the high end of the density range reported for exosomes (1.19–1.21 g/ml) (58). This may reflect unique properties of adipocytes and the lipid composition that may influence the precise characteristics of these vesicles. Our earlier observations demonstrated that plasma aP2 levels increase ∼4-fold in mice with dietary or genetic obesity (15). However, in the circulation, the majority of aP2 is in the free form, and based on therapeutic efficacy of an aP2 neutralizing antibody, free aP2 may be the biologically active form, at least in the contexts in which it has been studied so far. While the precise calculation of the ratio of the vesicular and free pools of aP2 is challenging, we predict that the vesicular fraction represents <5% of total aP2. At the moment, whether the free form is derived from breakdown of the vesicles exported through MVBs and/or is contributed through an independent secretory pathway remains unclear. It will be interesting to evaluate these possibilities as well as potential distinct functional properties of free and vesicle-bound aP2 in different target tissues in the future. For example, it was previously reported that adipose-derived exosome-like vesicles from ob/ob mice have proinflammatory effects on macrophages (59), suggesting that they may play a role in the metabolically driven inflammatory aspects of diabetes.
The regulation of aP2 secretion by nutritional status and lipolysis, and its function in promoting hepatic glucose production and insulin resistance, fits well with our current understanding of blood glucose regulation during prolonged fasting, when the release of gluconeogenic substrates (i.e., glycerol) and signals from adipose tissue are synchronized. One condition in which specific organisms are challenged with prolonged fasting is hibernation. A recent study has demonstrated that during hibernation, grizzly bears exhibit reversible insulin resistance coupled with elevated lipolysis and markedly increased aP2 expression, and their glucose levels are well maintained at this stage (60). In the posthibernation period when bears are no longer reliant on fat stores, adipose aP2 levels are dramatically decreased and insulin sensitivity is increased. However, how circulating aP2 levels correlate with this remarkable regulation remains to be studied.
Finally, in obesity, adipose tissue becomes resistant to insulin-mediated suppression of lipolysis, which will drive aP2 release and contribute to increased liver glucose output, leading to hyperglycemia and diabetes. Hence, although aP2 carries the properties of a glucoregulatory hormone of fasting or catabolism, it is paradoxically elevated to signal a “pseudofasting” condition in obesity. Interestingly, the synthetic status of liver in obesity resembles that of fasting in a healthy animal (61). Lipase inhibitors have been suggested as therapeutic tools for inhibiting toxic effects of lipids resulting from increased lipolysis in patients with metabolic disease (37, 62, 63). Our data suggest that regulation of aP2 secretion or its neutralization may be appealing alternatives or additive therapeutic approaches that would circumvent limitation of inhibition of lipases and the potential for broader and undesired consequences.
Acknowledgments
The authors thank Kathryn Claiborn for editorial input and discussion; James Hester, Megan Washack, David M. Ndegwa, Keith Doucet, Karen Inouye, and Motohiro Sekiya for administrative and experimental help; and Ping Li, Suneng Fu, and Motohiro Sekiya for technical advice and scientific discussions. The authors also thank the Confocal Microscopy Facility at the University of Oslo for help with imaging studies; Dr. Erin Kershaw (University of Pittsburgh, Pittsburgh, PA) for providing Atglflox/flox mice; Dr. Rudolf Zechner (University of Graz, Graz, Austria) for Atglistatin and Hslflox/flox and Maglflox/flox mice; Dr. Evan Rosen (BIDMC, Harvard Medical School) for Adipoq-Cre mice; and Dr. Peter Kurtzhals and Dr. Christian Fledelius (Novo Nordisk, Denmark) for 76-0079.
Footnotes
Abbreviations:
- Adipoq-Cre
- adiponectin-Cre
- ATGL
- adipose triglyceride lipase
- CCS
- Cosmic Calf Serum
- CL
- cell lysate
- CM
- conditioned media
- DAG
- diacylglycerol
- DAPI
- 4,6-diamidino-2-phenylindole
- EV
- extracellular vesicle
- FABP
- fatty acid binding protein
- FSK
- forskolin
- GFP
- green fluorescent protein
- HSL
- hormone-sensitive lipase
- IBMX
- 3-isobutyl-1-methylxanthine
- MAG
- monoacylglycerol
- MAGL
- monoacylglycerol lipase
- MVB
- multivesicular body
- PKA
- protein kinase A
- TAG
- triacylglycerol
- TL
- tissue lysate
This work is supported in part by National Institutes of Health Grant DK064360 (G.S.H.). M.E.E. was supported by the Roadmap Grant R90 DK071507 from the National Institutes of Health and the Harvard Merit/Term Time Research Fellowship (John Parker Bequest Award). F.S. and J.S. are supported by grants from the Norwegian Research Council and Norwegian Cancer Society.
REFERENCES
- 1.Kershaw E. E., Flier J. S. 2004. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 2.Scherer P. E. 2006. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 55: 1537–1545. [DOI] [PubMed] [Google Scholar]
- 3.Furuhashi M., Hotamisligil G. S. 2008. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 7: 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertzel A. V., Bernlohr D. A. 2000. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol. Metab. 11: 175–180. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil G. S., Johnson R. S., Distel R. J., Ellis R., Papaioannou V. E., Spiegelman B. M. 1996. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 274: 1377–1379. [DOI] [PubMed] [Google Scholar]
- 6.Uysal K. T., Scheja L., Wiesbrock S. M., Bonner-Weir S., Hotamisligil G. S. 2000. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 141: 3388–3396. [DOI] [PubMed] [Google Scholar]
- 7.Boord J. B., Maeda K., Makowski L., Babaev V. R., Fazio S., Linton M. F., Hotamisligil G. S. 2004. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 110: 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao H., Maeda K., Gorgun C. Z., Kim H. J., Park S. Y., Shulman G. I., Kim J. K., Hotamisligil G. S. 2006. Regulation of metabolic responses by adipocyte/macrophage fatty acid-binding proteins in leptin-deficient mice. Diabetes. 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 9.Furuhashi M., Tuncman G., Gorgun C. Z., Makowski L., Atsumi G., Vaillancourt E., Kono K., Babaev V. R., Fazio S., Linton M. F., et al. 2007. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 447: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., Hotamisligil G. S. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makowski L., Boord J. B., Maeda K., Babaev V. R., Uysal K. T., Morgan M. A., Parker R. A., Suttles J., Fazio S., Hotamisligil G. S., et al. 2001. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 7: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuncman G., Erbay E., Hom X., De Vivo I., Campos H., Rimm E. B., Hotamisligil G. S. 2006. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc. Natl. Acad. Sci. USA. 103: 6970–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saksi J., Ijäs P., Mäyränpää M. I., Nuotio K., Isoviita P. M., Tuimala J., Lehtonen-Smeds E., Kaste M., Jula A., Sinisalo J., et al. 2014. The low-expression variant of fatty acid-binding protein 4 favors reduced manifestations of atherosclerotic disease and increased plaque stability. Circ Cardiovasc Genet. 7: 588–598. [DOI] [PubMed] [Google Scholar]
- 15.Cao H., Sekiya M., Erikci Ertunc M., Burak M. F., Mayers J. R., White A., Inouye K., Rickey L. M., Ercal B. C., Furuhashi M., et al. 2013. Adipocyte lipid chaperone aP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi T., Onoue G., Hirohata A., Hirohata S., Usui S., Hina K., Kawamura H., Doi M., Kusano K. F., Kusachi S., et al. 2010. Serum adipocyte fatty acid-binding protein is independently associated with coronary atherosclerotic burden measured by intravascular ultrasound. Atherosclerosis. 211: 164–169. [DOI] [PubMed] [Google Scholar]
- 17.Xu A., Wang Y., Xu J. Y., Stejskal D., Tam S., Zhang J., Wat N. M., Wong W. K., Lam K. S. 2006. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 52: 405–413. [DOI] [PubMed] [Google Scholar]
- 18.Yoo H. J., Kim S., Park M. S., Choi H. Y., Yang S. J., Seo J. A., Kim S. G., Kim N. H., Baik S. H., Choi D. S., et al. 2011. Serum adipocyte fatty acid-binding protein is associated independently with vascular inflammation: analysis with (18)F-fluorodeoxyglucose positron emission tomography. J. Clin. Endocrinol. Metab. 96: E488–E492. [DOI] [PubMed] [Google Scholar]
- 19.Furuhashi M., Ishimura S., Ota H., Hayashi M., Nishitani T., Tanaka M., Yoshida H., Shimamoto K., Hotamisligil G. S., Miura T. 2011. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS ONE. 6: e27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu B. G., Chen Y. C., Lee R. P., Lee C. C., Lee C. J., Wang J. H. 2010. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ. J. 74: 327–331. [DOI] [PubMed] [Google Scholar]
- 21.Bao Y., Lu Z., Zhou M., Li H., Wang Y., Gao M., Wei M., Jia W. 2011. Serum levels of adipocyte fatty acid-binding protein are associated with the severity of coronary artery disease in Chinese women. PLoS ONE. 6: e19115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M., Zhou M., Bao Y., Xu Z., Li H., Zhang H., Zhu W., Zhang J., Xu A., Wei M., et al. 2013. Circulating adipocyte fatty acid-binding protein levels are independently associated with heart failure. Clin. Sci. (Lond.). 124: 115–122. [DOI] [PubMed] [Google Scholar]
- 23.Ishimura S., Furuhashi M., Watanabe Y., Hoshina K., Fuseya T., Mita T., Okazaki Y., Koyama M., Tanaka M., Akasaka H., et al. 2013. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE. 8: e81318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Eynatten M., Breitling L. P., Roos M., Baumann M., Rothenbacher D., Brenner H. 2012. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler. Thromb. Vasc. Biol. 32: 2327–2335. [DOI] [PubMed] [Google Scholar]
- 25.Terra X., Quintero Y., Auguet T., Porras J. A., Hernandez M., Sabench F., Aguilar C., Luna A. M., Del Castillo D., Richart C. 2011. FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. Eur. J. Endocrinol. 164: 539–547. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y. C., Cho Y. K., Lee W. Y., Kim H. J., Park J. H., Park D. I., Sohn C. I., Jeon W. K., Kim B. I., Park S. E., et al. 2011. Serum adipocyte-specific fatty acid-binding protein is associated with nonalcoholic fatty liver disease in apparently healthy subjects. J. Nutr. Biochem. 22: 289–292. [DOI] [PubMed] [Google Scholar]
- 27.Lamounier-Zepter V., Look C., Alvarez J., Christ T., Ravens U., Schunck W. H., Ehrhart- Bornstein M, Bornstein S. R, Morano I. 2009. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ. Res. 105: 326–334. [DOI] [PubMed] [Google Scholar]
- 28.Wu L. E., Samocha-Bonet D., Whitworth P. T., Fazakerley D. J., Turner N., Biden T. J., James D. E., Cantley J. 2014. Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol Metab. 3: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilherme A., Virbasius J. V., Puri V., Czech M. P. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arner P., Langin D. 2014. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol. Metab. 25: 255–262. [DOI] [PubMed] [Google Scholar]
- 31.Petersen T. N., Brunak S., von Heijne G., Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 8: 785–786. [DOI] [PubMed] [Google Scholar]
- 32.Bendtsen J. D., Jensen L. J., Blom N., Von Heijne G., Brunak S. 2004. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 17: 349–356. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T., Furuhashi M., Li P., Cao H., Tuncman G., Sonenberg N., Gorgun C. Z., Hotamisligil G. S. 2010. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 140: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffiths G., Simons K., Warren G., Tokuyasu K. T. 1983. Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins. Methods Enzymol. 96: 466–485. [DOI] [PubMed] [Google Scholar]
- 35.Cox B., Emili A. 2006. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat. Protoc. 1: 1872–1878. [DOI] [PubMed] [Google Scholar]
- 36.Lass A., Zimmermann R., Oberer M., Zechner R. 2011. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50: 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer N., Schweiger M., Romauch M., Grabner G. F., Eichmann T. O., Fuchs E., Ivkovic J., Heier C., Mrak I., Lass A., et al. 2013. Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat. Chem. Biol. 9: 785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. 2006. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 281: 40236–40241. [DOI] [PubMed] [Google Scholar]
- 39.Muccioli G. G., Labar G., Lambert D. M. 2008. CAY10499, a novel monoglyceride lipase inhibitor evidenced by an expeditious MGL assay. ChemBioChem. 9: 2704–2710. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Botas J., Anderson J. B., Tessier D., Lapillonne A., Chang B. H., Quast M. J., Gorenstein D., Chen K. H., Chan L. 2000. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 26: 474–479. [DOI] [PubMed] [Google Scholar]
- 42.Tansey J. T., Sztalryd C., Gruia-Gray J., Roush D. L., Zee J. V., Gavrilova O., Reitman M. L., Deng C. X., Li C., Kimmel A. R., et al. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA. 98: 6494–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H. H., Souza S. C., Muliro K. V., Kraemer F. B., Obin M. S., Greenberg A. S. 2003. Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J. Biol. Chem. 278: 51535–51542. [DOI] [PubMed] [Google Scholar]
- 44.Garcia A., Subramanian V., Sekowski A., Bhattacharyya S., Love M. W., Brasaemle D. L. 2004. The amino and carboxyl termini of perilipin a facilitate the storage of triacylglycerols. J. Biol. Chem. 279: 8409–8416. [DOI] [PubMed] [Google Scholar]
- 45.Sha R. S., Kane C. D., Xu Z., Banaszak L. J., Bernlohr D. A. 1993. Modulation of ligand binding affinity of the adipocyte lipid-binding protein by selective mutation. Analysis in vitro and in situ. J. Biol. Chem. 268: 7885–7892. [PubMed] [Google Scholar]
- 46.Prydz K., Tveit H., Vedeler A., Saraste J. 2013. Arrivals and departures at the plasma membrane: direct and indirect transport routes. Cell Tissue Res. 352: 5–20. [DOI] [PubMed] [Google Scholar]
- 47.Simons M., Raposo G. 2009. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21: 575–581. [DOI] [PubMed] [Google Scholar]
- 48.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J. J., Lotvall J. O. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 49.Andaloussi E. L., Mager S. I., Breakefield X. O, Wood M. J. 2013. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12: 347–357. [DOI] [PubMed] [Google Scholar]
- 50.Yajima H., Komatsu M., Schermerhorn T., Aizawa T., Kaneko T., Nagai M., Sharp G. W., Hashizume K. 1999. cAMP enhances insulin secretion by an action on the ATP-sensitive K+ channel- independent pathway of glucose signaling in rat pancreatic islets. Diabetes. 48: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 51.Vitalis E. A., Costantin J. L., Tsai P. S., Sakakibara H., Paruthiyil S., Iiri T., Martini J. F., Taga M., Choi A. L., Charles A. C., et al. 2000. Role of the cAMP signaling pathway in the regulation of gonadotropin-releasing hormone secretion in GT1 cells. Proc. Natl. Acad. Sci. USA. 97: 1861–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kucka M., Bjelobaba I., Tomic M., Stojilkovic S. S. 2013. The role of cyclic nucleotides in pituitary lactotroph functions. Front. Endocrinol. (Lausanne). 4: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye F., Than A., Zhao Y., Goh K. H., Chen P. 2010. Vesicular storage, vesicle trafficking, and secretion of leptin and resistin: the similarities, differences, and interplays. J. Endocrinol. 206: 27–36. [DOI] [PubMed] [Google Scholar]
- 54.Shen B., Wu N., Yang J. M., Gould S. J. 2011. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J. Biol. Chem. 286: 14383–14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., Simons M. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 56.Eichmann T. O., Kumari M., Haas J. T., Farese R. V., Jr, Zimmermann R., Lass A., Zechner R. 2012. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 287: 41446–41457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kranendonk M. E., Visseren F. L., van Balkom B. W., Nolte-'t Hoen E. N., van Herwaarden J. A., de Jager W., Schipper H. S., Brenkman A. B., Verhaar M. C., Wauben M. H., et al. 2014. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 22: 1296–1308. [DOI] [PubMed] [Google Scholar]
- 58.Théry C., Ostrowski M., Segura E. 2009. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9: 581–593. [DOI] [PubMed] [Google Scholar]
- 59.Deng Z. B., Poliakov A., Hardy R. W., Clements R., Liu C., Liu Y., Wang J., Xiang X., Zhang S., Zhuang X., et al. 2009. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 58: 2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson O. L., Jansen H. T., Galbreath E., Morgenstern K., Gehring J. L., Rigano K. S., Lee J., Gong J., Shaywitz A. J., Vella C. A., et al. 2014. Grizzly bears exhibit augmented insulin sensitivity while obese prior to a reversible insulin resistance during hibernation. Cell Metab. 20: 376–382. [DOI] [PubMed] [Google Scholar]
- 61.Fu S., Fan J., Blanco J., Gimenez-Cassina A., Danial N. N., Watkins S. M., Hotamisligil G. S. 2012. Polysome profiling in liver identifies dynamic regulation of endoplasmic reticulum translatome by obesity and fasting. PLoS Genet. 8: e1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kusunoki J., Kanatani A., Moller D. E. 2006. Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine. 29: 91–100. [DOI] [PubMed] [Google Scholar]
- 63.Girousse A., Tavernier G., Valle C., Moro C., Mejhert N., Dinel A. L., Houssier M., Roussel B., Besse-Patin A., Combes M., et al. 2013. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 11: e1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]