Abstract
Indirect evidence suggests that impaired triglyceride storage in the subcutaneous fat depot contributes to the development of insulin resistance via lipotoxicity. We directly tested this hypothesis by measuring, in vivo, TG synthesis, de novo lipogenesis (DNL), adipocyte proliferation, and insulin suppression of lipolysis in subcutaneous adipose tissue of BMI-matched individuals classified as insulin resistant (IR) or insulin sensitive (IS). Nondiabetic, moderately obese subjects with BMI 25–35 kg/m2, classified as IR or IS by the modified insulin suppression test, consumed deuterated water (2H2O) for 4 weeks. Deuterium incorporation into glycerol, palmitate, and DNA indicated TG synthesis, DNL, and adipocyte proliferation, respectively. Net TG synthesis and DNL in adipose cells were significantly lower in IR as compared with IS subjects, whereas adipocyte proliferation did not differ significantly. Plasma FFAs measured during an insulin suppression test were 2.5-fold higher in IR subjects, indicating resistance to insulin suppression of lipolysis. Adipose TG synthesis correlated directly with DNL but not with proliferation. These results provide direct in vivo evidence for impaired TG storage in subcutaneous adipose tissue of IR as compared with IS. Relative inability to store TG in the subcutaneous depot may represent a mechanism contributing to the development of insulin resistance in the setting of obesity.
Keywords: obesity, insulin resistance, subcutaneous adipose, triglycerides, de novo, lipogenesis, lipolysis
The biologic mechanism linking obesity to insulin resistance is incompletely understood. We were one of the first groups (1, 2) to hypothesize that impaired TG storage in subcutaneous adipose tissue contributes to obesity-induced insulin resistance in humans. Support for this hypothesis can be found in mouse models in which complete absence of adipose tissue results in ectopic fat deposition and severe metabolic dysfunction but is reversed by surgical implantation of only 100 mg of subcutaneous fat tissue (3) and in which overexpression of adiponectin leads to adipocyte hyperplasia, providing greater fat storage availability in the subcutaneous adipose tissue, preventing adipocyte hypertrophy, ectopic fat deposition, and insulin resistance (4). Evidence in humans is largely indirect. We have previously shown that insulin-resistant (IR) individuals, as compared with similarly obese insulin-sensitive (IS) individuals, have increased visceral (relative to subcutaneous) abdominal and thigh fat, accumulation of very small adipose cells, and decreased expression of lipogenic genes (1, 5). These data point to impaired differentiation and lipid storage in subcutaneous adipose cells of IR as compared with IS individuals. We sought to extend these findings by directly measuring adipose TG synthesis, de novo lipogenesis (DNL), and adipocyte proliferation in vivo through oral administration of deuterated water (2H2O) and measurement of deuterium incorporation into newly synthesized TG-glycerol, TG-palmitate, and mature adipocyte DNA-deoxyribose, respectively (6). Results reveal adipocyte dysfunction through differences in TG deposition and DNL, providing a potential mechanism for obesity-induced insulin resistance.
MATERIALS AND METHODS
Subjects
Healthy, weight-stable individuals (BMI 25–35 kg/m2) were recruited from the San Francisco Bay area. Subjects with a history of major organ disease, chronic inflammatory conditions, malignancy, bariatric surgery, liposuction, heavy alcohol consumption (>2 drinks/day for female subjects or >4 drinks/day for male subjects), smoking, pregnancy/lactation, or use of diabetogenic or weight loss medications were excluded. Patients taking prescription cholesterol-lowering and antihypertensive medications were instructed to not change dosage during the study. The study was approved by the Stanford University Human-Subjects Committee; all subjects gave written, informed consent during screening in the Stanford Clinical and Translational Research Unit. Weekly visits with dietitian and 3-day dietary recalls ensured consistent weight and macronutrient intake during the course of the study.
Measurement of insulin-mediated glucose uptake and insulin suppression of lipolysis
Subjects were admitted to the Stanford Clinical and Translational Research Unit after a 12-h fast for quantification of insulin-mediated glucose uptake and insulin suppression of lipolysis via the octreotide-modified insulin suppression test as previously described (8). Briefly, insulin was infused at 25 mU/(m2/min) to yield a plasma insulin concentration of 60 μU/ml; glucose was infused at 240 mg/(m2/min) until steady-state was reached. Steady-state plasma glucose concentration (SSPG), representing the relative ability of insulin to dispose of a glucose load, was used to classify individuals as IR or IS based on values falling within the highest or lowest 40th percentile of the normal distribution (1, 9). FFA concentrations during steady-state indicated relative insulin suppression of lipolysis as previously described (8).
Adipose tissue biopsy and isolation of adipose cells
Subcutaneous periumbilical adipose tissue was obtained via scalpel biopsy as previously described (1); fresh adipocytes were isolated via collagenase digestion and flotation using the method described by Rodbell (10).
2H2O body water enrichment
Subjects consumed 2H2O for 4 weeks before adipose tissue biopsy to achieve and maintain a total body water enrichment of 1.0–2.0%, as previously validated (6). Total body water enrichment was measured from plasma obtained at weeks 0, 2, and 4.
Compliance was checked by counting empty vials at weekly visits.
Lipid dynamics measured with 2H2O
TGs were separated from isolated adipocytes using the Folch technique (6). Fractional TG synthesis and DNL were calculated based on deuterium (2H) incorporation into TG-glycerol and TG-palmitate, respectively (6). The use of 2H2O for the quantification of newly synthesized glycerol, representing net TG synthesis retained in adipose tissue over the course of deuterium exposure, has been well documented in humans and is generally referred to as “TG synthesis” (6, 7). All subjects were weight stable and of similar BMIs during the 4 weeks of 2H2O labeling, thus, fractional TG and DNL synthesis here represents turnover. Adipocyte proliferation was measured via deuterium incorporation into the deoxyribose moiety of extracted mature adipocyte DNA (Qiagen). To account for slight variances in labeling times between subjects, all measures were expressed as the rate of synthesis per day (k) [k = −ln(1 − f)/labeling time]. Isotope enrichments were measured by GC-MS analysis (models 5971 and 5973; Hewlett-Packard).
Statistical analysis
Data are presented as mean ± SD. Due to skewed distributions, all lipid dynamics variables and FFA concentrations were log-transformed for statistical tests. Student’s t-tests and chi2 test were used to compare clinical and demographic characteristics or IR versus IS subgroups. By-group comparisons of lipid dynamics variables and FFA used ANCOVA with adjustment for BMI. Linear regression with adjustment for BMI was used to assess independent associations between FFA and lipid dynamics measures. P < 0.05 was considered statistically significant.
RESULTS
Fifteen subjects (seven IR and eight IS) were studied. Each group contained six men, and there were no significant differences in sex or race (Table 1). Despite attempts to match groups, BMI was slightly higher in the IR subgroup (31.3 ± 3.2 vs. 28.5 ± 1.7 kg/m2; P = 0.07). By definition, SSPG was significantly higher in the IR as compared with the IS subgroup (195.3 ± 29.1 vs. 83.9 ± 17.0 mg/dl; P < 0.001). IR subjects demonstrated marginally higher fasting plasma glucose (98.4 ± 5.0 vs. 100.3 ± 9.3 mg/dl; P = 0.06) and significantly higher fasting plasma TG (141 ± 37.2 vs. 86.5 ± 15 mg/dl; P = 0.007) as well as a trend toward greater visceral adipose tissue (VAT) (70.3 ± 25.7 vs. 46.9 ± 23.7 cm3; P = 0.09). Other lipid/lipoprotein measures and blood pressure did not differ significantly between groups.
TABLE 1.
Demographic and clinical characteristics of insulin-resistant and insulin-sensitive moderately obese human subjects
| Characteristic | Insulin Sensitive (n = 8) | Insulin Resistant(n = 7) | a |
| Age (years) | 56.9 ± 7.5 | 54.7 ± 4.2 | 0.53 |
| Sex (M/F) | 6/2 | 6/1 | 0.64 |
| Race (white/non-white) | 7/1 | 5/2 | 0.10 |
| BMI (kg/m2) | 31.3 ± 3.2 | 28.5 ± 1.7 | 0.07 |
| SSPG (mg/dl) | 83.9 ± 17.0 | 195 ± 29.1 | <0.001 |
| Fasting glucose (mg/dl) | 98.4 ± 5.0 | 100.3 ± 9.3 | 0.06 |
| Fasting TG (mg/dl) | 86.5 ± 15.0 | 141 ± 37.2 | 0.007 |
| HDL (mg/dl) | 56.1 ± 10.8 | 48.6 ± 14.4 | 0.28 |
| LDL (mg/dl) | 114.9 ± 16.5 | 116.0 ± 30.6 | 0.93 |
| SAT (cm3) | 113.0 ± 38.3 | 135.3 ± 31.1 | 0.24 |
| VAT (cm3) | 46.9 ± 23.7 | 70.3 ± 25.7 | 0.09 |
| VAT/SAT ratio | 0.43 ± 0.22 | 0.52 ± 0.16 | 0.34 |
| Thigh fat (cm3) | 52.8 ± 21.0 | 44.7 ± 15.3 | 0.40 |
Values are means ± SD. SAT, subcutaneous adipose tissue; SSPG, steady-state plasma glucose; VAT, visceral adipose tissue.
Unpaired Student’s t-test for continuous variables; chi2 test for categorical variables.
Of the 15 subjects, two IR subjects and one IS subject were taking statins, and two IR patients were on antihypertensive medications (atenolol or lisinopril). Dietary surveys and weight checks showed no significant differences in macronutrient composition or change in body weight throughout the study.
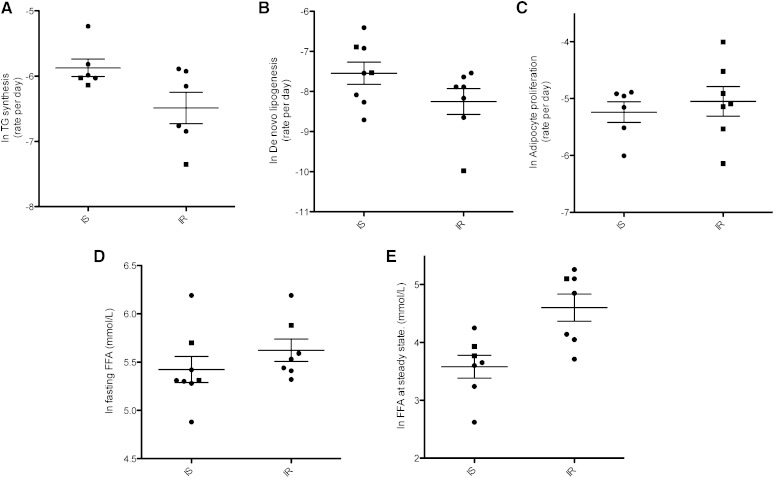
The rate of TG synthesis (Fig. 1A) in subcutaneous abdominal adipocytes was 33% lower in IR as compared with IS subjects (0.17% vs. 0.30%/day; P < 0.001). DNL (Fig. 1B) was 63% lower in IR subjects as compare with IS subjects (0.05 vs. 0.08%/day; P < 0.001). DNL accounted for 23 and 29% of total TG-palmitate synthesis, respectively, in the IR and IS subgroups subgroups. Differences were not statistically significant (P = 0.29). Adipocyte proliferation (0.7 and 0.6%/day, respectively) in the IR and IS subgroups did not differ significantly (P = 0.80) (Fig. 1C). Fasting plasma FFA concentrations in IR compared with IS subjects (Fig. 1D) did not differ significantly (P = 0.11). During steady-state of the insulin suppression test, during which insulin concentrations are the same in all subjects (60 μU/ml), plasma FFA concentrations were 65% higher in the IR subgroup (P = 0.059) (Fig. 1E), consistent with impaired insulin suppression of lipolysis among the IR subgroup, as has been shown previously (8). Possible effects of medications were also considered. Two IR subjects and one IS subject were taking statins, a class of cholesterol-lowering drugs that reduces HMG-CoA reductase, the rate-limiting enzyme for cholesterol synthesis. HMG-CoA does not regulate adipose TG and fatty acid synthesis, so it is unlikely that these medications affected lipid dynamics in our subjects. Two IR patients were on antihypertensive medications (atenolol or lisinopril). Although atenolol has been shown to have lipolysis lowering effects, this was not evident in the FFA levels of the patient taking this medication, who had greater FFA levels compared with the other subjects. Lisinopril has been shown to have a low binding affinity to PPAR-γ and thus has the potential to stimulate lipid uptake. However, lipid synthesis was not greater in this subject compared with those within the group.
Fig. 1.
TG synthesis and DNL in subcutaneous adipose tissue of obese IS and IR patients. A: Rate of TG synthesis per day (k), based on incorporation of 2H into glycerol moieties and labeling time (P < 0.001). B: Rate of de novo lipogenesis per day (k) based on incorporation of 2H into palmitate (P = 0.004). C: Rate of adipocyte proliferation per day (k) (P = 0.80). D: FFAs at fasting (P = 0.11). E: FFAs at steady-state (insulin infused at 60 μU/ml) (P = 0.059). Mean ± SD shown; P values were generated from log-transformed values and adjusted for BMI using ANCOVA analysis. Circles = male subjects; squares = female subjects.
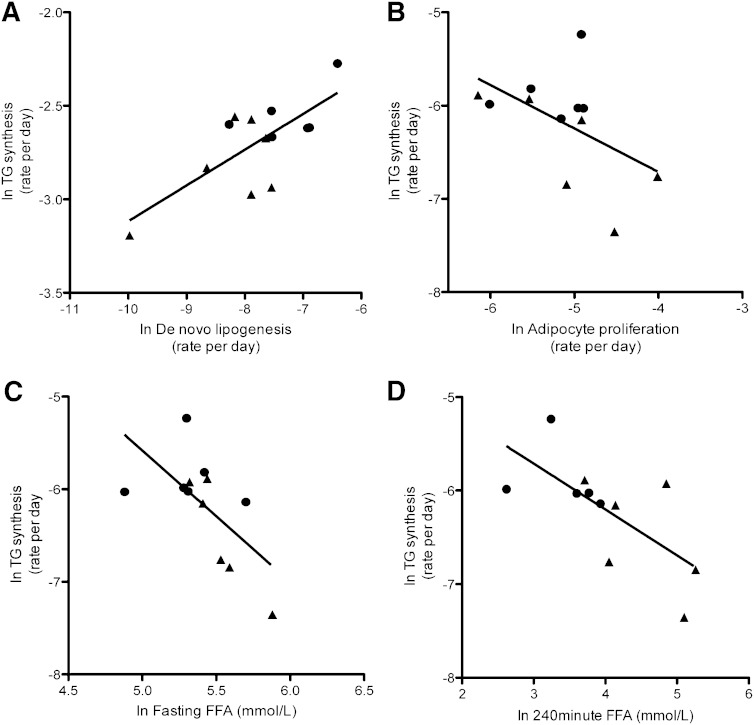
To determine whether adipocyte proliferation, DNL, and insulin suppression of lipolysis contributed to TG synthesis, we measured these relationships, adjusting for BMI. As shown in Fig. 2A, adipocyte DNL correlated significantly with TG synthesis (P = 0.006), but adipocyte proliferation (Fig. 2B) did not (P = 0.14). Plasma FFA, during fasting and steady-state (Fig. 2C and D), correlated inversely with adipocyte TG synthesis (P = 0.04 and 0.06, respectively). Although the latter measure did not quite reach statistical significance, taken together with the whole of FFA data and prior published work, this appears to represent a reportable trend.
Fig. 2.
Relationships between adipocyte lipid and cellular dynamics. A: DNL versus log- transformed TG synthesis (r = 0.80; P = 0.006). B: Adipocyte proliferation versus TG synthesis (r = −0.50; P = 0.14). C: Fasting FFAs versus TG synthesis (r = −0.65; P = 0.04). D) FFA at steady-state during SSPG test versus TG synthesis (r = −0.69; P = 0.06). P values generated from log-transformed values, adjusted for BMI using linear multiple regression analysis. Circles = IS; triangles = IR.
Discussion
Of primary importance in this study is a clear demonstration of reduced TG synthesis in subcutaneous adipose cells of insulin-resistant humans as compared with insulin-sensitive, BMI-matched controls. Unlike previous studies relying on static measures such as adipocyte size and gene expression, this investigation uses a stable isotope to confirm, in vivo, that TG synthesis and DNL are impaired in IR individuals, whereas adipocyte proliferation is not. Specifically, IR individuals exhibited 33% less newly synthesized adipose TG (TG-glycerol) and 60% less DNL (TG-palmitate) compared with BMI-matched IS subjects. Although newly synthesized TG could have undergone lipolysis and thus could have not been reflected in our measurements, lipolysis occurs throughout the stored TG pool, with newly synthesized TG comprising only 8 and 4.7% of total adipose TG in IR and IS, respectively. Therefore, this is unlikely to have altered the results substantially. The observed adipose TG synthesis rate of 0.3% per day in IS obese individuals, which is nearly twice the rate of similarly obese IR individuals, is similar to that observed in healthy lean individuals (6). Thus, this rate may reflect a healthy state regardless of body weight in which the ability to store TG protects the individual from lipotoxicity and insulin resistance. Impaired TG synthesis in subcutaneous fat, as observed in IR individuals, may indicate general dysfunction of adipocytes or specific inability of subcutaneous adipocytes to store TG. These findings, along with decreased DNL, lower insulin suppression of lipolysis, higher fasting FFA concentrations, and a trend toward greater VAT mass, support the notion that relative inability to store TG in the subcutaneous depot might promote insulin resistance via lipotoxicity.
Adipocyte DNL was significantly decreased in the IR versus the IS individuals. Furthermore, DNL correlated significantly with total TG synthesis. Although it is possible that DNL measured in adipose cells may include newly synthesized fatty acids from other sources, such as the liver, it is very unlikely that this is the case here. The relative hepatic DNL contribution to adipose DNL has been previously determined to be minor at a rate of 0.2–0.3% per day (6).
Other researchers have suggested that recruitment of new adipocytes allows for maintenance of lipid uptake, providing protection against obesity-induced insulin resistance (11, 12). Although total TG synthesis retained/stored in the subcutaneous adipose tissue was impaired in IR subjects, adipocyte proliferation was not impaired, nor did adipoycte proliferation correlate with TG synthesis. This suggests that impaired TG storage in weight-stable IR individuals is not a function of disordered adipocyte proliferation but rather of decreased potential of committed adipocytes to synthesize and store TG. Tuvdendorj and colleagues (13) found TG synthesis and adipocyte proliferation to be greater in healthy individuals than in individuals with metabolic syndrome; however, the authors noted that contamination by rapidly turning over stromal vascular cells may have contributed to the observed high adipocyte proliferation rates (6). Our adipocytes were thoroughly washed to avoid such contamination and clearly show that adipocyte proliferation did not differ in IR versus IS subjects.
In the setting of decreased TG storage, one would expect to see decreased evidence of lipolysis in a weight-stable state. We observed the contrary, with IR individuals exhibiting significant elevations in fasting plasma FFAs and decreased insulin suppression of lipolysis during fixed insulin concentrations of 60 μU/ml, which simulate the postprandial state. Although the latter comparison did not reach statistical significance (0.059), two prior studies in larger, women-only cohorts demonstrated this between-group difference to be highly statistically significant (8, 14). We further demonstrated that TG synthesis was inversely correlated with insulin suppression of lipolysis, suggesting that in the IR state, multiple insulin-stimulated pathways are impaired in adipose cells. Indeed, although whole-body lipolysis was not measured under ambient conditions and in the fasted (but not the steady-state, which was experimentally controlled) could have been suppressed by compensatory hyperinsulinimia in the IR subjects, the tendency for greater lipolysis, together with decreased TG synthesis and DNL in IR subjects, is suggestive of a mild form of lipodystrophy in which TG storage is reduced in abdominal subcutaneous fat. Indeed, other researchers have shown that fat deposition in visceral and ectopic depots is associated with whole-body insulin resistance and associated metabolic characteristics, such as hypertriglyceridemia and hepatic gluconeogenesis (15).
The current results were obtained in a largely male and Caucasian sample and might not apply to other races or to female subjects. Furthermore, we sampled only abdominal subcutaneous adipose tissue, so it remains to be seen if the differences observed between IR and IS subjects are limited to a single adipose depot or are more diffuse. Although our study was not powered to detect between group differences in regional fat mass, we have previously shown that, when adjusted for BMI, IR subjects have a greater proportion of visceral to subcutaneous abdominal fat (5), and other researchers have demonstrated greater intrahepatic and intramyocellular fat (16) in those with insulin resistance. Although intramyocellular and intrahepatic fat were not measured in the current study, VAT tended to be higher in the IR cohort in this study. Despite these limitations, our findings lend support to the hypothesis that inadequate TG synthesis in subcutaneous adipose tissue is a potential mechanism underlying relationships between obesity and systemic insulin resistance, possibly through ectopic fat deposition or other mechanisms, such as cellular hypertrophy and cellular stress-mediated inflammation.
In conclusion, the current findings provide direct, novel, in vivo evidence that impaired TG synthesis/storage in abdominal subcutaneous adipose tissue is a feature of insulin resistance in weight-stable, overweight/moderately obese individuals. This is an important extension of limited static and nonhuman data supporting the hypothesis that dysfunctional fat storage in subcutaneous adipose tissue contributes to obesity-associated insulin resistance. Future identification of molecular targets responsible for decreased lipogenesis in subcutaneous adipose cells may lead to new treatments for systemic insulin resistance and prevention of type 2 diabetes.
Acknowledgments
The authors thank Mark Fitch for assistance in preparing sterile and safe 2H2O for all subjects.
Footnotes
Abbreviations:
- DNL
- de novo lipogenesis
- IR
- insulin resistant
- IS
- insulin sensitive
- SSPG
- steady-state plasma glucose
- VAT
- visceral adipose tissue
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK080436 and R01DK071309.
REFERENCES
- 1.McLaughlin T., Sherman A., Tsao P., Gonzalez O., Yee G., Lamdendola C., Reaven G. M., Cushman S. W. 2007. Enhanced proportion of small adipose cells in insulin-resistance vs insulin sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 50: 1707–1715. [DOI] [PubMed] [Google Scholar]
- 2.Gray S. L., Vidal-Puig A. J. 2007. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr. Rev. 65: S7–S12. [DOI] [PubMed] [Google Scholar]
- 3.Kim J. K., Gavrilova O., Chen Y., Reitman M. L., Shulman G. I. 2000. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 275: 8456–8460. [DOI] [PubMed] [Google Scholar]
- 4.Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M., Hofmann S., Schraw T., Durand J., Li H., Li G., et al. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117: 2621–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin T., Lamendola C., Liu A., Abbasi F. 2011. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab. 96: E1756–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strawford A., Antelo F., Christiansen M., Hellerstein M. K. 2004. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am. J. Physiol. Endocrinol. Metab. 286: E577–E588. [DOI] [PubMed] [Google Scholar]
- 7.Turner S. M., Murphy E. J., Neese R. A., Antelo F., Thomas T., Agarwal A., Go C., Hellerstein M. K. 2003. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am. J. Physiol. Endocrinol. Metab. 285: E790–E803. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin T., Yee G., Glassford A., Lamendola C. 2011. Use of a two-stage insulin suppression test to assess the relationship between insulin-suppression of lipolysis and insulin-mediated glucose uptake in overweight/obese, nondiabetic women. Metabolism. 60: 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLaughlin T., Allison G., Abbasi F., Lamendola C., Reaven G. 2004. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 53: 495–499. [DOI] [PubMed] [Google Scholar]
- 10.Rodbell M. 1964. Metabolism of isolated fat cells: Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 239: 375–380. [PubMed] [Google Scholar]
- 11.Gustafson B., Hammarstedt A., Hedjazifar S., Smith U. 2013. Restricted adipogenesis in hypertrophic obesity. Diabetes. 62: 2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Jansson P. A., Nagaev I., Jack M. M., Carvalho E., Sunnerhagan K. S., Cam M. C., Cushman S. W., Smith U. 2004. Evidence of impaired adipogenesis in insulin resistance. Biochem. Biophys. Res. Commun. 317: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 13.Tuvdendorj D., Chandalia M., Batbayar T., Saraf M., Beysen C., Murphy E. J., Abate N. 2013. Altered subcutaneous abdominal adipose tissue lipid synthesis in obese, insulin-resistant humans. Am. J. Physiol. Endocrinol. Metab. 305: E999–E1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magkos F., Fabbrini E., Conte C., Patterson B. W., Klein S. 2012. Relationship between adipose tissue lipolytic activity and skeletal muscle insulin resistance in non-diabetic women. J. Clin. Endocrinol. Metab. 97: E1219–E1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groop L. C., Saloranta C., Shank M., Bonadonna R. C., Ferrannini E., DeFronzo R. A. 1991. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 72: 96–107. [DOI] [PubMed] [Google Scholar]
- 16.Shulman G.I. 2014. Ectopic fat insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med 371: 1131–1141. [DOI] [PubMed] [Google Scholar]