Abstract
Context
The neurokinin B (NKB) receptor, encoded by TACR3, is widely expressed within the central nervous system, including hypothalamic nuclei involved in regulating GnRH release. We have recently reported two mutations in transmembrane segments of the receptor and a missense mutation in NKB in patients with normosmic isolated hypogonadotropic hypogonadism (nIHH).
Patients and Methods
We sequenced the TACR3 gene in a family in which three siblings had nIHH. The novel mutant receptor thus identified was studied in a heterologous expression system using calcium flux as the functional readout.
Results
All affected siblings were homozygous for the His148Leu mutation, in the first extracellular loop of the NKB receptor. The His148Leu mutant receptor exhibited profoundly impaired signaling in response to NKB (EC50 = 3 ± 0.1 nm and >5 μm for wild-type and His148Leu, respectively). The location of the mutation in an extracellular part of the receptor led us also to test whether senktide, a synthetic NKB analog, may retain ability to stimulate the mutant receptor. However, the signaling activity of the His148Leu receptor in response to senktide was also severely impaired (EC50 = 1 ± 1 nm for wild-type and no significant response of His148Leu to 10 μm).
Conclusions
Homozygosity for the TACR3 His148Leu mutation leads to failure of sexual maturation in humans, whereas signaling by the mutant receptor in vitro in response to either NKB or senktide is severely impaired. These observations further strengthen the link between NKB, the NKB receptor, and regulation of human reproductive function.
Full understanding of the regulation of GnRH release, and hence of human reproductive function, has proved elusive despite several decades of investigation (1). Major insights have been gained, however, through elucidation of novel genetic defects causing hypogonadotropic hypogonadism (HH) (2). The genes implicated may be divided into two groups—those whose normal function relates to the ontogeny of the system, and those whose function is concerned instead with regulating GnRH release from anatomically normal GnRH neurons (3).
The clinical hallmark of defects in genes with developmental roles is the presence of abnormalities such as anosmia or hyposmia due to impaired neuronal migration, survival, or branching (KAL1, FGFR1, FGF8, NELF, PROK2, PROK2R, CDH7), although it is now known that milder defects in several of these genes may also produce HH with no olfactory abnormality (4). An interesting aspect of these developmental defects has been the identification of pathogenic mutations in both receptors and their cognate ligands (FGFR1/FGF8 and PROKR2/PROK2) (4). In contrast, defects in genes with roles in regulating GnRH secretion from anatomically normal GnRH neurons only produce HH with neither anosmia nor other developmental abnormalities, known as normosmic isolated HH (nIHH). Those mutations with the most readily explicable pathogenic link to isolated HH lie in the gene encoding the GnRH receptor (GNRHR) (5, 6) or its ligand GnRH (GNRH1, in which mutations were first reported during the writing of this manuscript) (7, 8).
Most informative about the physiological regulation of anatomically normal GnRH neurons have been mutations in the gene encoding the kisspeptin receptor, or KISS1R (formerly GPR54). The establishment in 2003, through a confluence of murine and human genetics, that loss of KISS1R function produces nIHH (9, 10) energized the field, and subsequent studies in humans and model organisms have demonstrated that kisspeptin, acting through KISS1R on GnRH neurons, is a highly conserved regulator of GnRH secretion (11). However, despite the value of the insights gained from human KISS1R mutations, they are an extremely rare cause of nIHH, with only around 19 cases described to date (12).
We have recently added to this body of knowledge by demonstrating loss-of-function mutations in either TAC3, encoding neurokinin B (NKB), or TACR3, encoding its endogenous receptor (the NK3R), associated with isolated HH in eight patients from four families (13). We now report the identification of a third loss-of-function TACR3 mutation in three Kurdish siblings with nIHH, further confirming the critical role for TACR3 in the control of human gonadal function.
Patients and Methods
Patients
Studies were performed with approval of the Ethics Committee of the Cukurova University Faculty of Medicine, Adana, Turkey. Each participant provided written informed consent, and all studies were conducted in accordance with the principles of the Declaration of Helsinki. Karyotypes were normal in all affected patients, and mutations in GNRHR, GNRH1, KISS1R, KISS1, KAL1, NELF, PROK2, PROK2R, FGFR1, and TAC3 were excluded by sequencing. None of the affected siblings had impaired sense of smell, obesity, other pituitary deficiencies, or adrenal failure.
Biochemical assays and endocrine testing
Plasma ACTH, serum FSH, LH, estradiol, dehydroepiandrosterone sulfate, cortisol, and testosterone levels were analyzed by commercial kits based on solid-phase, two-site sequential, or competitive chemiluminescent immunometric assay or electro-chemiluminescence immunoassay.
GnRH stimulation testing was undertaken using a standard protocol, with gonadotropins determined at baseline and after 30, 60, 90, and 120 min following an iv bolus injection of GnRH (2.5 μg/kg, maximum 100 μg). A peak LH of more than 4 U/liter was taken to be indicative of central activation of puberty (14).
Mutational analysis
DNA was extracted from blood leukocytes using standard methods. PCR-amplified exons and splice junctions of TACR3 were sequenced on an ABI PRISM 3130 autosequencer (Applied Biosystems, Foster City, CA). Primer sequences, annealing temperatures, and PCR and sequencing conditions have been described previously (13).
Recreation of mutant TACR3 alleles and functional studies in vitro
TACR3 cDNA in pcDNA3.1+ was purchased from the Missouri S&T cDNA Resource Center (Rolla, MO), and mutagenesis was undertaken using the QuickChange Site Directed Mutagenesis kit (Stratagene, La Jolla, CA). After sequence verification, all constructs were subcloned into pIRES2-AcGFP1 vector (Clontech, Palo Alto, CA) tandemly expressing green fluorescent protein (GFP) from an internal ribosomal entry site downstream from the inserted TACR3 coding sequence. This allowed identification of transfected cells by virtue of GFP expression without the necessity to fuse the TACR3 constructs to GFP, which could potentially impair signaling function. HEK293A cells (Qbiogene, Carlsbad, CA), cultured in DMEM supplemented with 5% fetal calf serum, were transiently transfected with the resulting expression constructs using 5μl of polyethylenimine (Sigma, St. Louis, MO) per microgram of DNA. Transfected cells were seeded into poly-l-lysine-coated glass-bottomed dishes (MatTec, Loveland, OH) 16 h later. At 24 h, cells were loaded with Fura2-AM for 30 min. We performed experiments on an inverted fluorescence microscope (Eclipse TE2000; Nikon, Melville, NY) with a ×40 oil-immersion objective. Fura2 was excited at 340, 360, and 380 nm, and GFP at 475 nm, using a 75 W xenon arc lamp and a monochromator (Cairn Research, Kent, UK) and MetaFluor software (Molecular Devices, Sunnyvale, CA). Emission, filtered at 510/80 and 535/25 for Fura2 and GFP, respectively, was recorded with a QuantEM CCD camera (Photometrics; Roper Scientific, Tucson, AZ). Solutions of NKB (Sigma) and senktide (Tocris Bioscience, Ellisville, MO) were prepared on the day of experimentation in standard saline containing: 138 mm NaCl, 4.5 mm KCl, 4.2 mm NaHCO3, 1.2 mm NaH2PO4, 2.6 mm CaCl2, 1.2 mm MgCl2, 10 mm HEPES, and 10 mm glucose. A stock solution of 5 mm Fura2-AM (Invitrogen, San Diego, CA) was prepared in dimethylsulfoxide and diluted in standard saline to give a working concentration of 5 μm.
NKB or senktide was perfused in standard saline at approximately 1 ml/min with a chamber volume of approximately 0.2 ml. Cells were excited at 340 and 380 nm every 2 sec. Free Ca2+ concentrations for individual cells were calculated from background-subtracted fluorescence ratios assuming a dissociation constant of 224 nm. Minimum and maximum signals were recorded in the presence of 5 μm ionomycin, in 5 mm EGTA plus 0 mm Ca2+, and in 5 mm Ca2+, respectively, at the end of the experiment. Peak [Ca2+]i from at least five experiments per construct was averaged over 30 sec, and the resulting dose response data fitted using the logistic function [Ca2+]i = (A1-A2)/(1+([agonist]/EC50))+A2 and Origin software (Microcal, Northampton, MA).
To assess expression of the mutant TACR3 compared with wild-type, enhanced GFP fusions of wild-type or mutant TACR3 were generated by subcloning in frame from pIRES2-AcGFP1 into pEGFP-C1 (Clontech) with sequence verification. BOSC cells grown on poly-l-lysine-coated microscope slides in DMEM supplemented with 10% fetal bovine serum and 4 mm glutamine were transiently transfected using FuGENE 6 (Roche, Indianapolis, IN) before fixing 48 h posttransfection with 4% formaldehyde (Sigma) in PBS for 20 min and mounting in ProLong Antifade Reagent with DAPI (Molecular Probes, Eugene, OR). Images were obtained on a Zeiss LSM 510 META confocal microscope (Carl Zeiss MicroImaging, Oberkochen, Germany).
Results
Clinical description
The proband was a 14.5-yr-old Kurdish boy with no clinical evidence of puberty: his testes were 1 ml, and his phallus was 4.4 cm long and 0.6 cm in diameter. He was born at term with normal birthweight after an uncomplicated pregnancy, although microphallus was noted. He had unilateral cryptorchidism, which resolved spontaneously at the age of 2. His medical history was otherwise unremarkable. His height was 168 cm [midparental target height (MPH), 166.5 cm], his upper/lower segment ratio was 1.0, and his body mass index was 22.5 kg/m2. His bone age was 13 yr. Endocrine evaluation revealed HH (Table 1). After 1 yr of testosterone treatment, therapy was temporarily withdrawn, and evaluation off treatment (FSH, 0.45 U/liter; LH, <0.1 U/liter; total testosterone, 3 ng/dl) indicated persisting HH.
TABLE 1.
Biochemical profiles of affected family members
| Family member | II-2 (♀) | II-3 (♂) | II-5 (♂) | Normal range |
|---|---|---|---|---|
| Age (yr) | 26 | 25 | 14.5 | |
| FSH (mIU/ml) (U/liter) | 0.9 (0.9) | 5.3 (5.3) | 0.7 (0.7) | M, 1.4–18.1 (1.4–18.1); F, 2.5–10.2 (2.5–10.2) |
| LH (mIU/ml) (U/liter) | 0.1 (0.1) | 2.7 (2.7) | 0.1 (2.7) | M, 1.5–9.3 (1.5–9.3); F, 1.9–12.5 (1.9–12.5) |
| Estradiol (ng/dl) (pmol/liter) | 0.6 (22) | ND | ND | M, 0.8–3.5 (26–128); F, 6.3–16.5 (231–605) |
| Testosterone (ng/dl) (nmol/liter) | ND | 72.0 (2.4)a | 2.0 (0.06) | M, 350–1030 (11.9–35); F, 14–76 (0.47–2.58) |
| GnRH stimulation testb | ||||
| Maximum FSH (mIU/ml) (U/liter) | 11.0 (11.0) | ND | 2.9 (2.9) | |
| Maximum LH (mIU/ml) (U/liter) | 4.0 (4.0) | ND | 0.5 (0.5) | |
| Prolactin (pg/ml) (nmol/liter) | 5.5 (0.2) | 9.4 (0.4) | 5.0 (0.2) | M, 2.1–17.7 (0.08–0.74); F, 2.8–29.2 (0.11–1.22) |
| TSH (mIU/liter) (mIU/liter) | 0.74 (0.74) | 1.5 (1.5) | 2.2 (2.2) | 0.35–4.2 (0.35–4.2) |
| Free T4 (ng/dl) (pmol/liter) | 1.1 (14) | 1.3 (16.7) | 1.6 (20.6) | 0.89–1.8 (11.5–148.3) |
| Cortisol (μg/dl) (nmol/liter) | 8 (224) | 10.7 (300) | 12.5 (350) | 3–25 (85–700) |
| IGF-I (ng/ml) (μg/liter) | ND | ND | 175 (175) | M, 155–432 (155–432); F, 87–368 (87–368) |
Conversion factors to SI units are: × 1 U/liter, for FSH and LH; × 36.71 pmol/liter, for estradiol; ×0.0346 nmol/liter, for testosterone; ×0.0426 nmol/liter, for prolactin; ×1 mIU/liter, for TSH; ×12.9 pmol/liter, for free T4; ×27.59 nmol/liter, for cortisol; and ×1 μg/liter, for IGF-I. SI units are shown in italics. M, Male; F, female; ND, not determined.
On testosterone enanthate injection.
Ref. 6.
The 26-yr-old sister of the proband had primary amenorrhea and absent breast development. She was born at term with normal birthweight after an uncomplicated pregnancy. No problems were apparent until she was 16 yr old, when she sought medical attention for failure of breast development and primary amenorrhea. Breasts and pubic and axillary hair were at Tanner stage 1, and physical examination was otherwise normal. Her height was 149 cm (MPH, 153.5 cm), with an upper to lower segment ratio of 1.0 and bone age 14 yr. Pelvic ultrasonography revealed hypoplastic ovaries and uterus. Gonadotropin and estradiol levels indicated HH at presentation (Table 1). She was then given estrogen and progesterone replacement. Three years later, FSH, LH, and estradiol levels were 5.7 mIU/ml, 1.2 mIU/ml, and 0.1 ng/dl off treatment, indicating persistent HH.
The third affected sibling was their 25-yr-old brother, who had a microphallus noted in early infancy and who subsequently failed to develop any signs of puberty. His medical history was otherwise unremarkable. He had no history of cryptorchidism. He was 173 cm tall (MPH, 166.5 cm), with an upper to lower segment ratio of 1.0. Endocrine investigation confirmed HH (Table 1). At the age of 17, testosterone and human chorionic gonadotropin replacement was commenced, resulting in appearance of male characteristics, testicular (to 4 ml) and penile (to 8 cm) growth.
All three affected siblings were of normal intelligence, with no hypo/anosmia, dysmorphic facial features, midline anomalies, retinitis pigmentosa, iris colobomata, hearing loss, hypotonia, ataxia, dementia, or polyneuropathy. They had otherwise normal anterior pituitary function and normal findings on magnetic resonance imaging of the brain.
Their parents are not known to be related. Their mother experienced menarche at age 12.5, and their father started shaving at age 14. Their 29-yr-old sister experienced menarche at 13 yr of age with a regular cycle subsequently, and one 20-yr-old brother had normal adult male physical characteristics.
Mutational analysis
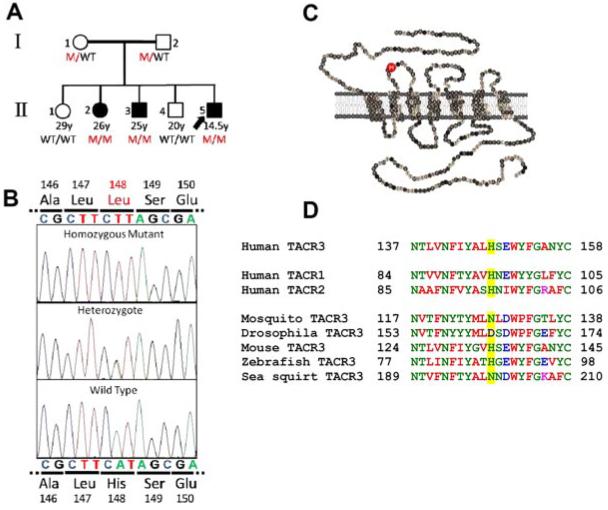
All three affected individuals were found to be homozygous for an A to T transversion at cDNA nucleotide 442 of TACR3. Both parents were heterozygous for the mutation, and both unaffected siblings carried homozygous wild-type alleles (Fig. 1A). This variant was not detected on sequencing of 117 Kurdish control individuals. The novel mutation leads to substitution of histidine 148 of the mature NKB receptor for leucine (His148Leu) (Fig. 1B). His148 is located in the first extracellular loop of the G protein-coupled receptor (Fig. 1C), and it is conserved among the three human tachykinin receptors, and in many, but not all, TACR3 orthologs and paralogs (Fig. 1D).
FIG. 1.
Identification of a TACR3 mutation in affected patients. A, Pedigree of the family with nIHH due to TACR3 (H148L) mutation. The index case is indicated by the arrow; solid black symbols depict affected individuals. M, TACR3 His148Leu; B, Nucleotide sequence analysis of genomic DNA from a healthy control, heterozygous and affected family members with the novel missense TACR3 His148Leu mutation. C, Schematic of the human NK3R with the mutated histidine residue indicated in the red circle. D, Evolutionary conservation of His148 (yellow highlighting).
Characterization of mutant TACR3 function
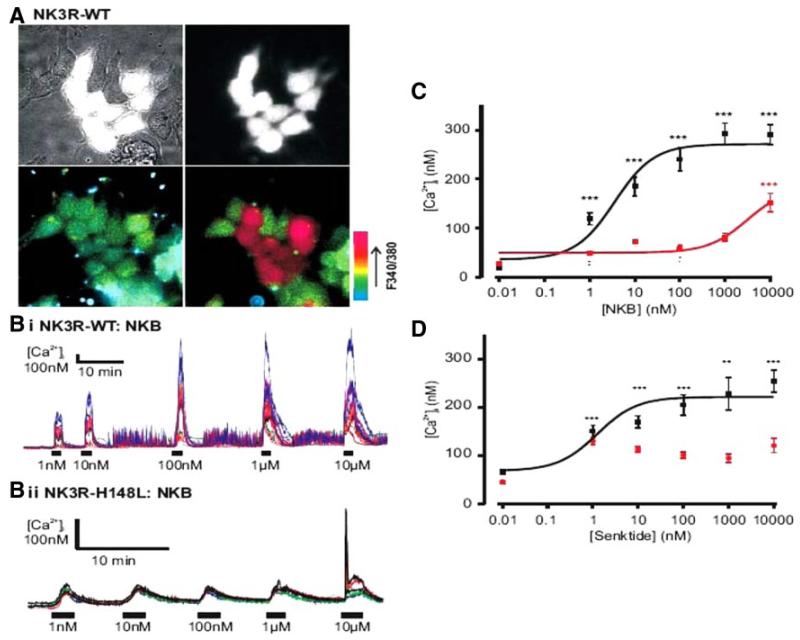
In HEK293 cells heterologously expressing wild-type TACR3, NKB evoked a dramatic increase in intracellular calcium, with an EC50 of 3 nm, whereas no significant response to NKB was seen in untransfected cells (Fig. 2A). The His148Leu mutant receptor showed markedly attenuated ability to respond to stimulation, with only 10μm NKB eliciting a response significantly above baseline, suggesting an EC50 for NKB of 5 μm or higher (Fig. 2, B and C). Because of the location of the mutation in an extracellular loop of the receptor, we speculated that the primary defect may lie in ligand binding, and therefore that NKB analogs of different structure may potentially still be able to elicit a response from the mutant. First, we confirmed qualitatively normal expression of GFP-tagged wild-type and mutant receptors, with no gross evidence of protein degradation or intra-endoplasmic reticulum retention of the mutant (Supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). We then tested the ability of senktide (suc-[Asp6,MePhe8]SP), a modified hexapeptide NKB analog, to activate the mutant receptor compared with wild-type NKB. However, although senktide elicited an increased intracellular calcium response in cells transfected with wild-type TACR3 with an EC50 of 1 nm, it failed to produce a significant increase in intracellular calcium in cells transfected with the mutant receptor even at 10μm (Fig. 2D). Thus, the His148Leu mutant NKB receptor found in homozygous form in all three affected siblings of the family studied exhibits dramatically impaired receptor signaling in response to both NKB and senktide.
FIG. 2.
NK3R-H148L mutated receptor results in loss of function. A, Brightfield (top left) and GFP-fluorescent cells only (top right) identifying TACR3 transfected cells. Bottom left and right images depict pseudocolored representations of the 340/380 nm ratio before and after application of 10 μm NKB, respectively. B, Representative [Ca2+]i traces of WT (i) and H148L TACR3 (ii) transfected cells in response to increasing concentrations of NKB. C, NKB dose response of peak [Ca2+]i for WT (black, n = 41–53) and H148L TACR3 (red, n = 71–76). The resulting logistic fit parameters for WT were A1 = 36 ± 17, A2 = 271 ± 11, and EC50 = 3 ± 0.1 nm. NKB only induced a significant increase in [Ca2+]i at 10 μm in the H48L-mutant receptor. D, Senktide dose response of peak [Ca2+]i for WT-TACR3 (black, n = 50-57). The resulting logistic fit parameters were: A1 = 68 ± 20, A2 = 221 ± 11, and EC50 = 1 ± 1 nm. Responses to senktide on the H148L-mutant TACR3 (red, n = 50–90) were not statistically significant from nontransfected cells. A minimum of five experiments were performed for each construct and agonist. **, P < 0.01; ***, P < 0.001, compared with nontransfected cells. Error bars represent sem values.
Discussion
This report describes three further related individuals with nIHH associated with homozygosity for a loss-of-function missense mutation—the novel His148Leu variant—in the first extracellular loop of the neurokinin 3 receptor (NK3R). In conjunction with our recent report of mutations in either TACR3 or TAC3, encoding the endogenous NK3R ligand NKB (13), this brings the total number of patients described to date with nIHH and genetic defects in the NKB/NK3R system to 11, spread across five of 10 multiplex families recruited for study in Turkey. However, none of 50 sporadic cases of nIHH harbored mutations in these genes(13). The identification of three different receptor mutations and one ligand mutation suggests that this does not reflect a founder effect in this geographical region.
Both of the mutations we have reported previously in TACR3 were localized in transmembrane segments of the receptor (13). In contrast, His148 lies in the first extra-cellular loop of the protein. Although small ligands such as catecholamines bind to G protein-coupled receptors at solute-isolated pockets within the transmembrane (TM) helices, binding of peptides usually involves epitopes on extracellular loops (EL) and the N terminus. In the case of the neurokinin receptor family, studies of NK1R-NK3R chimeras (15) revealed that the higher affinity of the NK3R for NKB can be transferred to the NK1R by swapping only the region C terminal to the third EL. In the same study, a different domain, encompassing TM II–IV, was identified as a critical determinant of senktide affinity. It was suggested that this reflected a bigger binding site for the decapeptide NKB, whereas the smaller hexapeptide, senktide, interacted with fewer points on the receptor. Taking this argument further, we speculated that the His148Leu mutation in EL1 might differentially affect binding of these two NK3R agonists. However, both NKB and senktide exhibited reduced potency at the mutant receptor, in keeping with other studies identifying residues in EL2 and TM2 of the NK1R that were critical for binding of the conserved C terminus of all tachykinin peptide agonists (16, 17). Two caveats do apply to these observations, however: although wild-type and His148Leu mutant receptor were expressed qualitatively normally, normal trafficking of the mutant receptor to the cell surface has not been proved, and even if trafficking is entirely normal, it remains formally possible that it is signal transduction that is specifically impaired rather than ligand binding per se in the mutant.
The current study further emphasizes that the dominant role of the NK3R in vivo in humans is in the regulation of reproductive function. However, the mechanism at play remains unclear. The NK3R is expressed in both central and peripheral tissues of the hypothalamo-pituitary-gonadal (HP1G) axis including ovaries, uterus, and hypothalamus (18), but study to date has focused on the hypothalamus. NKB and GnRH axons are closely apposed within the median eminence, and NK3Rs have been identified on GnRH axons (19). A neuroanatomical case has been made that NKB within the arcuate nucleus may mediate negative feedback by sex steroids together with kisspeptin (20). However, whether loss of this inferred role accounts for the phenotype of patients with genetic loss of NKB/NK3R function awaits further study, and indeed in humans NKB expression is detectable in many brain areas other than the hypothalamus (21), whereas in the hypothalamus the highest expression has been reported in the paraventricular nucleus (22).
The HPG endocrine axis in humans is fully active for the first few months of life before entering a state of quiescence until puberty (1) due to centrally determined suppression of GnRH release (23, 24). How this is exerted and how it is relieved at puberty remains among the great un-answered questions in human endocrinology. Recent attention has focused on a possible role for up-regulation of kisspeptin expression in peripubertal HPG reactivation in primates (25). However, kisspeptin is also able robustly to stimulate GnRH secretion in rodents (26, 27), which do not seem to exhibit a centrally determined juvenile pause (28-30). In contrast, Tacr3 knockout mice are fertile (Ref. 31, and Dr. Jeffrey Stock, Pfizer, personal communication), at odds with the profound nIHH seen in all humans to date with homozygous TACR3 mutations. The concordance between the primate specificity of the juvenile pause and the likely primate-specific reproductive consequences of loss of TACR3 function raises the intriguing possibility that NKB/NK3R may play a role instead of, or as well as, kisspeptin in prepubertal HPG suppression; however, further studies are required to address this.
In conclusion, we report a novel extracellular loss-of function mutation in the TACR3 gene associated with failure of initiation of sexual maturation and pubertal development in three related patients. This reinforces the importance of the NKB/NK3R system in normal human reproductive function and suggests a specific target for future therapy in affected patients.
Supplementary Material
Acknowledgments
This work was supported by grants to A.K.T. from the Scientific and Technological Research Council of Turkey (TÜBİTAK); from the Support Program for Scientific and Technological Research Projects (1001), project 106S276; and from the Cukurova University Scientific Research Projects Support Unit (Grant TF-2006-BAP20). T.G. received grants from the European Molecular Biology Organization and the European Society for Paediatric Endocrinology within the framework of the Visiting Research Fellowship program. The study was also supported by the Wellcome Trust[IntermediateClinicalFellowship080952/Z/06/Z (to R.K.S.); Senior Research Fellowship 084210/Z/07/Z (to F.R.); and Senior Research Fellowship 071187 (to F.M.G.)], the UK National Institute for Health Research Cambridge Biomedical Research Centre, and the UK Medical Research Council Centre for Obesity and Related Metabolic Diseases. G.T. is funded by a Lister Research Prize to F.M.G.
Abbreviations
- EL
Extracellular loop
- GFP
green fluorescent protein
- HH
hypogonadotropic hypogonadism
- HPG
hypothalamo-pituitary-gonadal
- MPH
midparental target height
- nIHH
normosmic isolated HH
- NKB
neurokinin B
- NK3R
neurokinin 3 receptor
- TM
transmembrane
Footnotes
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Plant TM. Hypothalamic control of the pituitary-gonadal axis in higher primates: key advances over the last two decades. J Neuroendocrinol. 2008;20:719–726. doi: 10.1111/j.1365-2826.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 2.Crowley WF, Jr, Pitteloud N, Seminara S. New genes controlling human reproduction and how you find them. Trans Am Clin Climatol Assoc. 2008;119:29–37. discussion 37-38. [PMC free article] [PubMed] [Google Scholar]
- 3.Layman LC. Hypogonadotropic hypogonadism. Endocrinol Metab Clin N Am. 2007;36:283–296. doi: 10.1016/j.ecl.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Hardelin JP, Dodé C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev. 2008;2:181–193. doi: 10.1159/000152034. [DOI] [PubMed] [Google Scholar]
- 5.de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- 6.Layman LC, Cohen DP, Jin M, Xie J, Li Z, Reindollar RH, Bolbolan S, Bick DP, Sherins RR, Duck LW, Musgrove LC, Sellers JC, Neill JD. Mutations in gonadotropin-releasing hormone receptor gene cause hypogonadotropic hypogonadism. Nat Genet. 1998;18:14–15. doi: 10.1038/ng0198-14. [DOI] [PubMed] [Google Scholar]
- 7.Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombès M, Millar RP, Guiochon-Mantel A, Young J. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med. 2009;360:2742–2748. doi: 10.1056/NEJMoa0900136. [DOI] [PubMed] [Google Scholar]
- 8.Chan YM, de Guillebon A, Lang-Muritano M, Plummer L, Cerrato F, Tsiaras S, Gaspert A, Lavoie HB, Wu CH, Crowley WF, Jr, Amory JK, Pitteloud N, Seminara SB. GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2009;106:11703–11708. doi: 10.1073/pnas.0903449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 11.Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- 12.Chan YM, Broder-Fingert S, Seminara SB. Reproductive functions of kisspeptin and Gpr54 across the life cycle of mice and men. Peptides. 2009;30:42–48. doi: 10.1016/j.peptides.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfield RL. Menstrual disorders and hyperandrogenism in adolescence. In: Radovick S, MacGillivray MH, editors. Pediatric endocrinology: a practical clinical guide. Humana Press; Totowa, NJ: 2003. pp. 451–478. [Google Scholar]
- 15.Gether U, Johansen TE, Schwartz TW. Chimeric NK1 (substance P)/NK3 (neurokinin B) receptors. Identification of domains determining the binding specificity of tachykinin agonists. J Biol Chem. 1993;268:7893–7898. [PubMed] [Google Scholar]
- 16.Fong TM, Huang RR, Strader CD. Localization of agonist and antagonist binding domains of the human neurokinin-1 receptor. J Biol Chem. 1992;267:25664–25667. [PubMed] [Google Scholar]
- 17.Huang RR, Yu H, Strader CD, Fong TM. Interaction of substance P with the second and seventh transmembrane domains of the neurokinin-1 receptor. Biochemistry. 1994;33:3007–3013. doi: 10.1021/bi00176a033. [DOI] [PubMed] [Google Scholar]
- 18.Patak E, Candenas ML, Pennefather JN, Ziccone S, Lilley A, Martín JD, Flores C, Mantecón AG, Story ME, Pinto FM. Tachykinins and tachykinin receptors in human uterus. Br J Pharmacol. 2003;139:523–532. doi: 10.1038/sj.bjp.0705279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chawla MK, Gutierrez GM, Young WS, 3rd, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1997;384:429–442. doi: 10.1002/(sici)1096-9861(19970804)384:3<429::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Koutcherov Y, Ashwell KW, Paxinos G. The distribution of the neurokinin B receptor in the human and rat hypothalamus. Neuroreport. 2000;11:3127–3131. doi: 10.1097/00001756-200009280-00018. [DOI] [PubMed] [Google Scholar]
- 23.Conte FA, Grumbach MM, Kaplan SL, Reiter EO. Correlation of luteinizing hormone-releasing factor-induced luteinizing hormone and follicle-stimulating hormone release from infancy to 19 years with the changing pattern of gonadotropin secretion in agonadal patients: relation to the restraint of puberty. J Clin Endocrinol Metab. 1980;50:163–168. doi: 10.1210/jcem-50-1-163. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson LE, Bhattacharya AN, Monroe SE, Dierschke DJ, Knobil E. Effects of gonadectomy on plasma LH concentration in the rhesus monkey. Endocrinology. 1970;87:847–849. doi: 10.1210/endo-87-5-847. [DOI] [PubMed] [Google Scholar]
- 25.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 27.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 28.Fraser MO, Plant TM. Further studies on the role of the gonads in determining the ontogeny of gonadotropin secretion in the guinea pig (Cavia porcelus) Endocrinology. 1989;125:906–911. doi: 10.1210/endo-125-2-906. [DOI] [PubMed] [Google Scholar]
- 29.Goldman BD, Grazia YR, Kamberi IA, Porter JC. Serum gonadotropin concentrations in intact and castrated neonatal rats. Endocrinology. 1971;88:771–776. doi: 10.1210/endo-88-3-771. [DOI] [PubMed] [Google Scholar]
- 30.Gupta D, Rager K, Zarzycki J, Eichner M. Levels of luteinizing hormone, follicle-stimulating hormone, testosterone and dihydrotestosterone in the circulation of sexually maturing intact male rats and after orchidectomy and experimental bilateral cryptorchidism. J Endocrinol. 1975;66:183–193. doi: 10.1677/joe.0.0660183. [DOI] [PubMed] [Google Scholar]
- 31.Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW. Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacol Res. 2004;50:611–615. doi: 10.1016/j.phrs.2004.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.