Abstract
Acrolein, a highly reactive unsaturated aldehyde, is a ubiquitous environmental pollutant and its potential as a serious environmental health threat is beginning to be recognized. Humans are exposed to acrolein per oral (food and water), respiratory (cigarette smoke, automobile exhaust, and biocide use) and dermal routes, in addition to endogenous generation (metabolism and lipid peroxidation). Acrolein has been suggested to play a role in several disease states including spinal cord injury, multiple sclerosis, Alzheimer’s disease, cardiovascular disease, diabetes mellitus, and neuro-, hepato-, and nephro-toxicity. On the cellular level, acrolein exposure has diverse toxic effects, including DNA and protein adduction, oxidative stress, mitochondrial disruption, membrane damage, endoplasmic reticulum stress, and immune dysfunction. This review addresses our current understanding of each pathogenic mechanism of acrolein toxicity, with emphasis on the known and anticipated contribution to clinical disease, and potential therapies.
Keywords: antioxidants, apoptosis, DNA adducts, inflammation, exposure, environmental, oxidative injury
Acrolein (CAS No 107-02-8) is listed by the Department of Homeland Security (DHS), Agency for Toxic Substances and Disease Registry (ATSDR), and Environmental Protection Agency (EPA) as a high priority toxic chemical (Toxicological Profile For Acrolein, 2007), with recommendations on acrolein exposure levels in the environment, food, and water. Acrolein is an industrial chemical and a biocide, and over 500 million pounds of acrolein are produced annually in the United States (Toxicological Profile For Acrolein, 2007). The past decade has witnessed a dramatic increase in research regarding acrolein, and this review attempts to assimilate this growing literature with a focus on the molecular mechanisms of acrolein toxicity pertaining to human disease. Although some early literature has been cited, this review is focused on more recent literature. The theme of this review is that there are multiple mechanisms involved in acrolein toxicity. In our extensive appraisal of the current literature, our goal was not only to characterize these various mechanisms, but also to identify an overarching pathway through which acrolein toxicity may be initiated and propagated. Our analysis suggests that such a common unifying pathway might not exist or is not currently discernable, placing even more onus on the need to further investigate acrolein toxicity in various disease conditions. This comprehensive review starts with succinct introductory comments on the generation, sources, and metabolism of acrolein, followed by a detailed discussion on the various mechanisms of acrolein toxicity, which include protein adduction, oxidative stress, mitochondrial dysfunction, DNA adduction, endoplasmic reticulum (ER) stress, inflammation and immune alterations, structural and membrane effects, and dysregulated signal transduction.
ACROLEIN SOURCES AND METABOLISM
Sources
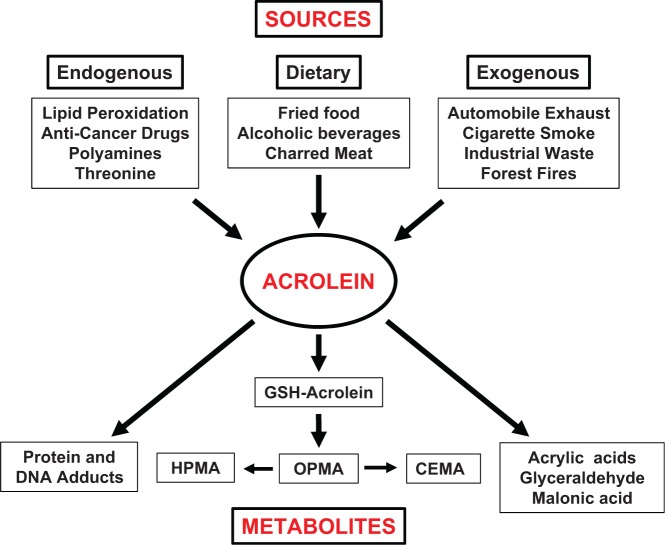
Acrolein is a common dietary and environmental pollutant and is also generated by cellular metabolism (Figure 1). It is an α,β-unsaturated aldehyde released during the combustion of petroleum fuels, biodiesel, plastic, paper and wood, and is a major component of tobacco smoke; such exposures are directly relevant in dermal and respiratory toxicity. Firefighters, acrolein industry workers, persons inhabiting densely polluted cities, and cigarette and hookah smokers (Kassem et al., 2014) face substantial exposures (reviewed in Cahill (2014); Stevens and Maier (2008)). Acrolein has been identified as a health hazard commonly found at significant levels in most homes and schools in the United States (Logue et al., 2012). Acrolein can be generated from overheated vegetable and animal fats; hence, cooked, fried, and charred foods are notable sources of acrolein, as are beer, wine, rum, and breads; the best current estimate for Tolerable Daily Intake (TDI) for acrolein is 7.5 µg/kg body weight (reviewed in Abraham et al. (2011)). Moreover, acrolein exposure from consumption of foods may be considerably underestimated (Watzek et al., 2012). Exposure to reactive unsaturated aldehydes like acrolein by dietary consumption is estimated at nearly 5 mg/kg of food (Wang et al., 2008). The gastrointestinal (GI) system is likely affected the most by ingested acrolein. Notably, acrolein can also be generated endogenously during cellular metabolism by (1) degradation of threonine by neutrophil-derived myeloperoxidase; (2) amine oxidase-mediated catabolism of polyamines such as spermine and spermidine; (3) metabolism of cancer drugs such as cyclophosphamide; and (4) lipid peroxidation (LPO) of polyunsaturated fatty acids (PUFAs) (Stevens and Maier, 2008; Uchida et al., 1998a,b). The levels of acrolein formed by metabolism are difficult to quantify, and may reach very high levels in certain cellular microenvironments; hence, a determination of adverse effects is challenging. Importantly, both acute and chronic acrolein exposures are described to have significant adverse effects (Centers for Disease and Prevention, 2013).
FIG. 1.
The sources and metabolic fate of acrolein. GSH: glutathione; CEMA: 2-carboxyethylmercapturic acid; HPMA: 3-hydroxypropylmercapturic acid; OPMA: S-(3-oxopropyl)-N-acetylcysteine.
Metabolism
Acrolein is highly water soluble and can rapidly enter bodily tissues. It is known to rapidly form conjugates with cellular glutathione (GSH), cysteine, N-acetylcysteine (NAC), and/or thioredoxin. Acrolein-GSH conjugation can occur without a catalyst or may be mediated by glutathione-S-transferases; these acrolein-GSH conjugates may be metabolized by enzymatic cleavage of γ-glutamic acid and glycine residues. Further metabolism via aldehyde dehydrogenase results in 2-carboxyethylmercapturic acid (CEMA), or catalyzed by aldo-keto reductase results in S-(3-hydroxypropyl)-N-acetylcysteine (3-HPMA), the main urinary metabolite of acrolein. Acrylic acid and glycidaldehyde are alternate acrolein by-products, should oxidation and epoxidation precede its conjugation with GSH; glycidaldehyde may represent a human health risk as it has carcinogenic properties in mice and rats when applied dermally. Acrolein may be also converted to malonic acid by aldehyde dehydrogenase. Finally, acrolein may form irreversible adducts with cellular nucleophiles, a metabolic reaction that may be the basis of several of its toxic effects (Figure 1) (reviewed in Stevens and Maier (2008)).
MECHANISMS OF TOXICITY
An interesting feature of acrolein is the diversity with which it exerts its toxic effects in different tissues and organs. Both disease- and organ-specific, as well as shared mechanisms have distinct value in the search for therapeutic targets; the former enables specific, targeted therapy, whereas the latter facilitates the broad management of acrolein toxicity. In the following sections, the various direct mechanisms of acrolein toxicity such as protein and DNA adduction, and indirect mechanisms including induction of oxidative, mitochondrial, and ER stress, are critically discussed with possible connection to affected organs and pathological conditions. Wherever known or tested, the effectiveness of acrolein scavengers and other compounds in mitigating acrolein toxicity has also been described (Table 1).
TABLE 1.
Acrolein Toxicity and Protective Agents
| Mechanisms of Acrolein Toxicity | Protective Agents |
|---|---|
| Protein adduction | Hydralazine |
| Oxidative stress | NAC, Hydralazine, MESNA, Lipoic acid, Hydroxytyrosol, Vitamin E, Diallyl disulfide, Rapamycin, L-Cysteine, Penicillamine, Aminoguanidine, Hydroxylamine, Sodium Borohydride, Sodium Bisulfite, Ascorbic acid, Trolox |
| Mitochondrial dysfunction | NAC, Polyethylene glycol-catalase, SOD mimetic-MnTBAP, Pifithrin- p53 inhibitor |
| Inflammation and Immune Alterations | NAC, MESNA, U0126- AKT inhibitor, FR180204 - ERK1/2 inhibitor, Tetramethylthiourea |
| ER stress | NAC, Caspase inhibitor, Phenylbutyric acid, JNK inhibitor |
| Structural and membrane effects | NAC, Hydralazine |
| Deregulated signal transduction | NAC, Rottlerin- PKC-δ inhibitor, JNK inhibitor, Simvastatin, Rosiglitazone, Sildenafil, NBHA (N-benzylhydroxylamine), SIS3- SMAD3 inhibitor |
Protein Adduction
Being a strong electrophile, acrolein shows high reactivity with cellular nucleophiles such as proteins, DNA and RNA. Acrolein readily targets the sulfhydryl group of cysteine, the imidazole group of histidine and the amino group of lysine, to form mainly Michael addition-type adducts or Schiff base cross-links (reviewed by Pizzimenti et al. (2013); Zarkovic et al. (2013)). These susceptible amino acid residues have major physiological importance and are involved in cellular processes including enzyme catalysis, redox signaling, reactive oxygen species (ROS) sensing, and cellular buffering; hence, adduction by acrolein may lead to significant alterations in protein function. Also, acrolein can interfere with its own metabolism by inhibiting two enzymes that metabolize acrolein-GSH conjugates, namely, alcohol dehydrogenase and aldehyde dehydrogenase. Furthermore, acrolein may augment the effects of other known targets via irreversible adduction and inactivation of arylamine N-acetyltransferases, a major family of xenobiotic-metabolizing enzymes involved in metabolizing many drugs and pollutants (Bui et al., 2013).
Acrolein protein adducts are assessed primarily by antibody-based techniques, high-performance liquid chromatography (HPLC), and mass spectrometry, and such adducts have been demonstrated in vitro and in vivo in several cell types and tissues (Aldini et al., 2011). Alterations in protein function caused by acrolein adduction have been demonstrated in several studies, albeit primarily in vitro. In 1998, Uchida et al. were the first to show that an acrolein-lysine adduct (FDP-Lys) was involved in the oxidation of human low-density lipoprotein (LDL); they also demonstrated the presence of specific antibodies against this adduct in vivo (Uchida et al., 1998b). Prabhu’s group showed that direct exposure to acrolein (as low as 0.01 µM) induced myofilament dysfunction in vitro and in vivo (1 mg/kg/day intravenous) in mice (Ismahil et al., 2011), with accumulation of acrolein-protein adducts and protein carbonyls within the myocardium. Burcham et al. showed in mouse hepatocytes that exposure to allyl alcohol and its metabolite acrolein led to protein carbonylation, which correlated with hepatocyte death. They later showed that the acrolein scavenger hydralazine effectively trapped acrolein adducts in vivo to reduce allyl alcohol-mediated hepatotoxicity in mice, and that mobilization of the chaperone protein HSP90 was protective against acrolein-induced carbonylation (Burcham et al., 2012). Acrolein was suggested to play a role in liver injury in a murine model of early alcoholic liver disease and two novel in vivo protein targets for acrolein were identified: neutral alpha-glucosidase AB and nesprin-1 (Galligan et al., 2012). Treatment of recombinant rat apoE with a 10-fold molar excess of acrolein resulted in an impairment in cholesterol efflux abilities, and a significant decrease in lipid-binding and LDLr- and heparin-binding capabilities, and overall stability of the protein (Tran et al., 2014). Recently, it was demonstrated that salivary lactate dehydrogenase (LDH) activity was reduced by cigarette smoke (34%), and more so by 10 µM acrolein (61%) via introducing a carbonyl group into the protein and causing dysfunction; this established acrolein as the main ingredient in cigarette smoke responsible for salivary LDH activity diminution (Avezov et al., 2014a).
A pathological role for acrolein in disease development/progression has been proposed based on the detection of higher levels of acrolein adducts in the sera of patients with various diseases, including diabetic nephropathy; renal disease; Sjogren’s syndrome autoimmune disorder (Brooks, 2013); cerebral stoke/infarction (Igarashi and Kashiwagi, 2011); colon carcinogenesis; and diabetes (reviewed by Aldini et al. (2011)). A recent study using sera from patients with diabetic nephropathy described the utility of “aldehyde capture”, a procedure used to calculate the protein carbonyl stress derived solely from LPO, suggesting future promise in the monitoring of renal disease and treatment (Medina-Navarro et al., 2011). The potential utility of plasma levels of protein-conjugated acrolein (along with amyloid β40/42 (Aβ40/42)) to detect and differentiate between mild cognitive impairment and Alzheimer’s disease was recently demonstrated (Mizoi et al., 2014). Thus, substantial evidence suggests that acrolein toxicity in major organ systems involves the common mechanism of formation of adducts with proteins that play critical functional roles in cellular processes. However, the question of causality versus association still remains, and the direct/indirect mechanisms by which the acrolein adducts may lead to injury remain to be determined.
Oxidative Stress
Aerobic metabolism and pathogen defense mechanisms produce ROS which can damage lipids, proteins, carbohydrates, and nucleic acids. To counter oxidative damage, the human body is equipped with antioxidant compounds and antioxidant enzymes. However, extensive ROS production can overwhelm these defense mechanisms, and results in oxidative stress (reviewed by Birben et al. (2012)). Acrolein is considered both a product and an initiator of LPO (Adams and Klaidman, 1993; Uchida et al., 1998a) and is thus a perpetrator of oxidative stress. Both in vitro and in vivo studies have shown that acrolein can itself cause oxidative damage, leading to membrane disruption, DNA and mitochondrial damage and can exacerbate apoptosis. Acrolein exposure (0.1–5 µM) in the human retinal epithelial cells (ARPE19) significantly increased oxidant levels by decreasing GSH, anti-oxidant enzymes (SOD and GSH-peroxidase), and total and nuclear levels of the antioxidant regulator-Nrf2, coincident with reduced mitochondrial function, suggesting a role for acrolein in retinal damage and age-related macular degeneration. Notably, lipoic acid was protective (Jia et al., 2007), as was hydroxytyrosol (antioxidant polyphenol in olives) via induction of phase II detoxifying enzymes and stimulation of mitochondrial biogenesis (Zhu et al., 2010), and α-tocopherol (Vitamin E) by upregulating Nrf2 (Feng et al., 2010).
With the abundance of PUFAs available for LPO and the lack of enzymes that metabolize reactive aldehydes, neurons and the central nervous system are particularly vulnerable to oxidative stress and acrolein toxicity (Hamann and Shi, 2009). Acrolein-induced neuronal damage was demonstrated in pigs and rats, suggesting that it may be a potential risk factor for spinal cord injury (SCI) (Due et al., 2014; Shi et al., 2011; Zheng et al., 2013). The neuroprotective role of hydralazine was shown in the attenuation of acrolein-mediated damage in SCI (Park et al., 2014). Plasma levels of protein-conjugated acrolein (along with acrolein producing enzymes—spermine oxidase and acetylpolyamine oxidase) were shown to be good biomarkers for human stroke. Interestingly, an inverse correlation was seen between stroke and 3-HPMA the urinary GSH-metabolite of acrolein; the authors proposed that the decrease in 3-HPMA reflects reduced GSH caused by increased acrolein generation at the region of brain infarct (Yoshida et al., 2012).
Acrolein-induced oxidative stress is associated with severe toxicity in the renal system due to the use of anticancer agents such as cyclophosphamide and ifosfamide that get metabolized to acrolein; the acrolein toxicity is prevented by Mesna (2-mercaptoethane sulfonate) which binds with and clears acrolein. A major contributor to acrolein toxicity in such cases appears to be the activation of intracellular ROS and nitric oxide production, either directly or via NF-κB and AP-1 (Korkmaz et al., 2007). A recent report showed that pretreatment with diallyl disulfide protected against cyclophosphamide-induced oxidative damage, histopathological lesions, and apoptosis; notably, the accumulation of acrolein-adducts in the bladder was reduced showing that the protection was by detoxifying acrolein (Kim et al., 2014). A recent review identifies acrolein-lysine adducts as a promising urinary biomarker of oxidative stress and oxidative damage (Il'yasova et al., 2012). Acrolein-induced oxidative damage is shown to have an indirect impact on cardiovascular health via apoptosis of adult mice cardiomyocytes in vitro (Wang et al., 2011); and via myocardial oxidative stress, increased apoptosis, and myocyte hypertrophy and dilated cardiomyopathy in vivo after long-term (48-day) oral exposure of mice to 1 mg/kg acrolein (Ismahil et al., 2011). Also, acrolein-induced oxidation of apolipoprotein A-I (apoA-I, the major high density lipoprotein) impaired cellular cholesterol efflux (Shao, 2012). The exposure of oral tissues to environmental hazards is immense, especially in smokers, and acrolein was shown to reduce GSH, and increase oxidative stress and protein carbonylation in oral keratinocytes in vitro (Avezov et al., 2014b). Studies in chronic obstructive pulmonary disease (COPD) have shown that cigarette smoke depletes total GSH levels in bronchial cells (van der Toorn et al., 2007) and human gingival fibroblasts (Colombo et al., 2012); in both cell types, mass spectrometric analyses demonstrated that the GSH was depleted by binding to acrolein. Rapamycin was demonstrated to inhibit acrolein-induced apoptosis in male germ cells by alleviating acrolein-induced ROS, and reducing ROS-driven mitochondrial dysfunction and apoptosis by increasing ratio of Bcl2/Bax and protecting mitochondrial membranes (He et al., 2014). Our work has shown that acrolein-induced oxidative stress was accompanied by mitochondrial dysfunction and ER stress in liver cells; acrolein toxicity was attenuated by the antioxidant and GSH-prodrug NAC (Mohammad et al., 2012). A recent article ranked the overall effectiveness of known protective agents in suppressing acrolein-induced ROS formation, protein damage, and toxicity in cell and cell-free models; starting with the most effective: reducing agents > thiol-containing compounds > carbonyl scavengers/amines > antioxidants/ROS scavengers (MacAllister et al., 2013).
Like many other inducers of oxidative stress, acrolein is known to activate cellular antioxidant systems, most likely as an adaptive response. These effects are seen primarily at low sublethal acrolein concentrations, and may be due to “hormesis” described for other toxins, wherein hormesis-stimulating compounds initiate an adaptive response that confers protection against harmful doses of the same or similar agents. One such example is the acrolein-induced activation of Nrf2 in vitro in endothelial cells leading to induction of heme-oxygenase-1, the enzyme that plays a major role in the cellular antioxidative response (Wu et al., 2006); and in vivo in the lungs of mice exposed to 5 ppm of acrolein vapor for 6 h/day for 14–17 days (Zhang and Forman, 2008). The effects of acrolein on two major redox regulators (thioredoxin and thioredoxin reductase) have been recently discussed (Myers et al., 2011). In the event that induction of cellular antioxidant systems fails or is subpar, acrolein may induce pathological oxidative mechanisms that contribute to disease development.
Mitochondrial Dysfunction
Mitochondria play a dynamic role in human cells, controlling oxidative phosphorylation to produce ATP, aiding in fatty acid breakdown and steroid synthesis, and playing an integral role in calcium signaling and apoptosis. Acrolein has been implicated as a mitochondrial toxin affecting several of these functions, most notably apoptosis, and whether apoptosis or necrosis ensues after acrolein exposure appears to be related to dose and cell type. The majority of these studies are in vitro, and the lack of much-needed in vivo data raises questions about their relevance; nonetheless, some key studies are discussed below.
Although the mitochondrial death pathway leading to apoptosis typically involves the activation of caspases, acrolein-induced apoptosis was shown to be both caspase-dependent in human neuroblastoma cells (Dong et al., 2013) and in A549 lung cells (Roy et al., 2010), and caspase-independent in CHO cells (Tanel and Averill-Bates, 2005). On the other hand, acrolein may inhibit caspases and ultimately inhibit apoptosis in some cells, such as murine proB lymphocytes (Kern and Kehrer, 2002) and in B lymphoblastoid SKW6.4 cells via direct alkylation of caspase active sites (Hristova et al., 2007).
Tanel et al. showed that NAC prevented apoptotic events in CHO cells exposed to low doses of acrolein (4 fmol/cell, 30 min), including Bad and Bcl-2 translocations, depolarization of the mitochondrial membrane potential, procaspase-9 processing, caspase-9, −7, −8 activation, and poly (ADP-ribose) polymerase cleavage (Tanel and Averill-Bates, 2007b); however, apoptosis was not prevented by the Fas receptor antagonist KP7-6 (Tanel and Averill-Bates, 2007a). Roy demonstrated in A549 human lung cells that low-dose acute acrolein exposures (3–27 µM, 30–60 min) could be overcome by an adaptive response involving activation of antiapoptosis survival factors AKT and cIAP1/2, despite triggering of early apoptotic changes in Bax and cytochrome c release; the survival proteins did not protect against higher acrolein exposures (10–50 µM) induced apoptosis (Roy et al., 2009). Most adverse changes were inhibited by catalase, the antioxidant polyethylene glycol-catalase, and the SOD mimetic-MnTBAP, reiterating the initiating role of acrolein-induced oxidative stress. Apoptosis in A549 lung cells was also reversible with pifithrin-a, an inhibitor of p53 (Roy et al., 2010). Acrolein is known to induce mitochondrial dysfunction leading to neuronal death in HT22 mouse hippocampal cells, and lithium was shown to protect against acrolein-induced neurotoxicity by enhancement of GSH, and attenuation of ROS and mitochondrial dysfunction caused by acrolein (Huang et al., 2014).
Acrolein also impacts the ability of the mitochondria to take part in cellular respiration. In rat liver mitochondria acrolein dose-dependently inhibited select components of the respiratory machinery, including complexes I and II, pyruvate dehydrogenase, and α-ketoglutarate dehydrogenase, over a broad range of acrolein exposures from 10 µM (relevant to human exposures) to 1000 µM (highly unlikely) (Sun et al., 2006). Mitochondrial dysfunction due to inhibition of key mitochondrial enzymes (pyruvate dehydrogenase and α-ketoglutarate dehydrogenase) has been observed in Alzheimer’s disease brain tissue; and lipoic acid, a cofactor for these enzymes, can be covalently bound and sequestered by acrolein, thereby decreasing enzyme activity. In keeping with this, supplementation with lipoic acid was shown to decrease oxidative stress and mitochondrial enzyme loss, partly by scavenging of acrolein (Maczurek et al., 2008). The presence of increased protein-carbonyl adducts in this study emphasizes that particular mitochondrial enzymes may be more susceptible to covalent modification, thereby acting as a wrench in the cogwheels of electron transport.
DNA Adduction
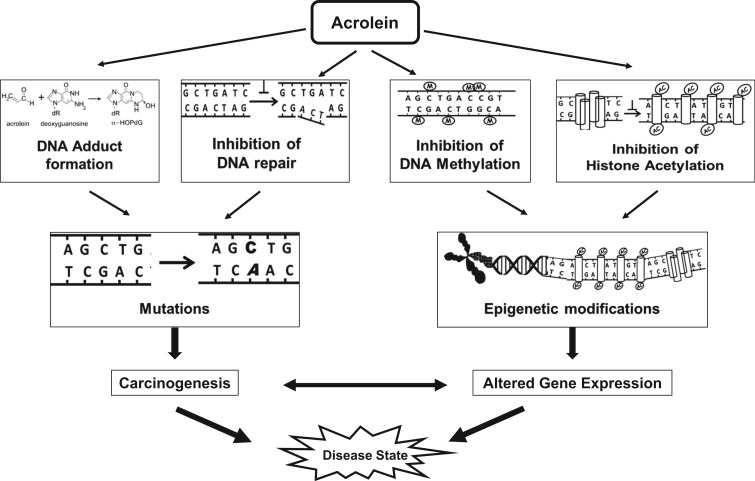
DNA damage can be induced by exposure to chemicals, ROS and reactive metabolites such as acrolein; DNA damage has been associated with mutations and carcinogenesis. Acrolein can produce interchain cross-links of double-stranded DNA as well as DNA-protein cross-links. Acrolein readily reacts with deoxyguanosine (dG) producing two exocyclic DNA adducts, α- and γ-hydroxy-1, N2-propano-2′-deoxyguanosine (α-HOPdG and γ-HOPdG), which are mutagenic to varying degrees (Tang et al., 2011). Additionally, acrolein adducts of 2′-deoxyadenosine (Pawlowicz et al., 2006a,b) and 2′-deoxycytidine DNA bases and thymidine (Pawlowicz and Kronberg, 2008) have been reported. Acrolein-DNA adducts have been characterized in vitro, and have also been detected in vivo in several different animal and human tissues and cells (Tang et al., 2011; Voulgaridou et al., 2011).
Acrolein adduction of DNA, if not repaired efficiently, has the potential to cause critical gene mutations, suggesting that acrolein may be mutagenic and may contribute to the process of carcinogenesis. Mutagenicity studies of acrolein have yielded inconsistent results, and both cell type and sequence context appear to play a role (reviewed in Liu et al. (2010)). A lack of mutagenicity by acrolein was demonstrated in various shuttle vector-based systems (Kim et al., 2007); in contrast, Demir’s group demonstrated dose-dependent in vitro mutagenic effects of acrolein (10 and 25 µM) using the mouse lymphoma and the Drosophila wing spot mutagenicity assay (Demir et al., 2013). Furthermore, CpG methylation, at CpG-rich hot spots that are prone to acrolein-DNA adduction, increased the acrolein-induced G-to-T and G-to-A mutation frequency to levels found in the CpG sites in the p53 gene, a known mutational hot spot in tobacco smoke-associated lung cancer (Wang et al., 2013). In the mitochondria, the natural absence of nucleotide excision repair enzymes permits accumulation of acrolein adducts in mitochondrial DNA, leading to transversion mutations which are thought to contribute to neurodegenerative disorders (Kasiviswanathan et al., 2013). Interestingly, acrolein not only induced DNA damage but also inhibited DNA repair by inhibition of repair enzymes, likely by acrolein-adduction leading to increased proteasome-dependent degradation (Wang et al., 2012) or slower repair activity (Choudhury et al., 2013). Additionally, acrolein inhibited DNA methylase by 30%–50% (Cox et al., 1988), and inhibited histone acetylation and compromised chromatin assembly suggesting that acrolein exposure may also cause epigenetic alterations in gene expression (Chen et al., 2013). However, in vivo evidence of mutagenicity has not been demonstrated thus far.
Acrolein-induced DNA damage and inhibition of DNA repair are likely to promote cancer development; however, the carcinogenicity of acrolein is controversial (reviewed in Abraham et al. (2011)). Intraperitoneal administration of acrolein (75 and 150 nmol per animal) increased DNA adducts but not tumor incidences in male or female B6C3F1 neonatal mice, suggesting that DNA adduction may not parallel cancer development (Von Tungeln et al., 2002). In contrast, a case has been made for the carcinogenicity of acrolein based on higher acrolein DNA adducts levels in lung (Feng et al., 2006), liver (Kawai et al., 2003), and bladder cancers (Lee et al., 2014). In an early study, acrolein was postulated to promote urinary bladder cancer since intraperitoneal and intravesicular administration of acrolein demonstrated abnormal cell proliferation and hyperplasia of the bladder epithelium and the urothelium (Cohen et al., 1992). Moreover, in vivo inhalation exposure of rodents to acrolein resulted in inflammation, hyperplasia, and squamous metaplasia of the respiratory airway epithelium (Dorman et al., 2008). In epidemiological studies, an increased lung cancer incidence was reported for nonsmoking Chinese women cooking in a traditional wok style. The women exhibited significantly higher levels of the mercapturic acid metabolites of acrolein, crotonaldehyde, and benzene, suggesting a link between lung cancer and volatile toxicants (such as acrolein) produced by high temperature wok cooking/frying (Wang et al., 2013).
A novel and contradictory hypothesis was recently proposed suggesting that acrolein may, in fact, be part of a physiological anticancer system that controls the growth/proliferation of cancer cells (Alarcon, 2012). This hypothesis is based on properties of acrolein such as GSH depletion and direct cytotoxicity, and the fact that acrolein is a metabolite of anticancer compounds, spermine and cyclophosphamide. The hypothesis is interesting but speculative; and needs further investigation. Detailed studies on the type and magnitude of DNA damage and mutations arising from endogenous and/or exogenous acrolein exposure, and the biological consequences are essential to understand the true role of acrolein in cancer (Figure 2).
FIG. 2.
Effects of acrolein on DNA.
Inflammation and Immune Alterations
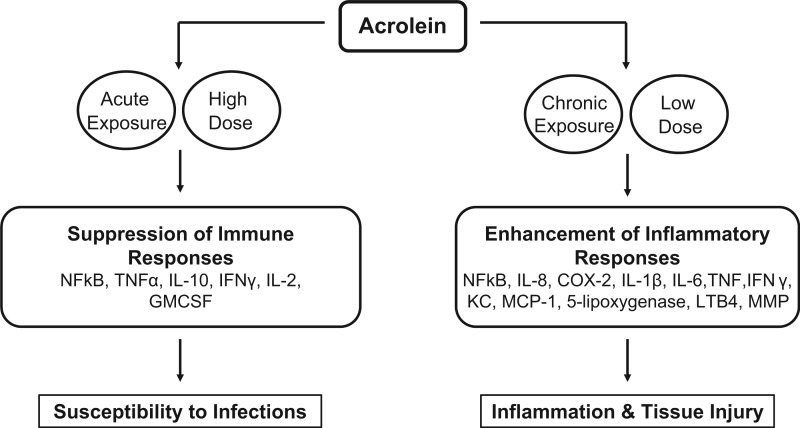
Adverse health effects of acrolein are anticipated to arise from its impact on innate and adaptive immune responses. Inflammation is an essential protective immune response for combating infections; however, uncontrolled or chronic inflammation is a major cause of tissue injury. Interestingly, reports of acrolein-induced alterations in inflammatory signaling and gene expression, particularly via the NF-κB pathway, are diverse and often conflicting, implying a significant multilevel complexity influenced by cell type, duration of acrolein exposure, and environmental context (reviewed by Yadav and Ramana (2013)).
Acrolein can potentially diminish defenses against bacterial and viral infections by inhibiting macrophage responses/function via suppression of NF-κB (Horton et al., 1999; Kehrer and Biswal, 2000; Kirkham et al., 2004; Lambert et al., 2005), or by direct alkylation of signaling proteins (Hristova et al., 2012; Kasahara et al., 2008; Valacchi et al., 2005), or by suppressing M1 proinflammatory reactions and favoring anti-inflammatory M2 responses, consistent with observations in smokers (Hristova et al., 2012). Acrolein is shown to suppress TNF-α-induced IL-8, a proinflammatory neutrophil chemoattractant, in both primary and immortalized human bronchial epithelial cells (Valacchi et al., 2005). Immunosuppressive effects of acrolein exposure were also seen in human T cells, in which acrolein directly alkylated Cys-61 and Arg-307 of NF-κB1 (p50), significantly reducing binding to the promoters of IL-2, IL-10, TNF-α, GMCSF, and IFNγ, with a notable exception being IL-8 (Lambert et al., 2007).
In contrast to these immunosuppressive effects, acrolein is demonstrated to activate NF-κB and induce proinflammatory mediators. In rat lung epithelial cells, acute acrolein exposure (20 µM, 6 h) induced the NF-κB dependent marker of inflammation COX-2 via calcium release and subsequent proteolytic degradation of IκBα. This was reversed with GW5074, a Ras/Raf-1/ERK pathway inhibitor (Sarkar and Hayes, 2007). Acrolein induced an inflammatory response and increased mucin gene expression in human middle ear epithelial cells supporting the hypothesis that acrolein may be a risk factor for otitis media (Song et al., 2013). IL-8 upregulation is associated with many inflammatory disorders, and IL-8 production due to acrolein exposure has been reported in vitro in multiple cell types, including normal human bronchial smooth muscle cells and in airway epithelial cells (Moretto et al., 2012), alveolar macrophages, cultured normal human lung fibroblasts, and in small airway epithelial cells which was prevented by antioxidants NAC and MESNA, and by U0126 and FR180204, inhibitors of Akt and ERK1/2 respectively, suggesting their mechanistic involvement in IL-8 induction (Moretto et al., 2009). Finkelstein showed acrolein-induced IL-8 production by isolated neutrophils which was accompanied by inhibition of apoptosis via suppressed caspase activation, extrapolating the potential for prolonged and increased inflammation within the lung (Finkelstein et al., 2001). Acrolein-induced IL-8 in human pulmonary fibroblasts was effectively suppressed by melatonin (Kim et al., 2012). Similar proinflammatory effects along with increased susceptibility to influenza-A virus was seen in vivo wherein mice exposed to acrolein for 4 days exhibited an accelerated inflammatory response with higher macrophages and neutrophils, and greater IL-1α, IL-1β, IL-6, TNF, IFN-γ, KC, and MCP-1 in the bronchial fluid; the effects were suppressed by the carbonyl scavenger bisulphite (Ong et al., 2012). In vitro acrolein exposure of nasal epithelial cells was inflammatory and cytotoxic (Comer et al., 2014). Also, intranasal acrolein exposure of mice caused both pulmonary inflammation and death of lung epithelial cells, with increased activated macrophages in the lungs and increased ROS formation via induction of NF-κB signaling; these effects were decreased by NAC (Sun et al., 2014).
Recent data indicate that acrolein may contribute to the highly atherogenic transformation of macrophages into foam cells via modification of LDL (Watanabe et al., 2013). In keeping with this, acrolein-lysine adducts in LDL are detected in atherosclerotic lesions in humans. Acrolein also increased macrophages expression of the atherogenic factors 5-lipoxygenase, leukotriene B4 and matrix metalloproteinase (Kim et al., 2010; O'Toole et al., 2009). Other proinflammatory effects of acrolein include mast cell degranulation which can increase inflammatory injury. Environmentally, relevant exposures of acrolein (1 ppm) promoted the generation of ROS and initiated degranulation of the airway mast cells (RBL-2H3); these effects were significantly reduced by antioxidants NAC and tetramethylthiourea (Hochman et al., 2014). These studies suggest a significant role for acrolein in chronic inflammatory disorders via dysregulation of inflammatory mediators both locally and systemically; however, such conclusions may be premature since in-vivo studies are limited. Although acrolein appears to have contradictory pro- and anti-inflammatory effects, they may not be incongruous. It is likely that the magnitude and duration of acrolein exposure may critically determine outcomes, wherein acute high-dose acrolein exposure might suppress innate immunity and inflammatory responses thereby increasing susceptibility to infections, whereas chronic low-dose exposures may increase inflammation leading to tissue injury (Figure 3).
FIG. 3.
Effects of acrolein on immune responses.
ER Stress
The ER is the site of synthesis and folding of proteins, and the accumulation of misfolded proteins can trigger ER stress and the unfolded protein response (UPR). ER stress is thought to contribute to several pathological conditions that may also be associated with increased acrolein, such as fatty liver disease (Vacaru et al., 2014), Alzheimer’s disease (Li et al., 2014), and inflammatory and cardiovascular diseases (Zhou and Tabas, 2013). Based on its proclivity to form protein adducts, acrolein is highly likely to trigger ER stress. Indeed, several recent studies demonstrate acrolein-induced ER stress and UPR. The effects of acrolein (2–25 µM) on ER stress and endothelial cell activation were studied in human umbilical vein endothelial cells (Haberzettl et al., 2009), wherein both the alarm and the adaptation pathways of UPR were triggered, and inflammatory genes TNF-α, IL-6, and IL-8 were upregulated, demonstrating the proinflammatory and atherogenic properties of acrolein. Notably, the acrolein effects were prevented by the chemical chaperone (phenylbutyric acid), suggesting its usefulness in treating pathologies associated with acrolein toxicity. The same group also demonstrated that the elimination of LPO products (eg acrolein) by aldose reductase diminishes ER stress and decreases ischemia-reperfusion injury (Keith et al., 2009). Our recent work demonstrates that acrolein-induced cytotoxicity in hepatocytes involves ER stress, in combination with mitochondrial disruption and oxidative stress, resulting in cell death (Mohammad et al., 2012) Acrolein-induced cell death was only partially attenuated by NAC, phenyl-butyric acid, and caspase or JNK inhibitors individually, indicating that combination therapies may be more effective against acrolein-induced toxicity. Interestingly, acrolein caused ER stress but failed to upregulate the protective ER-chaperones, GRP78 and GRP94. Acrolein is demonstrated to modify and reduce function of another chaperone protein disulfide isomerase; this may lead to the enhancement and perpetuation of ER stress (Carbone et al., 2005). Activation of protective versus injurious ER responses to acrolein exposure may be dependent on dose and time, as shown in A549 lung cells (Tanel et al., 2014).
In vitro acrolein exposure (<10 µM) of Swiss 3T3 cells was demonstrated to be primarily responsible for cigarette smoke-induced ER stress and activation of PERK, and ATF4 (Hengstermann and Muller, 2008). In vivo acute (a single intraperitoneal injection of acrolein 12 µmol/kg) and chronic (three weekly intraperitoneal injections of acrolein at 6, 12, or 24 µmol/kg) systemic acrolein exposure in rats caused ER stress and upregulation of ATF4, CHOP and GADD34, and VEGF, leading to emphysematous lung tissue remodeling and airspace enlargement; caspase-3 activation and apoptosis were concurrently observed in the septal cells in acrolein-treated rat lungs. Moreover, accumulated acrolein-protein adducts were observed in the lungs of smokers with COPD, indicating that acrolein may contribute to lung disorders like emphysema and COPD (Kitaguchi et al., 2012). Recent reports have demonstrated ER stress associated with cigarette smoke and the development of lung disorders such as emphysema (Gan et al., 2011) and COPD (Ribeiro and O'Neal, 2012). Thus, acrolein-induced ER stress may be a relevant mechanism of toxicity in various disorders.
Structural and Membrane Effects
Myelin is a critical structural component of the nervous system composed of lipids and proteins including myelin basic protein (MBP), and demyelination is a hallmark of neurodegenerative diseases, including multiple sclerosis (MS) and SCI. Increased acrolein levels are seen in the spinal cord in MS and SCI animal models, with acrolein levels correlating to neuronal membrane damage, loss of conduction, and even degeneration (Leung et al., 2011; Shi et al., 2011). The acrolein scavenger hydralazine lessened myelin damage and improved behavioral outcomes (Leunget al., 2011); reversed SCI-induced damage by alleviating superoxide production, preventing GSH depletion (Hamann et al., 2008); and effectively reducing acrolein levels, tissue damage, motor deficits, and neuropathic pain (Park et al., 2014). Acrolein exposure in vitro also elicited a concentration-dependent modification and aggregation of neurofilament-L (NF-L) which may contribute to neurodegeneration. The acrolein concentrations used (100–500 µM) correspond to concentrations of 1.0–5.2 nmol/mg protein, comparable to levels observed in Alzheimer’s disease, and thus are within the expected range of SCI and MS. These effects of acrolein were blocked by NAC and GSH (Jeong and Kang, 2008).
Acrolein exposures as low as 1 μM for 4 h resulted in increased permeability of cell membranes to ethidium bromide in ex vivo spinal cords; as the dose and duration increased, larger molecules such as horseradish peroxidase and LDH were able to pass through (Luo and Shi, 2004). Exposure to acrolein in vivo and in vitro was associated with reduced presynaptic neurotransmitter release; this effect involved inhibition of key proteins that regulate membrane-vesicle fusion, such as N-ethylmaleimide-sensitive fusion protein (NSF) and synaptosomal-associated protein of 25 kDa (SNAP-25). Acrolein, resulting from acrylamide, also inhibited presynaptic membrane neurotransmitter uptake and vesicular storage in vivo and in vitro (LoPachin et al., 2004, 2006); proteomic analyses showed that these dysfunctions were associated with the formation of acrolein adducts with the dopamine transporter and v-ATPase (Lopachin et al., 2007).
Acrolein exposure resulted in several structural changes including induced F-actin microfilament aggregation, and marginalization and regionalization in Sertoli cells, which are important in spermatogenesis; such effects may underlie the testicular toxicity of the anticancer drug cyclophosphamide which is metabolized into acrolein (Liu et al., 2012). Another membrane effect of acrolein was the stimulation of erythrocyte cell membrane scrambling, a typical feature of suicidal death or eryptosis. Membrane phospholipid scrambling was accompanied by the generation of ceramide, cell shrinkage, and apoptosis (Ahmed et al., 2013). Airborne acrolein was shown to induce hyperphosphorylation and damage of keratin-8 and ubiquitination of intermediate filament in bronchiolar lung cell monolayers (Burcham et al., 2014). Numerous studies have shown in vitro and in vivo toxicity of acrolein associated with structural (and functional) effects in the cardiovascular system, including increased susceptibility to vasospasm (Conklin et al., 2006), increased heart rate and blood pressure (Perez et al., 2013), increased atherosclerotic lesions (Srivastava et al., 2011), and decreased endothelial cell migration (O'Toole et al., 2014); however, the exact toxic mechanisms remain unclear.
Deregulated Signal Transduction
Protein kinases and phosphatases are critical components of intracellular signaling that regulate cellular function, and acrolein is reported to inactivate as well as activate them in a contextual manner, depending on dose and cell type. Acrolein, at a fairly high concentrations (100–1000 µM), was shown to be a potent, time-dependent inactivator of protein tyrosine phosphatase-1B via covalent modification of an active site cysteine (Seiner et al., 2007); and phosphatase PP2A was also inhibited by acrolein, with concurrent apoptosis (Fathi et al., 2000). On the other hand, a lower acrolein exposure (25 µM) in human hepatocytes led to a modest ∼ 2-fold increase in both serine and tyrosine phosphatase activity and reduced interferon-α antiviral activity, which may contribute to therapy resistance in hepatitis C virus infection (Joshi-Barve et al., 2009). Acrolein exposure (10–30 µM) in breast cancer cells altered the thioredoxin-dependent redox system and caused a G2-M cell cycle arrest, but did not inhibit phosphatase-CDC25 which controls this cell cycle checkpoint (Beillerot et al., 2012).
Several in vitro studies described below have demonstrated acrolein-induced alterations in cellular mitogen activated protein kinases (MAPKs) which are likely to contribute to other mechanisms of toxicity; however, few findings are validated in vivo. Acrolein-induced MAPK activation and cytotoxicity were observed in cultured neurons along with the activation of c-jun cAMP response element binding protein, CREB (Pugazhenthi et al., 2006), and in cultured rat vascular smooth muscle cells; however, in the latter study, MAPK inhibitors failed to block cell death, suggesting divergent mechanisms (Ranganna et al., 2002). Activation of p38 in human airway smooth muscle cells and lung fibroblasts exposed to subcytotoxic concentrations of acrolein (10–100 µM) resulted in the production of a potent proangiogenic factor, vascular endothelial growth factor (VEGF); the effects were prevented by p38 inhibitor and NAC (Volpi et al., 2011). Activation and membrane translocation of another protein kinase, PKC-δ, by allyl alcohol and its primary metabolite acrolein (up to 125 µM), resulted in cytotoxicity in hepatocytes, which was blocked by rottlerin, a selective PKC-δ inhibitor (Maddox et al., 2003). Our work showed that acrolein exposure of cultured hepatocytes led to a sustained activation of ERK1/2, JNK, and p38, which was associated with ER and mitochondrial stress and apoptosis; the cytotoxic effects of acrolein were decreased by JNK inhibitor, suggesting that kinase activation may be linked to cell death and liver injury (Mohammad et al., 2012). Via p38 activation, acrolein also caused upregulation of the muscle-specific E3 ligases atrogin-1 and MuRF1, suggesting a possible role in degradation of myosin heavy chain, myotube catabolism, and muscle wasting (Rom et al., 2013). In contrast, acrolein was shown to suppress phosphorylation of JNK and activation of c-Jun; moreover, JNK was a direct target for alkylation by acrolein at key cysteine residues in the active site (Hristova et al., 2012).
Acrolein-induced deregulated signaling is known to induce mucin production and inflammation (major contributors to lung disorders) via many upstream signals, including (1) MAPKs and epidermal growth factor receptor (Deshmukh et al., 2005); (2) matrix metalloproteinase-9 and the Ras/extracellular signal-regulated kinase pathway, which was attenuated by simvastatin (Chen et al., 2010); (3) Peroxisome proliferator-activated receptor-gamma (PPAR-gamma), which was reduced by rosiglitazone (Liu et al., 2009); and (4) Phosphodiesterase 5 that selectively degrades cyclic guanosine 3′,5′-monophosphate (cGMP), which was prevented by sildenafil (Wang et al., 2009). Acrolein has also been implicated in the demise of retinal pigment epithelium (ARPE-19) cells, with overproduction of the proangiogenic cytokines, TGFβ2 and VEGF that are important in macular degeneration and in diabetic retinopathy. These effects were potentiated by high glucose and were attenuated by NBHA (N-benzylhydroxylamine, a hydroxylamine that sequesters aldehydes) and by SIS3, a specific inhibitor of SMAD3 that blocks TGFβ2 signaling (Grigsby et al., 2012; Vidro-Kotchan et al., 2011). Recently, a novel mechanism of acrolein cytotoxicity involving the acrolein-conjugation and inactivation of glyceraldehyde-3-phosphate dehydrogenase, an enzyme with many diverse functions, was described in vitro (Martyniuk et al., 2011) and in mouse mammary carcinoma FM3A cells (Nakamura et al., 2013). Thus, acrolein-induced changes in cellular signaling and gene expression may alter function and physiology, and potentially contribute to the disease process in multiple organ systems.
CONCLUSIONS AND FUTURE DIRECTON
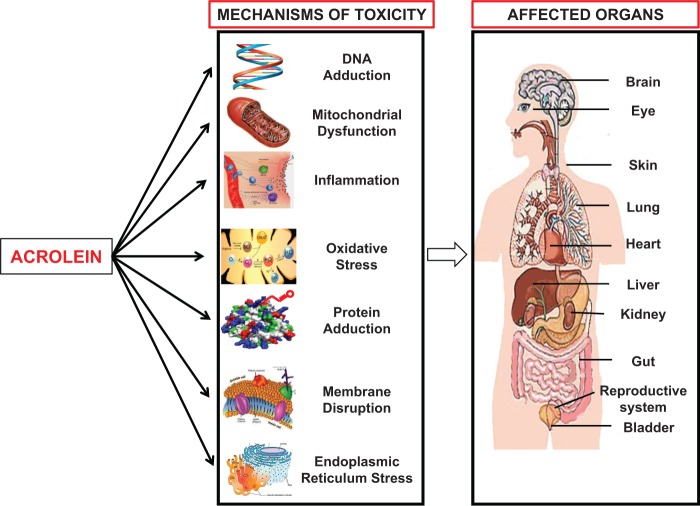
In this review, we focused on the myriad effects mediated by acrolein with an emphasis on the molecular mechanisms of toxicity that provide evidence to suggest that acrolein plays a critical role in disease pathogenesis (Figure 4). Acrolein operates via disruption of cell organelles such as mitochondria, ER, and membranes. In addition, it increases oxidative stress and apoptosis, alters proinflammatory mediators, and changes cellular signal transduction leading to a variety of defects that may cause or predispose to disease in various organs. Moreover, acrolein may have cooperative effects with other toxic insults. Many of the studies described here were done in vitro or in cultured cells, rather than in vivo; hence, despite strong links to several diseases, the causal role of acrolein in disease pathogenesis, though highly likely, can only be surmised. A limitation of this review stems from the fact that levels of acrolein are difficult to quantify, particularly in vivo, while the in vitro literature describes acrolein concentrations in various units that are problematic to interconvert and challenging to compare.
FIG. 4.
Toxicity mechanisms and organ systems affected by acrolein (images courtesy Google).
Several unanswered questions remain regarding acrolein toxicology. Detailed studies are necessary to determine not only the temporal sequence of molecular events, but also the inter-dependence, cross-talk, and the relative contributions of different mechanisms of acrolein toxicity to the pathogenesis/progression of disease. The development of small and large mammalian models of exogenous and endogenous acrolein exposures, both acute and chronic, will promote detailed mechanistic investigations and toxicity studies. Research, particularly in humans, has been limited by availability of techniques to quantify acrolein and acrolein-adducts in body fluids and tissues. Hence, the development of sensitive, high-throughput and quantitative techniques will facilitate large population-based human studies. Acrolein scavengers with proven effectiveness in small in vitro/in vivo studies may provide effective and safe means to mitigate acrolein toxicity and associated disorders; however, their bioavailability, effective concentrations, and delivery to the affected areas would require extensive investigations. A true determination of causality and the contribution of acrolein need to be established by detailed in vivo studies in animal models and in human populations. Moreover, it will be important to examine the impact of age, sex, lifestyle, and genetic variation on the susceptibility to acrolein exposures in correlation with disease states.
FUNDING
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) and National Institute of Environmental Health Sciences (NIEHS) at the National Institutes of Health (NIH); and U.S. Department of Veterans Affairs. Grant numbers are: R01AA018869-02 (CJM), T35ES014559-06 (CJM), P01AA017103-01 (CJM), K01 ES017105-01A1 (S.J.-B.) and (BX000350 (CJM).
ACKNOWLEDGMENTS
The authors would like to acknowledge Ms Marion McClain for proof-reading the article. Nothing to disclose.
REFERENCES
- Abraham K., Andres S., Palavinskas R., Berg K., Appel K. E., Lampen A. (2011). Toxicology and risk assessment of acrolein in food. Mol. Nutr. Food Res. 55, 1277–1290. [DOI] [PubMed] [Google Scholar]
- Adams J. D., Jr., Klaidman L. K. (1993). Acrolein-induced oxygen radical formation. Free Radic. Biol. Med. 15, 187–193. [DOI] [PubMed] [Google Scholar]
- Ahmed M. S., Langer H., Abed M., Voelkl J., Lang F. (2013). The uremic toxin acrolein promotes suicidal erythrocyte death. Kidney Blood Press. Res. 37, 158–167. [DOI] [PubMed] [Google Scholar]
- Alarcon R. A. (2012). Anticancer system created by acrolein and hydroxyl radical generated in enzymatic oxidation of spermine and other biochemical reactions. Med. Hypotheses 79, 522–530. [DOI] [PubMed] [Google Scholar]
- Aldini G., Orioli M., Carini M. (2011). Protein modification by acrolein: relevance to pathological conditions and inhibition by aldehyde sequestering agents. Mol. Nutr. Food Res. 55, 1301–1319. [DOI] [PubMed] [Google Scholar]
- Avezov K., Reznick A. Z., Aizenbud D. (2014a). LDH enzyme activity in human saliva: the effect of exposure to cigarette smoke and its different components. Arch. Oral Biol. 59, 142–148. [DOI] [PubMed] [Google Scholar]
- Avezov K., Reznick A. Z., Aizenbud D. (2014b). Oxidative damage in keratinocytes exposed to cigarette smoke and aldehydes. Toxicol. in vitro 28, 485–491. [DOI] [PubMed] [Google Scholar]
- Beillerot A., Battaglia E., Aline B., Bagrel D. (2012). Protection of CDC25 phosphatases against oxidative stress in breast cancer cells: evaluation of the implication of the thioredoxin system. Free Radic. Res. 46, 674–689. [DOI] [PubMed] [Google Scholar]
- Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. (2012). Oxidative stress and antioxidant defense. World Allergy Organ. J. 5, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks W. H. (2013). Increased polyamines alter chromatin and stabilize autoantigens in autoimmune diseases. Front. Immunol. 4, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui L. C., Manaa A., Xu X., Duval R., Busi F., Dupret J. M., Rodrigues Lima F., Dairou J. (2013). Acrolein, an alpha, beta-unsaturated aldehyde, irreversibly inhibits the acetylation of aromatic amine xenobiotics by human Nat1. Drug Metab. Dispos. 41, 1300-1305. [DOI] [PubMed] [Google Scholar]
- Burcham P. C., Raso A., Henry P. J. (2014). Airborne acrolein induces keratin-8 (Ser-73) hyperphosphorylation and intermediate filament ubiquitination in bronchiolar lung cell monolayers. Toxicology 319, 44–52. [DOI] [PubMed] [Google Scholar]
- Burcham P. C., Raso A., Kaminskas L. M. (2012). Chaperone heat shock protein 90 mobilization and hydralazine cytoprotection against acrolein-induced carbonyl stress. Mol. Pharmacol. 82, 876–886. [DOI] [PubMed] [Google Scholar]
- Cahill T. M. (2014). Ambient acrolein concentrations in coastal, remote, and urban regions in California. Environ. Sci. Technol. 48, 8507–8513. [DOI] [PubMed] [Google Scholar]
- Carbone D. L., Doorn J. A., Kiebler Z., Petersen D. R. (2005). Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem. Res. Toxicol. 18, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Centers for Disease, C., and Prevention (2013). Notes from the field: acute pesticide-related illness resulting from occupational exposure to acrolein - washington and california, 1993-2009. MMWR 62, 313–314. [PMC free article] [PubMed] [Google Scholar]
- Chen Y. J., Chen P., Wang H. X., Wang T., Chen L., Wang X., Sun B. B., Liu D. S., Xu D., An J., Wen F. Q. (2010). Simvastatin attenuates acrolein-induced mucin production in rats: involvement of the Ras/extracellular signal-regulated kinase pathway. Int. Immunopharmacol. 10, 685–693. [DOI] [PubMed] [Google Scholar]
- Chen D., Fang L., Li H., Tang M. S., Jin C. (2013). Cigarette smoke component acrolein modulates chromatin assembly by inhibiting histone acetylation. J. Biol. Chem. 288, 21678–21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S., Dyba M., Pan J., Roy R., Chung F. L. (2013). Repair kinetics of acrolein- and (E)-4-hydroxy-2-nonenal-derived DNA adducts in human colon cell extracts. Mutat. Res. 751–752, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Garland E. M., St John M., Okamura T., Smith R. A. (1992). Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 52, 3577–3581. [PubMed] [Google Scholar]
- Colombo G., Dalle-Donne I., Orioli M., Giustarini D., Rossi R., Clerici M., Regazzoni L., Aldini G., Milzani A., Butterfield D. A., Gagliano N. (2012). Oxidative damage in human gingival fibroblasts exposed to cigarette smoke. Free Radic. Biol. Med. 52, 1584–1596. [DOI] [PubMed] [Google Scholar]
- Comer D. M., Elborn J. S., Ennis M. (2014). Inflammatory and cytotoxic effects of acrolein, nicotine, acetylaldehyde and cigarette smoke extract on human nasal epithelial cells. BMC Pulm. Med. 14, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin D. J., Bhatnagar A., Cowley H. R., Johnson G. H., Wiechmann R. J., Sayre L. M., Trent M. B., Boor P. J. (2006). Acrolein generation stimulates hypercontraction in isolated human blood vessels. Toxicol. Appl. Pharmacol. 217, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Goorha S., Irving C. C. (1988). Inhibition of DNA methylase activity by acrolein. Carcinogenesis 9, 463–465. [DOI] [PubMed] [Google Scholar]
- Demir E., Turna F., Kaya B., Creus A., Marcos R. (2013). Mutagenic/recombinogenic effects of four lipid peroxidation products in Drosophila. Food Chem. Toxicol. 53, 221–227. [DOI] [PubMed] [Google Scholar]
- Deshmukh H. S., Case L. M., Wesselkamper S. C., Borchers M. T., Martin L. D., Shertzer H. G., Nadel J. A., Leikauf G. D. (2005). Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am. J. Respir. Crit. Care Med. 171, 305–314. [DOI] [PubMed] [Google Scholar]
- Dong L., Zhou S., Yang X., Chen Q., He Y., Huang W. (2013). Magnolol protects against oxidative stress-mediated neural cell damage by modulating mitochondrial dysfunction and PI3K/Akt signaling. J. Mol. Neurosci. 50, 469–481. [DOI] [PubMed] [Google Scholar]
- Dorman D. C., Struve M. F., Wong B. A., Marshall M. W., Gross E. A., Willson G. A. (2008). Respiratory tract responses in male rats following subchronic acrolein inhalation. Inhal. Toxicol. 20, 205–216. [DOI] [PubMed] [Google Scholar]
- Due M. R., Park J., Zheng L., Walls M., Allette Y. M., White F. A., Shi R. (2014). Acrolein involvement in sensory and behavioral hypersensitivity following spinal cord injury in the rat. J. Neurochem. 128, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi A. R., Krautheim A., Kaap S., Eger K., Steinfelder H. J. (2000). Michael adducts of ascorbic acid as inhibitors of protein phosphatase 2A and inducers of apoptosis. Bioorg. Med. Chem. Lett. 10, 1605–1608. [DOI] [PubMed] [Google Scholar]
- Feng Z., Hu W., Hu Y., Tang M. S. (2006). Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. U.S.A. 103, 15404–15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Liu Z., Li X., Jia H., Sun L., Tian C., Jia L., Liu J. (2010). Alpha-tocopherol is an effective Phase II enzyme inducer: protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. J. Nutr. Biochem. 21, 1222–1231. [DOI] [PubMed] [Google Scholar]
- Finkelstein E. I., Nardini M., Van der Vliet A. (2001). Inhibition of neutrophil apoptosis by acrolein: a mechanism of tobacco-related lung disease?. Am. J. Physiol. Lung C 281, L732–L739. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Smathers R. L., Fritz K. S., Epperson L. E., Hunter L. E., Petersen D. R. (2012). Protein carbonylation in a murine model for early alcoholic liver disease. Chem. Res. Toxicol. 25, 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan G., Hu R., Dai A., Tan S., Ouyang Q., Fu D., Jiang D. (2011). The role of endoplasmic reticulum stress in emphysema results from cigarette smoke exposure. Cell. Physiol. Biochem. 28, 725–732. [DOI] [PubMed] [Google Scholar]
- Grigsby J., Betts B., Vidro-Kotchan E., Culbert R., Tsin A. (2012). A possible role of acrolein in diabetic retinopathy: involvement of a VEGF/TGFbeta signaling pathway of the retinal pigment epithelium in hyperglycemia. Curr. Eye Res. 37, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P., Vladykovskaya E., Srivastava S., Bhatnagar A. (2009). Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol. Appl. Pharmacol. 234, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann K., Nehrt G., Ouyang H., Duerstock B., Shi R. (2008). Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J. Neurochem. 104, 708–718. [DOI] [PubMed] [Google Scholar]
- Hamann K., Shi R. (2009). Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury. J. Neurochem. 111, 1348–1356. [DOI] [PubMed] [Google Scholar]
- He X., Song W., Liu C., Chen S., Hua J. (2014). Rapamycin inhibits acrolein-induced apoptosis by alleviating ROS-driven mitochondrial dysfunction in male germ cells. Cell Prolif. 47, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstermann A., Muller T. (2008). Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein-response-dependent PERK pathway of cell survival. Free Radic. Biol. Med. 44, 1097–1107. [DOI] [PubMed] [Google Scholar]
- Hochman D. J., Collaco C. R., Brooks E. G. (2014). Acrolein induction of oxidative stress and degranulation in mast cells. Environ. Toxicol. 29, 908–915. [DOI] [PubMed] [Google Scholar]
- Horton N. D., Biswal S. S., Corrigan L. L., Bratta J., Kehrer J. P. (1999). Acrolein causes inhibitor kappaB-independent decreases in nuclear factor kappaB activation in human lung adenocarcinoma (A549) cells. J. Biol. Chem. 274, 9200–9206. [DOI] [PubMed] [Google Scholar]
- Hristova M., Heuvelmans S., van der Vliet A. (2007). GSH-dependent regulation of Fas-mediated caspase-8 activation by acrolein. Febs Lett. 581, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristova M., Spiess P. C., Kasahara D. I., Randall M. J., Deng B., van der Vliet A. (2012). The tobacco smoke component, acrolein, suppresses innate macrophage responses by direct alkylation of c-Jun N-terminal kinase. Am. J. Respir. Cell Mol. Biol. 46, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Qin J., Chen M., Chao X., Chen Z., Ramassamy C., Pi R., Jin M. (2014). Lithium prevents acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurochem. Res. 39, 677–684. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K. (2011). Protein-conjugated acrolein as a biochemical marker of brain infarction. Mol. Nutr. Food Res. 55, 1332–1341. [DOI] [PubMed] [Google Scholar]
- Il'yasova D., Scarbrough P., Spasojevic I. (2012). Urinary biomarkers of oxidative status. Clin. Chim. Acta 413, 1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismahil M. A., Hamid T., Haberzettl P., Gu Y., Chandrasekar B., Srivastava S., Bhatnagar A., Prabhu S. D. (2011). Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 301, H2050–H2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong M. S., Kang J. H. (2008). Acrolein, the toxic endogenous aldehyde, induces neurofilament-L aggregation. BMB Rep. 41, 635–639. [DOI] [PubMed] [Google Scholar]
- Jia L., Liu Z., Sun L., Miller S. S., Ames B. N., Cotman C. W., Liu J. (2007). Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-alpha-lipoic acid. Invest. Ophthalmol. Vis. Sci. 48, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Barve S., Amancherla K., Patil M., Bhatnagar A., Mathews S., Gobejishvili L., Cave M., McClain C., Barve S. (2009). Acrolein, a ubiquitous pollutant and lipid hydroperoxide product, inhibits antiviral activity of interferon-alpha: relevance to hepatitis C. Free Radic. Biol. Med. 47, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara D. I., Poynter M. E., Othman Z., Hemenway D., van der Vliet A. (2008). Acrolein inhalation suppresses lipopolysaccharide-induced inflammatory cytokine production but does not affect acute airways neutrophilia. J. Immunol. 181, 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiviswanathan R., Minko I. G., Lloyd R. S., Copeland W. C. (2013). Translesion synthesis past acrolein-derived DNA adducts by human mitochondrial DNA polymerase gamma. J. Biol. Chem. 288, 14247–14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem N. O., Daffa R. M., Liles S., Jackson S. R., Kassem N. O., Younis M. A., Mehta S., Chen M., Jacob P., 3rd, Carmella S. G., et al. (2014). Children's exposure to secondhand and thirdhand smoke carcinogens and toxicants in homes of hookah smokers. Nicotine Tobacco Res. 16, 961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y., Furuhata A., Toyokuni S., Aratani Y., Uchida K. (2003). Formation of acrolein-derived 2'-deoxyadenosine adduct in an iron-induced carcinogenesis model. J. Biol. Chem. 278, 50346–50354. [DOI] [PubMed] [Google Scholar]
- Kehrer J. P., Biswal S. S. (2000). The molecular effects of acrolein. Toxicol. Sci. 57, 6–15. [DOI] [PubMed] [Google Scholar]
- Keith R. J., Haberzettl P., Vladykovskaya E., Hill B. G., Kaiserova K., Srivastava S., Barski O., Bhatnagar A. (2009). Aldose reductase decreases endoplasmic reticulum stress in ischemic hearts. Chem. Biol. Int. 178, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J. C., Kehrer J. P. (2002). Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem. Biol. Int. 139, 79–95. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Lee I. C., Baek H. S., Shin I. S., Moon C., Bae C. S., Kim S. H., Kim J. C., Kim H. C. (2014). Mechanism for the protective effect of diallyl disulfide against cyclophosphamide acute urotoxicity in rats. Food Chem. Toxicol. 64, 110–118. [DOI] [PubMed] [Google Scholar]
- Kim G. D., Lee S. E., Kim T. H., Jin Y. H., Park Y. S., Park C. S. (2012). Melatonin suppresses acrolein-induced IL-8 production in human pulmonary fibroblasts. J. Pineal Res. 52, 356–364. [DOI] [PubMed] [Google Scholar]
- Kim C. E., Lee S. J., Seo K. W., Park H. M., Yun J. W., Bae J. U., Bae S. S., Kim C. D. (2010). Acrolein increases 5-lipoxygenase expression in murine macrophages through activation of ERK pathway. Toxicol. Appl. Pharmacol. 245, 76–82. [DOI] [PubMed] [Google Scholar]
- Kim S. I., Pfeifer G. P., Besaratinia A. (2007). Lack of mutagenicity of acrolein-induced DNA adducts in mouse and human cells. Cancer Res. 67, 11640–11647. [DOI] [PubMed] [Google Scholar]
- Kirkham P. A., Spooner G., Rahman I., Rossi A. G. (2004). Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophys. Res. Commun. 318, 32–37. [DOI] [PubMed] [Google Scholar]
- Kitaguchi Y., Taraseviciene-Stewart L., Hanaoka M., Natarajan R., Kraskauskas D., Voelkel N. F. (2012). Acrolein induces endoplasmic reticulum stress and causes airspace enlargement. PloS One 7, e38038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz A., Topal T., Oter S. (2007). Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol. Toxicol. 23, 303–312. [DOI] [PubMed] [Google Scholar]
- Lambert C., Li J., Jonscher K., Yang T. C., Reigan P., Quintana M., Harvey J., Freed B. M. (2007). Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J. Biol. Chem. 282, 19666–19675. [DOI] [PubMed] [Google Scholar]
- Lambert C., McCue J., Portas M., Ouyang Y., Li J., Rosano T. G., Lazis A., Freed B. M. (2005). Acrolein in cigarette smoke inhibits T-cell responses. J. Allergy Clin. Immunol. 116, 916–922. [DOI] [PubMed] [Google Scholar]
- Lee H. W., Wang H. T., Weng M. W., Hu Y., Chen W. S., Chou D., Liu Y., Donin N., Huang W. C., Lepor H., et al. (2014). Acrolein- and 4-aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget 5, 3526–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G., Sun W., Zheng L., Brookes S., Tully M., Shi R. (2011). Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune encephalomyelitis mouse. Neuroscience 173, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Q., Yu J. T., Jiang T., Tan L. (2014). Endoplasmic reticulum dysfunction in Alzheimer's Disease. Mol. Neurobiol. doi:10.1007/s12035-014-8695-8. [DOI] [PubMed] [Google Scholar]
- Liu D. S., Liu W. J., Chen L., Ou X. M., Wang T., Feng Y. L., Zhang S. F., Xu D., Chen Y. J., Wen F. Q. (2009). Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates acrolein-induced airway mucus hypersecretion in rats. Toxicology 260, 112–119. [DOI] [PubMed] [Google Scholar]
- Liu F., Li X. L., Lin T., He D. W., Wei G. H., Liu J. H., Li L. S. (2012). The cyclophosphamide metabolite, acrolein, induces cytoskeletal changes and oxidative stress in Sertoli cells. Mol. Biol. Rep. 39, 493–500. [DOI] [PubMed] [Google Scholar]
- Liu X. Y., Zhu M. X., Xie J. P. (2010). Mutagenicity of acrolein and acrolein-induced DNA adducts. Toxicol. Mech. Methods 20, 36–44. [DOI] [PubMed] [Google Scholar]
- Logue J. M., Price P. N., Sherman M. H., Singer B. C. (2012). A method to estimate the chronic health impact of air pollutants in U.S. residences. Environ. Health Persp. 120, 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopachin R. M., Barber D. S., Geohagen B. C., Gavin T., He D., Das S. (2007). Structure-toxicity analysis of type-2 alkenes: in vitro neurotoxicity. Toxicol. Sci. 95, 136–146. [DOI] [PubMed] [Google Scholar]
- Luo J., Shi R. (2004). Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem. Int. 44, 475–486. [DOI] [PubMed] [Google Scholar]
- MacAllister S. L., Martin-Brisac N., Lau V., Yang K., O'Brien P. J. (2013). Acrolein and chloroacetaldehyde: an examination of the cell and cell-free biomarkers of toxicity. Chem. Biol. Int. 202, 259–266. [DOI] [PubMed] [Google Scholar]
- Maczurek A., Hager K., Kenklies M., Sharman M., Martins R., Engel J., Carlson D. A., Munch G. (2008). Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer's disease. Adv. Drug Deliv. Rev. 60, 1463–1470. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Roth R. A., Ganey P. E. (2003). Allyl alcohol activation of protein kinase C delta leads to cytotoxicity of rat hepatocytes. Chem. Res. Toxicol. 16, 609–615. [DOI] [PubMed] [Google Scholar]
- Martyniuk C. J., Fang B., Koomen J. M., Gavin T., Zhang L., Barber D. S., Lopachin R. M. (2011). Molecular mechanism of glyceraldehyde-3-phosphate dehydrogenase inactivation by alpha, beta-unsaturated carbonyl derivatives. Chem. Res. Toxicol. 24, 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Navarro R., Nieto-Aguilar R., Alvares-Aguilar C. (2011). Protein conjugated with aldehydes derived from lipid peroxidation as an independent parameter of the carbonyl stress in the kidney damage. Lipids Health Dis. 10, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoi M., Yoshida M., Saiki R., Waragai M., Uemura K., Akatsu H., Kashiwagi K., Igarashi K. (2014). Distinction between mild cognitive impairment and Alzheimer's disease by CSF amyloid beta40 and beta42, and protein-conjugated acrolein. Clin. Chim. Acta 430, 150–155. [DOI] [PubMed] [Google Scholar]
- Mohammad M. K., Avila D., Zhang J., Barve S., Arteel G., McClain C., Joshi-Barve S. (2012). Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol. Appl. Pharmacol. 265, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto N., Bertolini S., Iadicicco C., Marchini G., Kaur M., Volpi G., Patacchini R., Singh D., Facchinetti F. (2012). Cigarette smoke and its component acrolein augment IL-8/CXCL8 mRNA stability via p38 MAPK/MK2 signaling in human pulmonary cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L929–L938. [DOI] [PubMed] [Google Scholar]
- Moretto N., Facchinetti F., Southworth T., Civelli M., Singh D., Patacchini R. (2009). Alpha, beta-unsaturated aldehydes contained in cigarette smoke elicit IL-8 release in pulmonary cells through mitogen-activated protein kinases. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L839–L848. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Myers J. M., Kufahl T. D., Forbes R., Szadkowski A. (2011). The effects of acrolein on the thioredoxin system: implications for redox-sensitive signaling. Mol. Nutr. Food Res. 55, 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Tomitori H., Suzuki T., Sakamoto A., Terui Y., Saiki R., Dohmae N., Igarashi K., Kashiwagi K. (2013). Inactivation of GAPDH as one mechanism of acrolein toxicity. Biochem. Biophys. Res. Commun. 430, 1265–1271. [DOI] [PubMed] [Google Scholar]
- O'Toole T. E., Abplanalp W., Li X., Cooper N., Conklin D. J., Haberzettl P., Bhatnagar A. (2014). Acrolein decreases endothelial cell migration and insulin sensitivity through induction of Let-7a. Toxicol. Sci. 140, 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole T. E., Zheng Y. T., Hellmann J., Conklin D. J., Barski O., Bhatnagar A. (2009). Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol. Appl. Pharmacol. 236, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong F. H., Henry P. J., Burcham P. C. (2012). Prior exposure to acrolein accelerates pulmonary inflammation in influenza A-infected mice. Toxicol. Lett. 212, 241–251. [DOI] [PubMed] [Google Scholar]
- Park J., Zheng L., Marquis A., Walls M., Duerstock B., Pond A., Vega-Alvarez S., Wang H., Ouyang Z., Shi R. (2014). Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J. Neurochem. 129, 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowicz A. J., Kronberg L. (2008). Characterization of adducts formed in reactions of acrolein with thymidine and calf thymus DNA. Chem. Biodivers. 5, 177–188. [DOI] [PubMed] [Google Scholar]
- Pawlowicz A. J., Munter T., Klika K. D., Kronberg L. (2006a). Reaction of acrolein with 2'-deoxyadenosine and 9-ethyladenine–formation of cyclic adducts. Bioorg. Chem. 34, 39–48. [DOI] [PubMed] [Google Scholar]
- Pawlowicz A. J., Munter T., Zhao Y., Kronberg L. (2006b). Formation of acrolein adducts with 2'-deoxyadenosine in calf thymus DNA. Chem. Res. Toxicol. 19, 571–576. [DOI] [PubMed] [Google Scholar]
- Perez C. M., Ledbetter A. D., Hazari M. S., Haykal-Coates N., Carll A. P., Winsett D. W., Costa D. L., Farraj A. K. (2013). Hypoxia stress test reveals exaggerated cardiovascular effects in hypertensive rats after exposure to the air pollutant acrolein. Toxicol. Sci. 132, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzimenti S., Ciamporcero E., Daga M., Pettazzoni P., Arcaro A., Cetrangolo G., Minelli R., Dianzani C., Lepore A., Gentile F., Barrera G. (2013). Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 4, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugazhenthi S., Phansalkar K., Audesirk G., West A., Cabell L. (2006). Differential regulation of c-jun and CREB by acrolein and 4-hydroxynonenal. Free Radic. Biol. Med. 40, 21–34. [DOI] [PubMed] [Google Scholar]
- Ranganna K., Yousefipour Z., Nasif R., Yatsu F. M., Milton S. G., Hayes B. E. (2002). Acrolein activates mitogen-activated protein kinase signal transduction pathways in rat vascular smooth muscle cells. Mol. Cell. Biochem. 24, 83–98. [DOI] [PubMed] [Google Scholar]
- Ribeiro C. M., O'Neal W. K. (2012). Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr. Mol. Med. 12, 872–882. [DOI] [PubMed] [Google Scholar]
- Rom O., Kaisari S., Aizenbud D., Reznick A. Z. (2013). The effects of acetaldehyde and acrolein on muscle catabolism in C2 myotubes. Free Radic. Biol. Med. 65C, 190–200. [DOI] [PubMed] [Google Scholar]
- Roy J., Pallepati P., Bettaieb A., Averill-Bates D. A. (2010). Acrolein induces apoptosis through the death receptor pathway in A549 lung cells: role of p53. Can. J. Physiol. Pharmacol. 88, 353–368. [DOI] [PubMed] [Google Scholar]
- Roy J., Pallepati P., Bettaieb A., Tanel A., Averill-Bates D. A. (2009). Acrolein induces a cellular stress response and triggers mitochondrial apoptosis in A549 cells. Chem. Biol. Int. 181, 154–167. [DOI] [PubMed] [Google Scholar]
- Sarkar P., Hayes B. E. (2007). Induction of COX-2 by acrolein in rat lung epithelial cells. Mol. Cell. Biochem. 301, 191–199. [DOI] [PubMed] [Google Scholar]
- Seiner D. R., LaButti J. N., Gates K. S. (2007). Kinetics and mechanism of protein tyrosine phosphatase 1B inactivation by acrolein. Chem. Res. Toxicol. 20, 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B. (2012). Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim. Biophys. Acta 1821, 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Sun W., McBride J. J., Cheng J. X., Shi R. (2011). Acrolein induces myelin damage in mammalian spinal cord. J. Neurochem. 117, 554–564. [DOI] [PubMed] [Google Scholar]
- Song J. J., Lee J. D., Lee B. D., Chae S. W., Park M. K. (2013). Effect of acrolein, a hazardous air pollutant in smoke, on human middle ear epithelial cells. Int. J. Pediatr. Otorhinolaryngol. 77, 1659–1664. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Sithu S. D., Vladykovskaya E., Haberzettl P., Hoetker D. J., Siddiqui M. A., Conklin D. J., D'Souza S. E., Bhatnagar A. (2011). Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis 215, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. F., Maier C. S. (2008). Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 52, 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Luo C., Long J., Wei D., Liu J. (2006). Acrolein is a mitochondrial toxin: effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Mitochondrion 6, 136–142. [DOI] [PubMed] [Google Scholar]
- Sun Y., Ito S., Nishio N., Tanaka Y., Chen N., Isobe K. I. (2014). Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol. Lett. 229, 384–392. [DOI] [PubMed] [Google Scholar]
- Tanel A., Averill-Bates D. A. (2005). The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochim. Biophys. Acta 1743, 255–267. [DOI] [PubMed] [Google Scholar]
- Tanel A., Averill-Bates D. A. (2007a). Activation of the death receptor pathway of apoptosis by the aldehyde acrolein. Free Radic. Bio. Med. 42, 798–810. [DOI] [PubMed] [Google Scholar]
- Tanel A., Averill-Bates D. A. (2007b). Inhibition of acrolein-induced apoptosis by the antioxidant N-acetylcysteine. J. Pharmacol. Exp. Ther. 321, 73–83. [DOI] [PubMed] [Google Scholar]
- Tanel A., Pallepati P., Bettaieb A., Morin P., Averill-Bates D. A. (2014). Acrolein activates cell survival and apoptotic death responses involving the endoplasmic reticulum in A549 lung cells. Biochim. Biophys. Acta 1843, 827–835. [DOI] [PubMed] [Google Scholar]
- Tang M. S., Wang H. T., Hu Y., Chen W. S., Akao M., Feng Z., Hu W. (2011). Acrolein induced DNA damage, mutagenicity and effect on DNA repair. Mol. Nutr. Food Res. 55, 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxicological Profile For ACROLEIN. (2007). Available at: http://www.atsdr.cdc.gov/ToxProfiles/tp124.pdf. Accessed November 10, 2014.
- Tran T. N., Kosaraju M. G., Tamamizu-Kato S., Akintunde O., Zheng Y., Bielicki J. K., Pinkerton K., Uchida K., Lee Y. Y., Narayanaswami V. (2014). Acrolein modification impairs key functional features of rat apolipoprotein E: identification of modified sites by mass spectrometry. Biochemistry 53, 361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Kanematsu M., Morimitsu Y., Osawa T., Noguchi N., Niki E. (1998a). Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 273, 16058–16066. [DOI] [PubMed] [Google Scholar]
- Uchida K., Kanematsu M., Sakai K., Matsuda T., Hattori N., Mizuno Y., Suzuki D., Miyata T., Noguchi N., Niki E., Osawa T. (1998b). Protein-bound acrolein: potential markers for oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 95, 4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacaru A. M., Di Narzo A. F., Howarth D. L., Tsedensodnom O., Imrie D., Cinaroglu A., Amin S., Hao K., Sadler K. C. (2014). Molecularly defined unfolded protein response subclasses have distinct correlations with fatty liver disease in zebrafish. Dis. Model. Mech. 7, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valacchi G., Pagnin E., Phung A., Nardini M., Schock B. C., Cross C. E., van der Vliet A. (2005). Inhibition of NFkappaB activation and IL-8 expression in human bronchial epithelial cells by acrolein. Antioxid. Redox Signal. 7, 25–31. [DOI] [PubMed] [Google Scholar]
- van der Toorn M., Smit-de Vries M. P., Slebos D. J., de Bruin H. G., Abello N., van Oosterhout A. J., Bischoff R., Kauffman H. F. (2007). Cigarette smoke irreversibly modifies glutathione in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1156–L1162. [DOI] [PubMed] [Google Scholar]
- Vidro-Kotchan E., Yendluri B. B., Le-Thai T., Tsin A. (2011). NBHA reduces acrolein-induced changes in ARPE-19 cells: possible involvement of TGFbeta. Curr. Eye Res. 36, 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi G., Facchinetti F., Moretto N., Civelli M., Patacchini R. (2011). Cigarette smoke and alpha, beta-unsaturated aldehydes elicit VEGF release through the p38 MAPK pathway in human airway smooth muscle cells and lung fibroblasts. Br. J. Pharmacol. 163, 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Tungeln L. S., Yi P., Bucci T. J., Samokyszyn V. M., Chou M. W., Kadlubar F. F., Fu P. P. (2002). Tumorigenicity of chloral hydrate, trichloroacetic acid, trichloroethanol, malondialdehyde, 4-hydroxy-2-nonenal, crotonaldehyde, and acrolein in the B6C3F(1) neonatal mouse. Cancer Lett. 185, 13–19. [DOI] [PubMed] [Google Scholar]
- Voulgaridou G. P., Anestopoulos I., Franco R., Panayiotidis M. I., Pappa A. (2011). DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat. Res. 711, 13–27. [DOI] [PubMed] [Google Scholar]
- Wang G. W., Guo Y., Vondriska T. M., Zhang J., Zhang S., Tsai L. L., Zong N. C., Bolli R., Bhatnagar A., Prabhu S. D. (2008). Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J. Mol. Cell. Cardiol. 44, 1016–1022. [DOI] [PubMed] [Google Scholar]
- Wang H. T., Hu Y., Tong D., Huang J., Gu L., Wu X. R., Chung F. L., Li G. M., Tang M. S. (2012). Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J. Biol. Chem. 287, 12379–12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu Y., Chen L., Wang X., Hu X. R., Feng Y. L., Liu D. S., Xu D., Duan Y. P., Lin J., et al. (2009). Effect of sildenafil on acrolein-induced airway inflammation and mucus production in rats. Eur. Respir. J. 33, 1122–1132. [DOI] [PubMed] [Google Scholar]
- Wang L., Sun Y., Asahi M., Otsu K. (2011). Acrolein, an environmental toxin, induces cardiomyocyte apoptosis via elevated intracellular calcium and free radicals. Cell Biochem. Biophys. 61, 131–136. [DOI] [PubMed] [Google Scholar]
- Wang H. T., Weng M. W., Chen W. C., Yobin M., Pan J., Chung F. L., Wu X. R., Rom W., Tang M. S. (2013). Effect of CpG methylation at different sequence context on acrolein- and BPDE-DNA binding and mutagenesis. Carcinogenesis 34, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Nakazato Y., Saiki R., Igarashi K., Kitada M., Ishii I. (2013). Acrolein-conjugated low-density lipoprotein induces macrophage foam cell formation. Atherosclerosis 227, 51–57. [DOI] [PubMed] [Google Scholar]
- Watzek N., Scherbl D., Feld J., Berger F., Doroshyenko O., Fuhr U., Tomalik-Scharte D., Baum M., Eisenbrand G., Richling E. (2012). Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps. Mol. Nutr. Food Res. 56, 1825–1837. [DOI] [PubMed] [Google Scholar]
- Wu C. C., Hsieh C. W., Lai P. H., Lin J. B., Liu Y. C., Wung B. S. (2006). Upregulation of endothelial heme oxygenase-1 expression through the activation of the JNK pathway by sublethal concentrations of acrolein. Toxicol. Appl. Pharmacol. 214, 244–252. [DOI] [PubMed] [Google Scholar]
- Yadav U. C., Ramana K. V. (2013). Regulation of NF-kappaB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid. Med. Cell. Longev. 2013, 690545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Mikami T., Higashi K., Saiki R., Mizoi M., Fukuda K., Nakamura T., Ishii I., Nishimura K., Toida T., et al. (2012). Inverse correlation between stroke and urinary 3-hydroxypropyl mercapturic acid, an acrolein-glutathione metabolite. Clin. Chim. Acta 413, 753–759. [DOI] [PubMed] [Google Scholar]
- Zarkovic N., Cipak A., Jaganjac M., Borovic S., Zarkovic K. (2013). Pathophysiological relevance of aldehydic protein modifications. J. Proteomics 92, 239–247. [DOI] [PubMed] [Google Scholar]
- Zhang H., Forman H. J. (2008). Acrolein induces heme oxygenase-1 through PKC-delta and PI3K in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 38, 483–490. [DOI] [PubMed] [Google Scholar]
- Zheng L., Park J., Walls M., Tully M., Jannasch A., Cooper B., Shi R. (2013). Determination of urine 3-HPMA, a stable acrolein metabolite in a rat model of spinal cord injury. J. Neurotrauma 30, 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A. X., Tabas I. (2013). The UPR in atherosclerosis. Semin. Immunopathol. 35, 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Liu Z., Feng Z., Hao J., Shen W., Li X., Sun L., Sharman E., Wang Y., Wertz K., et al. (2010). Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 21, 1089–1098. [DOI] [PubMed] [Google Scholar]