Abstract
In the past decade, autologous platelet-rich plasma (PRP) therapy has seen increasingly widespread integration into medical specialties. PRP application is known to accelerate wound epithelialization rates, and may also reduce postoperative wound site pain. Recently, we observed an increase in patient satisfaction following PRP gel (Angel, Cytomedix, Rockville, MD) application to split-thickness skin graft (STSG) donor sites. We assessed all patients known to our university-based hospital service who underwent multiple STSGs up to the year 2014, with at least one treated with topical PRP. Based on these criteria, five patients aged 48.4±17.6 (80% male) were identified who could serve as their own control, with mean time of 4.4±5.1 years between operations. In both therapies, initial dressing changes occurred on postoperative day (POD) 7, with donor site pain measured by Likert visual pain scale. Paired t-tests compared the size and thickness of harvested skin graft and patient pain level, and STSG thickness and surface area were comparable between control and PRP interventions (p>0.05 for all). Donor site pain was reduced from an average of 7.2 (±2.6) to 3 (±3.7), an average reduction in pain of 4.2 (standard error 1.1, p=0.0098) following PRP use. Based on these results, the authors suggest PRP as a beneficial adjunct for reducing donor site pain following STSG harvest.
Keywords: skin grafts, platelet-rich plasma, diabetic foot, pain reduction
Over the past three decades, greater understanding into the physiologic pathways involved in wound healing has yielded a number of novel wound treatment products (1). One of these products, autologous platelet-rich plasma (PRP), has seen increasingly widespread use across medical specialties, finding regular application in the fields of chronic neuropathic wounds (2), maxillofacial bone defects (3), and cosmetic (4), spinal (5), and reconstructive surgery (6).
The extraction of PRP begins with any peripheral venous or access. The extraction of platelet concentrate from patient blood occurs via plasmapheresis, whereby PRP is concentrated to 300% of normal blood levels (7). This extracted plasma is then activated to a gel-like substance consisting of a fibrin matrix scaffold with platelet releasate contents and multiple growth factors. The degranulation of platelets by proteins such as thrombin initiates the release of different growth factors such as platelet-derived growth factor-AB, transforming growth factor beta-1, and vascular endothelial growth factor (8). These growth factors are thought to be responsible for the observed increased rate of epithelialization and pain reduction at wound sites (2).
In the developed and developing nations alike, the treatment of high-risk diabetic wounds remains costly, complicated, and may often require the adjunctive use of split-thickness skin grafts (STSG) to expedite wound healing (9–13). However, graft donor sites, especially those of the dorsal thigh, are often a primary source of discomfort postoperatively. Currently, the focus of clinical research with regard to PRP therapy on graft donor sites emphasizes wound healing and epithelialization rates. Pain reduction is typically only mentioned secondarily, pertaining strictly to dressing changes, or in the acute trauma setting (7). In this study, we used autologous PRP gel to treat soft tissue wounds created by STSG harvest. Our aim is to preliminarily assess if treating these wounds with topical PRP gel mitigates patient pain following a STSG harvest from the thigh.
Materials and methods
This pilot study reviewed all patients known to our university-based limb salvage service who underwent multiple STSGs up to the year 2014. Inclusion criteria: patients of either sex, aged 18–80, with one STSG donor site treated with PRP, and one without. Five patients aged 48.4±17.6 and 80% male met inclusion criteria with a mean time between operations of 4.4±5.1 years. Three out of five patients were positive for longstanding history of type 2 diabetes mellitus with diabetic peripheral neuropathy and previous lower extremity amputations in the foot.
All treatments were highly standardized. Control donor sites were dressed with Xeroform (Medline Industries, Mundelein, IL), Tegaderm (3M, Two Harbors, MN), gauze padding, and light ACE compression bandage (Johnson & Johnson, New Brunswick, NJ). Interventional donor sites were treated with intraoperative autologous PRP gel and dressed with SorbaView (Centurion Medical, Williamston, MI), Tegaderm, gauze padding, and light ACE compression bandage, in ascending order. In both therapies, initial dressing changes occurred on postoperative day (POD) 7 and donor site pain was measured using a Likert visual pain scale. Table 1 further summarizes the patient demographics in this cohort.
Table 1.
Cohort demographics
| Patient | Age, gender | Past Medical History |
|---|---|---|
| 1 | 33, M | Diabetes mellitus type 2 with diabetic foot infection and amputation, diabetic peripheral neuropathy, hypertension |
| 2 | 38, M | Pulmonary hypertension and 20-year history of chronic painful venous stasis ulcers |
| 3 | 43, M | Diabetes mellitus type 2 with diabetic foot infection and amputation, diabetic peripheral neuropathy, hypertension, hyperlipidemia, hypercholesterolemia, and alcohol dependency |
| 4 | 55, M | Diabetes mellitus type 2, hypertension, peripheral arterial disease, Fournier's gangrene, depression |
| 5 | 70, F | Ovarian cancer and vasculitis |
STSG extraction and application method
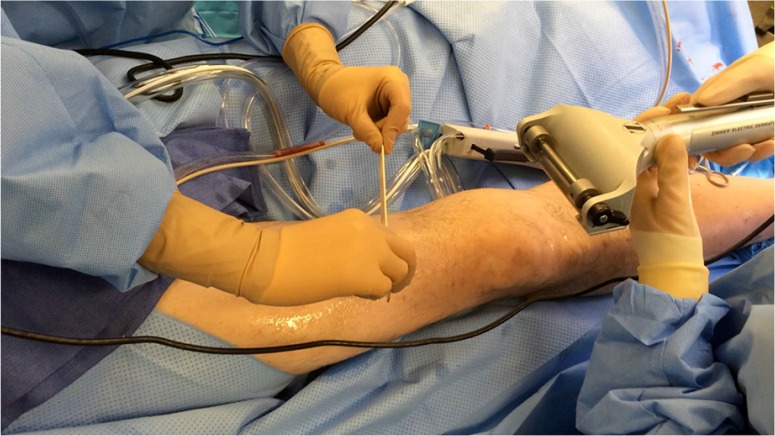
Our university-based limb salvage service's standard STSG protocol is as follows. Donor sites receive 15–30 mL of local anesthesia subdermally, typically either 1% lidocaine with 1:100,000 epinephrine or 0.25 or 0.5% bupivacaine with 1:200,000 epinephrine. Copious mineral oil is applied to the donor site and Zimmer dermatome (Zimmer Inc., Warsaw, IN) used for extraction (Fig. 1). All patient grafts were harvested with a thickness of 0.016-0.020 in., and meshed to a ratio of 1.5:1 or 2:1. Following preparation, the grafts are applied to the wound site and secured using either chromic gut suture or skin staples.
Fig. 1.
A sample thigh is prepared for skin graft harvesting. The selected area has been cleansed, shaved, and all equipment covered in copious mineral oil. In this picture, the surgeons are preparing for a two-person harvest technique using a Zimmer dermatome.
Preparation and application of PRP gel
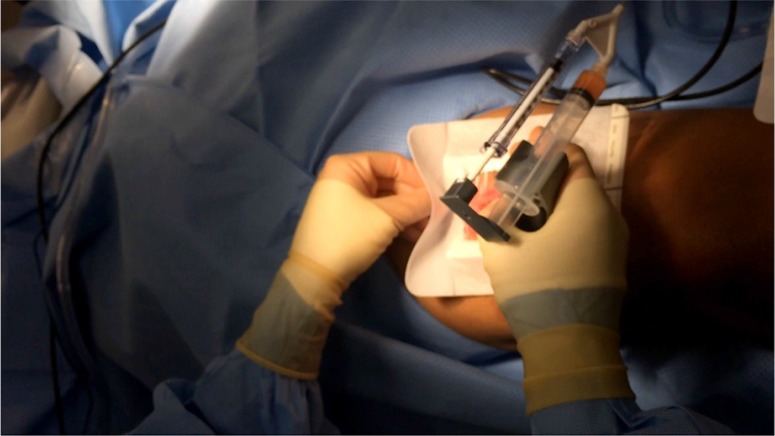
A variety of extraction and preparation methods exist for PRP gel. In this case series, the following procedure was followed. After general anesthesia induction in the operating room, 40–180 mL of blood was drawn from the patient's peripheral access per anticipated graft size. This blood is transferred into an Angel (Cytomedix Inc., Gaithersburg, MD) centrifuge chamber (Fig. 2) and spun for 15–30 min, concentrating the platelets to 10–12× patient baseline levels. PRP is then extracted from the buffy coat into an empty, sterile syringe, including a 2% dip into the red blood cell layer. This syringe is attached to a rationed dual-liquid tip adapter, the second chamber containing a previously prepared 1:1 mixture of 5,000 units thrombin and 0.5 g of calcium chloride. Using this dual applicator device, both syringes may be administered in tandem, creating a PRP gel in a 10:1 ratio of the PRP to Calcium Chloride/Thrombin mixture.
Fig. 2.
The machine used to centrifuge the extracted blood samples.
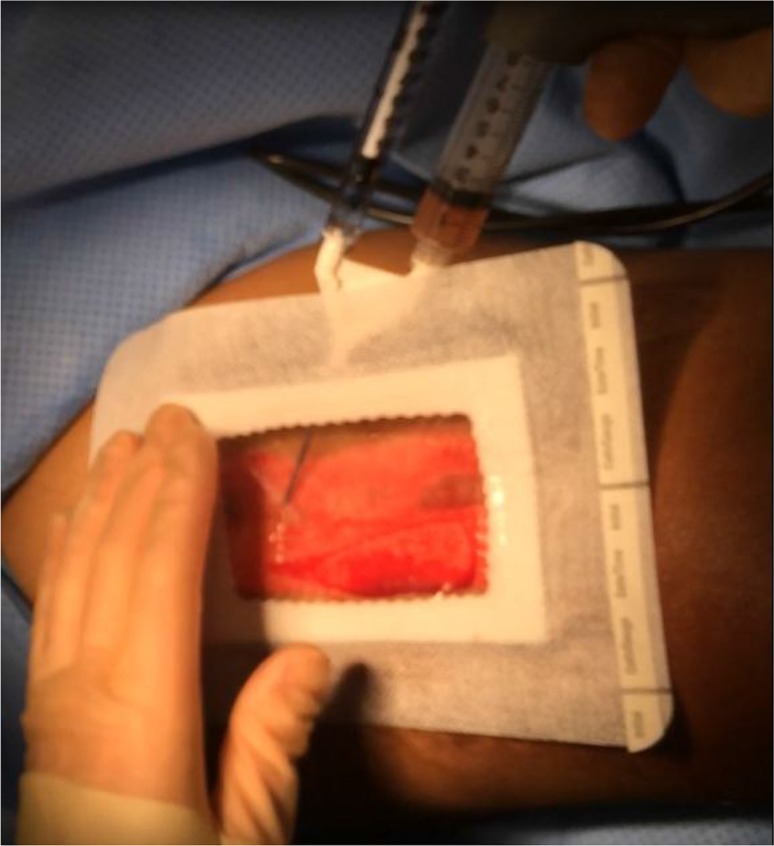
The resulting mixture is then liberally applied to the harvest site and covered with SorbaView dressing (Figs. 3 and 4). Dressings with non-adhesive backing in the central portion are ideal, keeping the PRP mixture in direct contact with the wound while preventing leakage via a semi-hydrophobic border. The area is then covered with an additional large Tegaderm dressing, 4×4 gauze, and light elastic bandage compression to be removed on POD 7.
Fig. 3.
With the dual-chambered PRP and thrombin syringe mixture prepared, the non-adhesive dressing is applied over the donor site. This is secured on three of the four sides, windowing the dressing to facilitate the application of PRP directly to the wound.
Fig. 4.
PRP is applied through the remaining windowed portion of the dressing. This dressing will then be sealed, covered in a large Tegaderm, gauze padding, and light compression via elastic bandage. First dressing change scheduled on postoperative day 7.
Results
Information on patients’ age, gender, past medical history, graft size, postoperative management, and pain scales for both with and without PRP application to the harvest sites are presented in Tables 1 and 2. For each patient, STSG thickness and surface area were comparable between control and PRP intervention (P>0.05). Donor site pain was reduced from an average of 7.2±2.6 to 3±3.7. The average reduction in pain with PRP was 4.2 with a standard error of 1.1 (p=0.0098). STSG thickness and surface area were comparable between control and PRP interventions (p>0.05 for all). No adverse reaction, infection, or additional complication was observed in those receiving topical PRP.
Table 2.
STSG with and without PRP therapy
| Patient | Graft size | Local anesthesia | Pain | Pain management |
|---|---|---|---|---|
| STSG with PRP therapy | ||||
| 1 | 55 cm2 at 0.16 in. thickness | 18 mL bupivacaine 0.25% with 1:200,000 epinephrine | 1 | Oxycodone 30 mg every 6 h and MS Contin 200 mg three times a day |
| 2 | 500 cm2 at 0.18 in. thickness | 30 mL lidocaine 1% with 1:100,000 epinephrine | 8 | Continuous epidural catheter of bupivacaine, PCA of hydromorphone 0.2 mg q10 min, gabapentin 400 mg |
| 3 | 24 cm2 at 0.18 in. thickness | 15 mL bupivacaine 0.25% with 1:200,000 epinephrine | 0 | One 500 mg acetaminophen |
| 4 | 24 cm2 at 0.18 in. thickness | 20 mL bupivacaine 0.25% with 1:200,000 epinephrine | 6 | Oxycodone 20 mg every 12 h |
| 5 | 8 cm2 at 0.16 in. thickness | 10 mL bupivacaine 0.25% with 1:200,000 epinephrine | 0 | One Percocet 5/325 mg every 4–6 h |
| STSG without PRP therapy | ||||
| 1 | 40 cm2 at 0.16 in. thickness | 15 mL bupivacaine 0.25% with 1:200,000 epinephrine | 9 | Oxycodone 30 mg every 6 h and MS contin 200 mg three times a day. His pain was later managed with PCA morphine. |
| 2 | 616 cm2 at 0.20 in. thickness | 30 mL lidocaine 1% with 1:100,000 epinephrine | 10 | Percocet 5/325 mg 1–2 tablets every 4 h, nortriptyline HCL 25 mg once daily, gabapentin 600 mg twice daily. |
| 3 | 15 cm2 at 0.20 in. thickness | 15 mL lidocaine 1% with epinephrine 1:100,000 | 5 | Percocet 5/325 mg 1–2 tablets every 4 h |
| 4 | About 24 cm2 | Information not available | 8 | Information not available |
| 5 | About 8 cm2 | Information not available | 4 | Information not available |
Discussion
Previous studies correlate regular application of PRP with pain reduction during gauze changes (14). However, none seem to have evaluated the overall postoperative pain of the donor site. Pain is a subjective finding that can be difficult to objectively quantify across individuals, but the use of each participant as their own control allowed us to eliminate some inter-participant variability. As seen in Table 3, we found patients’ postoperative pain to be better tolerated both by subjective Likert scale and by reduction in narcotic use following PRP application (Table 2). These results are similar to a study of PRP use in total knee arthroplasty, which demonstrated reduction in patients’ postoperative intravenous (P=0.024) and oral (P=0.063) narcotic use (15).
Table 3.
Extracted STSG pain survey – Likert pain scale
| Patient | Without PRP | With PRP |
|---|---|---|
| 1 | 9 | 1 |
| 2 | 10 | 8 |
| 3 | 5 | 0 |
| 4 | 8 | 6 |
| 5 | 4 | 0 |
Of note, while all patients noted a reduction in pain from the PRP intervention, two patients (patients 3 and 5) responded with a pain rating of zero or no pain at all. In addition, a third patient (patient 1) reportedly went from 9/10 (near maximum) with standard control dressings to 1/10 (near minimum) with PRP therapy.
In addition to pain management, previous attention has been directed toward healing rates and scar reduction in STSG donor sites by various methods (16–20). Acceptance of PRP in promoting epithelialization has been a point of contention; however, it seems that regular dressing changes have a synergistic effect with PRP (3, 14, 21, 22). This reinforces the connection between proper postoperative wound maintenance, and positive wound outcomes.
Postoperative topical PRP for STSG donor sites is cost effective, safe, and easy to use. Previous works estimate an $18,000 cost reduction per patient over 5 years when comparing topical PRP intervention with a control (saline gel) (23). In addition, during the course of our study, no adverse reactions, infections, or additional complications were observed in the patients receiving topical PRP; likely the result of its autology. Time required to centrifuge blood in PRP preparation was consistent with other researchers, between 15 min for 40 mL of blood and 30 min for 180 mL, and requiring little coordination and additional staff to implement (7).
Several limitations are inherent in this study. This was a retrospective analysis of a small, comorbid cohort. Furthermore, patients who were able to compare their previous standard STSG with this novel interventional approach may have had some memory distortion due to the varying length of time in between operations. It is possible the second occurrence of a similar surgical procedure may seem less painful as an effect of ‘learned experience’. Future work should continue with larger, prospective, and randomized control trials.
Conclusion
These provisional data suggest that PRP may provide some degree of STSG donor site pain relief. By reducing donor site pain, PRP may also have the potential to reduce analgesic usage postoperatively and shorten hospital stay. We look forward to further works that might confirm or refute these initial findings.
Conflict of interest and funding
The authors declare no conflict of interest or having received any funding or benefits from industry to conduct this study.
References
- 1.Isaac AL, Armstrong DG. Negative pressure wound therapy and other new therapies for diabetic foot ulceration: the current state of play. Med Clin North Am. 2013;97:899–909. doi: 10.1016/j.mcna.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2001;24:483–8. doi: 10.2337/diacare.24.3.483. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 4.Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–37. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- 5.Hee HT, Majd ME, Holt RT, Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12:400–7. doi: 10.1007/s00586-003-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352–60. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 7.Kazakos K, Lyras DN, Verettas D, Tilkeridis K, Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury. 2009;40:801–5. doi: 10.1016/j.injury.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Kushida S, Kakudo N, Morimoto N, Hara T, Ogawa T, Mitsui T, et al. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. J Artif Organs. 2014;17:186–92. doi: 10.1007/s10047-014-0761-5. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong DG, Lavery LA, Harkless LB, Van Houtum WH. Amputation and reamputation of the diabetic foot. J Am Podiatr Med Assoc. 1997;87:255–9. doi: 10.7547/87507315-87-6-255. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DG, Harkless LB. Outcomes of preventative care in a diabetic foot specialty clinic. J Foot Ankle Surg. 1998;37:460–6. doi: 10.1016/s1067-2516(98)80022-7. [DOI] [PubMed] [Google Scholar]
- 11.Mills JL, Sr, Conte MS, Armstrong D, Pomposelli F, Schanzer A, Sidawy AN, et al. The Society of Vasulcar Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on Wound, Ischemia and foot Infection (WIfI) J Vasc Surg. 2014;59:220–34. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Rose JF, Giovinco N, Mills JL, Najafi B, Pappalardo J, Armstrong DG. Split-thickness skin grafting the high-risk diabetic foot. J Vasc Surg. 2014;59:1657–63. doi: 10.1016/j.jvs.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 13.Ramanujam CL, Han D, Fowler S, Kilpadi K, Zgonis T. Impact of diabetes and comorbidities on split-thickness skin grafts for foot wounds. J Am Podiatr Med Assoc. 2013;103:223–32. doi: 10.7547/1030223. [DOI] [PubMed] [Google Scholar]
- 14.Kakudo N, Kushida S, Minakata T, Suzuki K, Kusumoto K. Platelet-rich plasma promotes epithelialization and angiogenesis in a split-thickness skin graft donor site. Med Mol Morphol. 2011;44:233–6. doi: 10.1007/s00795-010-0532-1. [DOI] [PubMed] [Google Scholar]
- 15.Gardner MJ, Demetrakopoulos D, Klepchick PR, Mooar PA. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty. An analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop. 2007;31:309–13. doi: 10.1007/s00264-006-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson M, Lindgren M, Jarnhed-Andersson I, Tarpila E. Dressing the split-thickness skin graft donor site: a randomized clinical trial. Adv Skin Wound Care. 2014;27:20–5. doi: 10.1097/01.ASW.0000437786.92529.22. [DOI] [PubMed] [Google Scholar]
- 17.Uraloglu M, Livaoglu M, Agdogan O, Mungan S, Alhan E, Karacal N. An evaluation of five different dressing materials on split-thickness skin graft donor site and full-thickness cutaneous wounds: an experimental study. Int Wound J. 2014;11:85–92. doi: 10.1111/j.1742-481X.2012.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuangsuwanich A, Arunakul S, Kamnerdnakta S. The efficacy of combined herbal extracts gel in reducing scar development at a split-thickness skin graft donor site. Aesthetic Plast Surg. 2013;37:770–7. doi: 10.1007/s00266-013-0140-2. [DOI] [PubMed] [Google Scholar]
- 19.Lairet KF, Baer D, Leas ML, Renz EM, Cancio LC. Evaluation of an oxygen-diffusion dressing for accelerated healing of donor-site wounds. J Burn Care Res. 2014;35:214–18. doi: 10.1097/BCR.0b013e31829b3338. [DOI] [PubMed] [Google Scholar]
- 20.Blome-Eberwein S, Abboud M, Lozano DD, Sharma R, Eid S, Gogal C. Effect of subcutaneous epinephrine/saline/local anesthetic versus saline-only injection on split-thickness skin graft donor site perfusion, healing, and pain. J Burn Care Res. 2013;34:e80–6. doi: 10.1097/BCR.0b013e31825d5414. [DOI] [PubMed] [Google Scholar]
- 21.Danielsen P, Jørgensen B, Karlsmark T, Jorgensen LN, Ågren MS. Effect of topical autologous platelet-rich fibrin versus no intervention on epithelialization of donor sites and meshed split-thickness skin autografts: a randomized clinical trial. Plast Reconstr Surg. 2008;122:1431–40. doi: 10.1097/PRS.0b013e318188202c. [DOI] [PubMed] [Google Scholar]
- 22.Carter MJ, Fylling CP, Parnell LKS. Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty. 2011;11:e38. [PMC free article] [PubMed] [Google Scholar]
- 23.Dougherty EJ. An evidence-based model comparing the cost-effectiveness of platelet-rich plasma gel to alternative therapies for patients with nonhealing diabetic foot ulcers. Adv Skin Wound Care. 2008;21:568–75. doi: 10.1097/01.ASW.0000323589.27605.71. [DOI] [PubMed] [Google Scholar]