A considerable number of plants depend on structural support of other plants. To understand their diversity and ecology, it is essential to know how strongly potential host species differ in their suitability as hosts. This review focuses on vascular epiphytes, i.e. structurally dependent plants that do not parasitize their hosts. Despite a longstanding interest in the topic, our knowledge on the strength of their host specificity is still scanty. This is arguably due to conceptual confusion, but also because of the large complexity of the study system, which turns quantifying host specificity in the field into a challenge.
Keywords: Biodiversity, host bias, host preference, host specificity, specialization, structurally dependent plants, vascular epiphytes
Abstract
Information on the degree of host specificity is fundamental for an understanding of the ecology of structurally dependent plants such as vascular epiphytes. Starting with the seminal paper of A.F.W. Schimper on epiphyte ecology in the late 19th century over 200 publications have dealt with the issue of host specificity in vascular epiphytes. We review and critically discuss this extensive literature. The available evidence indicates that host ranges of vascular epiphytes are largely unrestricted while a certain host bias is ubiquitous. However, tree size and age and spatial autocorrelation of tree and epiphyte species have not been adequately considered in most statistical analyses. More refined null expectations and adequate replication are needed to allow more rigorous conclusions. Host specificity could be caused by a large number of tree traits (e.g. bark characteristics and architectural traits), which influence epiphyte performance. After reviewing the empirical evidence for their relevance, we conclude that future research should use a more comprehensive approach by determining the relative importance of various potential mechanisms acting locally and by testing several proposed hypotheses regarding the relative strength of host specificity in different habitats and among different groups of structurally dependent flora.
Introduction
The relative breadth of tolerance or utilization of a species along particular niche axes is one of the most fundamental topics in biology (Futuyma and Moreno 1988). Niche axes of interest may be climatic variables, abiotic resources, but also potential biotic interaction partners, e.g. the set of potential host species for a guild of host-dependent species. The degree of host specificity has been studied for many different interaction types, including antagonistic (e.g. herbivory, parasitism), mutualistic (e.g. pollination, mycorrhizae) and commensalistic (e.g. epiphytism) ones.
Understanding the degree of host specificity of such host-dependent species is an important piece of the ‘diversity jigsaw puzzle’ (Kitching 2006). In principle, the diversity of a host-dependent guild may be a function of the diversity of the host guild as suggested for herbivorous insects in tropical rain forests (Novotny et al. 2006). Strong host specificity opens the possibility of sympatric speciation and may allow species coexistence by niche complementarity. Determining the degree of host specificity is also important in a conservation context because specialist species are generally more vulnerable to habitat alterations and climate change (Clavel et al. 2011) than generalist species, and host specialists, in particular, are threatened by coextinction with their hosts (Tremblay et al. 1998; Colwell et al. 2012).
Specificity is generally assumed to be the result of trade-offs between adaptations that (i) allow organisms to cope with diverse environmental conditions or (ii) allow the exploitation of different resource types. Specialization and generalization (i.e. the evolutionary processes of decreasing or increasing niche axis breadth) depend on the environmental heterogeneity experienced by a population (Futuyma and Moreno 1988; Poisot et al. 2011; Kassen 2002). Evolutionary pressure for generalization should be high in spatially or temporally fine grained, heterogeneous habitats—i.e. whenever members of the same population are likely to encounter heterogeneous resources or environmental conditions. In contrast, whenever the spatio-temporal grain is large relative to the dispersal ability or longevity of individuals, specialization should occur. Active habitat or resource selection (i.e. preference or avoidance) may reinforce specialization when preference and performance bias are matching (Ravigné et al. 2009). The expected degree of ‘host’ specificity should also depend on the type of species interaction. Specialization may be reinforced by coevolution in (i) antagonistic relationships (e.g. plant–herbivore systems) because of an ‘arms race’ (Ehrlich and Raven 1964) and (ii) in the mutualistic plant–pollinator system because plants may evolve mechanisms to favour specialist pollinators which are more effective than generalists (Blüthgen et al. 2007). Commensalistic relationships lack such driving forces for coevolution.
Many plants use other plants (mostly trees) as hosts that offer substrate area, ‘a place in the sun’ and, in the case of mistletoes, also water and carbohydrates. Since tree species differ in many traits (e.g. bark properties and foliage density), the growing conditions for these structurally dependent plants may strongly depend on the particular host species. Host specificity might arise if trade-offs prevent structurally dependent plant species to adapt equally well to all types of conditions found on different host species. Indications for a certain degree of host specificity have indeed been observed for all groups of structurally dependent plants: non-vascular (e.g. Barkman 1958; Palmer 1986) and vascular epiphytes (e.g. Callaway et al. 2002; Laube and Zotz 2006), hemiepiphytes (sensu Zotz 2013a) (e.g. Todzia 1986; Male and Roberts 2005), climbers (e.g. Putz 1984; Ladwig and Meiners 2010) and mistletoes (e.g. Hawksworth and Wiens 1996; Okubamichael et al. 2013).
Host tree specificity should be weaker for structurally dependent plants than for arboreal herbivores because in contrast to animals, plants cannot actively search for appropriate hosts and only have the options of establishing or perishing at the location where diaspores were carried by chance (Pennings and Callaway 2002). Based on theoretical considerations on coevolution under different interaction types, different groups of structurally dependent plants should show different degrees of host specificity. Mistletoes, climbers and hemiepiphytes tend to have an adverse effect on their host tree's fitness (e.g. Todzia 1986; Schnitzer and Bongers 2002; Aukema 2003). Thus, in these groups host specialization may be reinforced by an evolutionary arms race resulting in a high degree of host specificity. Indeed, a number of resistance mechanisms against mistletoe establishment have been described (Hoffmann et al. 1986; Yan 1993). For example, Medel (2000) presented evidence that mistletoe infection exerts a selection pressure for long spines, which reduce perching of mistletoe dispersers, in Echinopsis chiloensis (Cactaceae). Epiphytes, in contrast, being defined as rooting on their hosts without parasitizing them (Zotz 2013b), are generally assumed to have hardly any negative effect on their host trees. Consequently, comparatively weak host specificity is expected. Hypotheses can also be formulated regarding the degree of host specificity of structurally dependent plants in different forest types. In mixed forests in which no tree species is exceedingly abundant (as in tropical rainforests with typical hyperdispersed tree distribution patterns), structurally dependent plant species should have weak host specificity, because of strong selection to cope with these diverse conditions while in habitats with a few dominant tree species host specificity would be less penalized as diaspores of specialists have a greater chance of landing on or, in the case of climbers, near the host they are positively associated with (Barlow and Wiens 1977; Norton and Carpenter 1998; Nieder et al. 2001; Garrido-Pérez and Burnham 2010). Finally, in climates with a high diurnal or annual temperature variation and/or a pronounced dry season species have relatively broad climatic tolerances. This exaptation (sensu Gould and Vrba 1982) should be beneficial for coping with the range of microclimatic conditions found on different tree species.
Vascular epiphytes represent ∼9 % of all vascular plant species (Zotz 2013b) and are a very important component of the plant assemblages of tropical wet forests (Gentry and Dodson 1987). However, notwithstanding their importance, our understanding of the mechanisms structuring epiphyte communities is still rather poor. Microclimate is a major determinant of the local distribution of vascular epiphytes as can be deduced from the vertical stratification of species documented in a large number of studies (e.g. Krömer et al. 2007; Zotz 2007). Host identity is another potential determinant, which has been invoked and/or investigated in >200 studies (Fig. 1, Appendix 1 [see Supporting Information]). However, while vertical gradients of microclimatic variables and epiphyte species distribution are relatively easy to document, the evidence for host specificity is much harder to obtain due to the complex vegetation structure and the multitude of candidate host traits.
Figure 1.
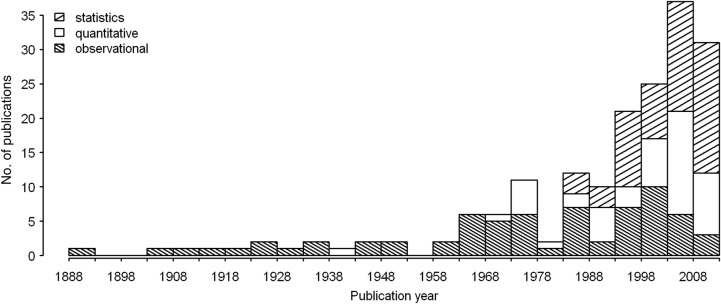
Histogram of publication dates (1888 through 2013 in 5-year intervals). Included were those publications, in which inference on host specificity of vascular epiphytes is based on own field observations. Excluded were studies that investigate mechanisms based on observations published in prior publications, articles only concerned with host specificity in the discussion section and secondary literature. Different shading lines indicate publication quality. Categories are: conclusions based on statistical tests (statistics), conclusions based on quantitative data (quantitative) and conclusions based on non-quantified observations (observational).
With this review we pursue several goals. Definitions of terms are a prerequisite to ensure communication about concepts. In view of the ambiguous definitions of host specificity in many papers on vascular epiphytes, a terminology is proposed that precisely differentiates between different facets and assemblage-based scenarios of host specificity. Second, we provide a comprehensive overview on the empirical evidence for host specificity and on the potential proximate mechanisms that cause host specificity. Finally, investigating epiphyte host specificity has many pitfalls, and it is one of the major goals of this review to identify these problems, propose adequate approaches to solve them, promoting meaningful future field studies, which we discuss in detail in the final section of this paper.
Terminology
For simplicity, ‘tree’ or ‘tree species’ are used when we refer to a potential epiphyte host individual or species although other growth forms (e.g. shrubs, lianas or columnar cacti) serve as epiphyte hosts as well. The terms ‘host’ and ‘host species’ imply that the focal epiphyte taxon has been observed on this tree individual or species. Note that, in unambiguous contexts, ‘host’ is often used as a short form of ‘host species’.
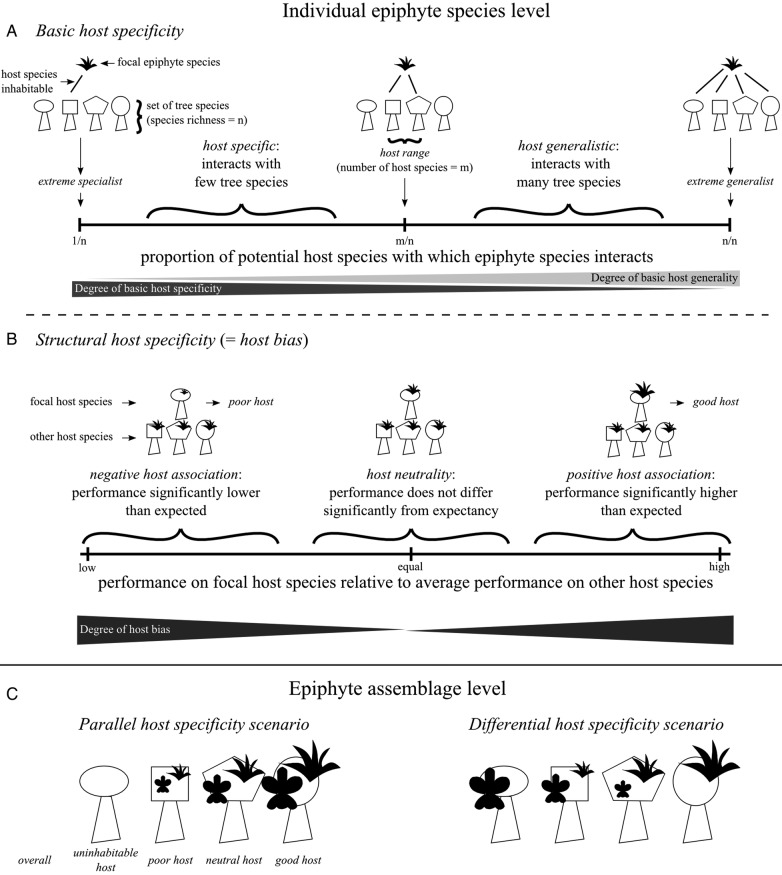
Ecological specificity can be separated into two components: the ‘range’ of occurrence along a niche axis and the ‘evenness’ of performance within this range (Devictor et al. 2010). In order to differentiate between these ‘facets of specificity’ we use the terms ‘basic’ and ‘structural host specificity’, which were introduced by Poulin et al. (2011). ‘Basic host specificity’ measures how many tree species are inhabitable by a focal epiphyte species (Fig. 2A). To allow comparison of figures from habitats with different numbers of tree species we suggest determining host specificity relative to the set of tree species. Depending on the scope of the study this may, for example, comprise all species occurring at the study site or throughout the distributional range of the epiphyte. As a proportion, basic specificity is a continuous trait ranging from ‘monospecificity’ (the epiphyte species can only inhabit a single tree species) over ‘intermediate basic specificity’ (it can inhabit a subset of tree species) to ‘complete basic generality’ (it can inhabit all tree species). The term ‘host range’ refers either to the raw number or to the set of host species. ‘Structural host specificity’ measures a ‘host bias’ (Gowland et al. 2013), i.e. differences in the performance (measured as occupancy, abundance or fitness parameters) of the focal epiphyte species on a given host species relative to other host species (Fig. 2B). The term ‘structural’ refers to the fact that population structure differs across host species (Poulin et al. 2011). When considering the association of a pair of epiphyte and tree species, the possibilities vary continuously from ‘uninhabitability’ over a ‘negative association’ and ‘host neutrality’ to a strong ‘positive association’. ‘Good’ and ‘poor host species’ are those on which epiphyte performance is disproportionally high or low. Note that, while all degrees of basic host specificity, with the exception of complete basic generality, imply structural host specificity, the reverse is not true: A species may be an extreme generalist concerning basic specificity (i.e. occur on all potential host species) and at the same time exhibit a host bias (with different performance on different host species).
Figure 2.
Terminology used throughout the text: (A) basic host specificity, (B) structural host specificity and (C) assemblage level scenarios. Different shapes represent different tree and epiphyte species, respectively. Epiphyte symbol size represents relative performance on a host species (measured as abundance, occupancy or fitness parameters of individual plants).
Although basic and structural host specificity are closely interrelated and should both be quantified by metrics of specificity, it is instructive which of these facets of host specificity have been investigated in different contexts. For example, while studies on mistletoes are mostly concerned with basic host specificity, i.e. the specific set of utilized host trees (e.g. Downey 1998), most vascular epiphyte studies are concerned with structural host specificity, i.e. a performance bias among different host trees (e.g. Callaway et al. 2002). These different foci probably reflect the much stronger host specificity of mistletoes. Unfortunately, terms are inconsistently used in the literature on host specificity of vascular epiphytes. For example, in some cases host specificity was explicitly or implicitly defined as monospecificity, i.e. as the extreme scenario of an epiphyte species being exclusively associated with a single tree species (e.g. Mehltreter et al. 2005; Martínez-Meléndez et al. 2008). A term that has been repeatedly used to describe structural host specificity of structurally dependent plants is ‘host preference’ (e.g. Mehltreter et al. 2005; Laube and Zotz 2006; Martínez-Meléndez et al. 2008). However, since the terms ‘preference’ and ‘avoidance’ are reserved to the outcome of active host selection behaviour in the context of host–animal interactions (e.g. Futuyma and Moreno 1988; Desjardins et al. 2010; Takken and Verhulst 2013), we agree with Zhou and Hyde (2001) not to use these terms for sessile organisms.
When analysing host specificity patterns at the species assemblage level, it is necessary to distinguish two scenarios, although a mixture of both is expected in nature: (i) the ‘parallel host specificity scenario’, in which all epiphyte species show the same low or high performance on a given tree species, and (ii) the ‘differential host specificity scenario’, in which individual epiphyte species differ strongly in their performance on individual tree species (Fig. 2C). Differences in host quality in the parallel host specificity scenario are expressed with the terms ‘overall’ poor/good hosts.
Investigating Epiphyte Host Specificity
A bestiary of methodological approaches
Approaches to detect structural host specificity are diverse. For example, sampling designs differ greatly (plot- or transect-based sampling contrasts with the sampling of equal numbers of different tree species of standardized size). Similarly variable is the number of studied epiphyte species: it ranges from one or few focal species to the entire set of species at a study site or within a geographical region (Table 1). We classified studies according to the main types of response variables: (i) occupancy, (ii) abundance, (iii) species richness, (iv) species composition, (v) fitness parameters and (vi) network topology.
Typically, only a fraction of trees in a forest is colonized by epiphytes and an even smaller fraction of trees is colonized by a particular epiphyte species. ‘Occupancy’ (i.e. presence/absence of epiphytes on a tree) is a widely used response variable in studies of host specificity (20 % of the 96 analyses in Table 1).
Epiphyte ‘abundance’ per tree is the most commonly used response variable to test for host biases: 38 % of the analyses use a measure of abundance (Table 1). It can be measured as the number of individuals, biomass or cover per tree. The last measure, i.e. the proportion of the substrate area of a tree used by epiphytes, implicitly takes into account the usually positive relationship of epiphytic abundance and host size (see the following subsection on the pitfalls of the analyses). In some cases, the pooled abundance of all epiphytes was used as response variable (Table 1), which allows the identification of overall good or poor host species. However, the pooled epiphyte abundance allows no inference on host biases of single species under the differential host specificity scenario because biases of single epiphyte species might cancel each other out.
‘Species richness’, i.e. the number of epiphyte species per tree, has been used as a response variable in 15 % of the analyses (Table 1). However, this response variable underestimates host biases under the differential host bias scenario and is only indicative of host bias strength under the parallel host bias scenario.
Epiphyte ‘species composition’ as response variable identifies differential host biases and was used as response variable in 11 % of the analyses (Table 1).
‘Fitness parameters’ (e.g. plant size, growth rate or physiological parameters) may be used to compare plant performance on different host species. This approach, which may be most useful for a mechanistic understanding of host specificity, was applied in very few studies (Callaway et al. 2001, 2002; Martin et al. 2007; Zhang et al. 2010; Gowland et al. 2011).
Tests for host specificity based on network analysis are indirect. The response variables are measures of ‘network topology’ (e.g. number of links) with host specificity assumed to cause a deviation of observed from expected topology of a null-model. The links between species in such a bipartite network can be qualitative (presence/absence of interactions between species) or quantitative (frequency of interactions between species). All network analyses (11 % of analyses in Table 1) have been published very recently.
Table 1.
Studies using statistical tests to detect structural host specificity. The table comprises all 55 publications that applied valid statistical tests for host biases. Methodological details of the literature search are described together with Appendix 1 [see Supporting Information]. The methods and outcomes of each test are specified separately if several tests were performed within a study, which leads to 97 entries.
| References | Epiphyte taxa | Tree taxa | RV | Test | Size | Host bias | Per cent cases with bias | Comments |
|---|---|---|---|---|---|---|---|---|
| Ackerman et al. (1996) | 1 | 16 | ocs | 8 | No | Yes | 100 | Inference: confidence interval |
| Addo-Fordjour et al. (2009) | 29 | 65 | ric | 3 | No | Yes | n/a | |
| Aguirre et al. (2010) | 21 | 21 | com | 7 | STA | Yes | n/a | Sabal palmetto vs. other trees; epiphytes and hemiepiphytes pooled |
| 21 | 21 | ric | 6 | STA | Yes | n/a | Sabal palmetto vs. other trees; epiphytes and hemiepiphytes pooled | |
| Andrade and Nobel (1996) | 1 | 22 | ocs | 1 | Ind | Yes | 100 | Unoccupied tree species excluded from analysis |
| Andrade and Nobel (1997) | 4 | 4 | abs | 1 | (STA) | Yes | n/a | |
| Azemi et al. (1996) | 61 | 4 | abs | 3 | No | Yes | 17 | Epiphytes pooled into taxonomic groups; response for some groups: cover |
| n/a | 4 | cop | 3 | COV | Yes | n/a | ||
| Benavides et al. (2011) | n/a | 8 | abs | 6 | OBS | Yes | 5 | Frequency of deviation from expectation (depending on plot): 0–20 %, mean = 5 % |
| 14 | n/a | ocs | 6 | CAV, SUA | Yes | 2 | Frequency of deviation from expectation (depending on plot): 0–12 %, mean = 2 % | |
| Bennett (1984) | 2 | 6 | abs | 3 | No | No | 0 | Methods not well described |
| Bennett (1987) | 4 | n/a | abs | 3 | Ind | Yes | 25 | Abundance/tree |
| 4 | n/a | abs | 3 | STA | No | 0 | Abundance/stem | |
| 4 | 3–5 | abs | 1 | STE | Yes | 25 | ||
| 4 | 3–5 | abs | 1 | No | Yes | 100 | ||
| 4 | 3–5 | abs | 1 | DBH | Yes | 100 | ||
| 4 | 3–5 | abs | 1 | BAA | Yes | 75 | ||
| Bernal et al. (2005) | 1 | 18 | abs | 1 | No | Yes | 100 | |
| 1 | 18 | abs | 1 | CAV | Yes | 100 | ||
| 1 | 18 | abs | 3 | No | Yes | 100 | ||
| Bittner and Trejos-Zelaya (1997) | 52 | 4 | com | 7 | No | Yes | n/a | Epiphytes and hemiepiphytes pooled |
| Blick and Burns (2009) | 19 | 7 | nec | 6 | No | No | n/a | |
| 19 | 7 | nes | 6 | No | ? | n/a | Weak evidence for nestedness | |
| Boelter et al. (2011) | n/a | 3 | abp | 3 | No | Yes | n/a | Trees in monospec. plantations |
| n/a | 3 | ric | 3 | No | (Yes) | n/a | Trees in monospec. plantations; no difference with richness estimators | |
| Breier (2005) | 22 | 12 | com | 7 | No | Yes | n/a | |
| Burns and Dawson (2005) | 6 | 7 | ocs | 2 | DBH | Yes | 33 | |
| 20 | 7 | ric | 5 | DBH | Yes | n/a | Significant interaction between dbh and host species | |
| Burns (2007) | 19 | 7 | ned | 4 | OBS | No | n/a | Linear regression between observed and expected network degree |
| Burns and Zotz (2010) | 77 | 8 | ned | 6 | OBS | Yes | n/a | |
| 77 | 8 | nes | 6 | No | ? | n/a | No nestedness | |
| 77 | 8 | nec | 6 | No | Yes | n/a | ||
| Callaway et al. (2001) | 1 | 8 | abs | 3 | (STA) | Yes | 100 | Essentially same results as in Callaway et al. (2002) |
| Callaway et al. (2002) | 2 | 8 | fit | 3 | n/a | Yes | 100 | Measurement of growth in transplants |
| Cardelús (2007) | 53 | 2 | cop | 3 | STA | Yes | n/a | |
| Castaño-Meneses et al. (2003) | 1 | 21 | abs | 3 | (STA) | Yes | 100 | Abies religiosa vs. several Quercus species |
| Dejean et al. (1995) | 51 | 4 | abs | 1 | No | Yes | 60 | Multiple comparisons problem; all Tillandsia pooled |
| Diaz Santos (2000) | 71 | 471 | com | 7 | No | Yes | n/a | Phorophytes: genus level, two different analyses |
| Einzmann et al. (2015) | 83 | 5 | com | 7 | DBH | Yes | n/a | |
| 83 | 5 | abp | 3 | (STA) | Yes | n/a | ||
| 83 | 5 | ric | 3 | (STA) | Yes | n/a | ||
| 2 | 3 | fit | 3 | n/a | Yes | 50 | ||
| Fontoura (1995) | 51 | 141 | ocs | 1 | No | Yes | n/a | Bromeliads: genus level; trees: family level |
| 51 | 141 | ocs | 7 | No | Yes | n/a | Bromeliads: genus level; trees: family level | |
| García-Franco and Peters (1987) | 6 | 5 | abs | 1 | No | Yes | n/a | |
| Gowland et al. (2011) | 3 | 5–10 | ocs | 1 | No | Yes | 100 | Test especially designed for this study; number of woody species depends on site |
| 3 | 21 | fit | 1,5 | n/a | Yes | 67 | Backhousia myrtifolia vs. other trees; resp.: flower, leaf length, no. of inflor. and leaves | |
| de Guaraldo et al. (2013) | 1 | 11 | ocs | 1 | No | Yes | 100 | |
| Hietz and Hietz-Seifert (1995a) | 22–53 | 2–15 | ric | 5 | BAA | (Yes) | n/a | Difference in only one out of six sites |
| 22–53 | 2–15 | abp | 5 | BAA | (Yes) | n/a | Difference in only one out of six sites | |
| Hietz and Hietz-Seifert (1995b) | 39 | n/a | ric | 3 | No | No | n/a | No. of tree taxa unclear |
| Hirata et al. (2009) | 21 | 10 | ric | 5 | DBH, HEI | Yes | n/a | |
| Köster et al. (2011) | 381, 383 | 12, 11 | ric | 3 | Ind | Yes | n/a | Species no.: 2 sites; trees: genus level (some) |
| Laube and Zotz (2006) | 103 | 3 | ocs | 6 | No | Yes | 11 | Depending on host species: 85–93 % of cases indistinguishable from chance |
| 103 | 3 | abs | 6 | OBS | Yes | 25 | Depending on host species: 69–81 % of cases indistinguishable from chance | |
| 43 | 3 | com | 7 | No | Yes | n/a | Only 5 % of variance explained | |
| Malizia (2003) | 23 | 6 | cos | 6 | COV | Yes | 52 | Epiphytes (18) and climbers (5) pooled, response: IV (occupancy + cover) |
| 23 | 6 | com | 7 | COV | Yes | n/a | Epiphytes (18) and climbers (5) pooled | |
| Martin et al. (2007) | 1 | 3 | fit | 3 | n/a | No | 0 | Chlorophyll concentration, CAM fluctuation, chlorophyll fluorescence |
| Martínez-Meléndez et al. (2008) | 19 | 6 | abs | 1 | BAA | Yes | 100 | Expected abundances based on ‘IV’ (host abundance + basal area) |
| Medeiros et al. (1993) | n/a | 21 | abp | 3 | (STA) | Yes | n/a | Native vs. alien tree ferns |
| Mehltreter et al. (2005) | 55 | 7 | abp | 3 | No | No | n/a | |
| 55 | 7 | ric | 3 | No | No | n/a | ||
| 55 | 2 | abp | 3 | STA | Yes | n/a | Tree ferns vs. angiosperms; dbh included by testing size classes separately | |
| 55 | 2 | ric | 3 | STA | Yes | n/a | Tree ferns vs. angiosperms; dbh included by testing size classes separately | |
| 19 | 2 | ocs | 1 | No | Yes | 32 | Tree ferns vs. angiosperms | |
| Merwin et al. (2003) | n/a | 3 | abp | 3 | No | Yes | n/a | Trees in monospec. plantations; biomass per plot; host association depends on plot age |
| Moran et al. (2003) | 31 | 2 | ocs | 1 | STA | Yes | 35 | Tree ferns vs. angiosperms |
| 31 | 2 | cos | 3 | STA | Yes | n/a | Tree ferns vs. angiosperms; tree size standardized by sampling comparable dbh | |
| 31 | 2 | ric | 3 | STA | Yes | n/a | Tree ferns vs. angiosperms; tree size standardized by sampling comparable dbh | |
| Moran and Russell (2004) | 1 | 21 | ocs | 1 | STA | Yes | 100 | Welfia georgii vs. dicot trees; tree size effect tested independently |
| 1 | 21 | cos | 3 | STA | Yes | 100 | Welfia georgii vs. dicot trees; tree size effect tested independently | |
| Muñoz et al. (2003) | 13 | 6 | ocs | 6 | No | Yes | 69 | |
| 13 | 6 | ric | 6 | No | (Yes) | n/a | Epiphytes and climbers pooled; richness deviating from expectation: 2 of 6 cases | |
| Otero et al. (2007a) | 1 | 22 | ocs | 1 | No | Yes | 100 | Unoccupied tree species excluded from analysis; test performed for 2 sites |
| Piazzon et al. (2011) | 51 | 22 | ned | 4 | No | Yes | n/a | Epiphytes and climbers pooled |
| 51 | 22 | nes | 6 | No | ? | n/a | Nestedness higher than expected: in ca. 5–9 (depending on null-model) of 16 transects | |
| Reyes-García et al. (2008) | 6 | n/a | abp | 5 | CAA, HEI | Yes | n/a | Explaining variable: leaf type (not host identity) |
| Roberts et al. (2005) | n/a | 2 | ocs | 2 | (STA) | Yes | n/a | Unclear how many epiphyte species tested |
| 97 | 2 | ric | 3 | (STA) | Yes | n/a | Vascular and non-vascular epiphytes pooled | |
| 97 | 2 | com | 7 | (STA) | Yes | n/a | Vascular and non-vascular epiphytes pooled | |
| Sáyago et al. (2013) | 12 | 50 | nes | 6 | No | ? | n/a | Nestedness higher than expected |
| 12 | 50 | nei | 8 | DBH | No | n/a | ||
| Silva et al. (2010) | 132 | 105 | nes | 6 | No | ? | n/a | |
| ter Steege and Cornelissen (1989) | 56 | 2 | abp | 1 | STA | Yes | n/a | |
| 56 | 2 | ocs | 1 | STA | Yes | 13 | ||
| Vergara-Torres et al. (2010) | 6 | 10 | abs | 1 | No | Yes | 100 | |
| 6 | 10 | abs | 1 | BAA | Yes | 100 | ||
| Wolf (1994) | 521 | 31 | com | 7 | COV | Yes | n/a | Vascular and non-vascular epiphytes pooled |
| Wyse and Burns (2011) | 16 | 4 | abp | 1 | SUA | Yes | n/a | |
| 16 | 4 | cop | 3 | COV | No | n/a | ||
| Zhang et al. (2010) | 1 | 95 | ocs | 2 | DBH | Yes | 100 | |
| 1 | 31 | fit | 5 | No | Yes | 100 | Hosts grouped in 3 association classes (bin. regression results); response: plant size | |
| Zotz and Schultz (2008) | n/a | 11 | com | 7 | DBH | Yes | n/a | |
| Zotz et al. (2014) | 15 | 6 | ocp | 1 | No | No | n/a | |
| 15 | 2 | ocp | 5 | DBH | No | n/a |
Abbreviations: n/a, not applicable or available.
Epiphyte taxa/Tree taxa gives the number of epiphyte or tree taxa included in the analysis.
RV notes the response variable used in the analysis. Categories: epiphyte abundance per tree–epiphyte species pooled (abp), epiphyte abundance per tree–single epiphyte taxa (abs), epiphyte species composition per tree (com), proportion of substrate area covered by epiphytes–epiphyte species pooled (cop), proportion of substrate area covered by epiphytes–single epiphyte taxa (cos), measure of fitness component or physiological parameter of epiphyte individuals (e.g. plant size, growth rate, chlorophyll fluorescence) rate (fit), network topology measure: checkerboard distribution, i.e. negative co-occurrence pattern in networks (nec), network topology measure: degree distribution (ned), network topology measure: interaction matrix (nei), network topology measure: nestedness, i.e. tendency for specialists to interact with perfect subsets of species interacting with generalists (nes), occupancy, i.e. presence/absence on tree–epiphyte species pooled (ocs), occupancy, i.e. presence/absence on tree–single epiphyte taxa(ocs), epiphyte richness, i.e. number of epiphyte species per tree (ric).
Test notes which kind of statistical test has been used. Categories: test for independence of variables in contingency tables: χ2 test, Fisher's exact test, G-test (1), response variable binary, explaining variable metric and/or nominal: Binary logistic regression, GLM (2), response variable metric, explaining variable nominal: ANOVA, Mann–Whitney U-test, Kruskal–Wallis test, t-test (3), response variable metric, explaining variable metric: Regression analysis (4), response variable metric, explaining variables: metric and nominal: ANCOVA, GLM (5), permutation tests (6), multivariate analyses: CA, CCA, Cluster Analysis, NMDS, PCA, SSHMS (7), other tests (8).
Size notes in which way problem of differential substrate area offered by different host species has been addressed. If appropriate gives proxy of tree size measured. Categories: basal area (BAA), canopy area (CAA), response data is per cent epiphyte cover, thus tree size already incorporated in response (COV), canopy volume (CAV), diameter at breast height (DBH), tree height (HEI), tree size not addressed in analysis of host specificity but independent test of correlation between tree size and response performed (ind), not necessary to account for size: response variable growth rate (n/a), tree size not addressed in analysis (no), permutation test, assumes that a tree can support the number of observed epiphyte individuals (OBS), size standardized by sampling design (STA), size roughly standardized by sampling design but still a lot of variation (STA), substrate area (SUA).
Host bias notes whether results indicate existence of non-random host epiphyte associations. Categories: analysis does not yield evidence for host bias (no), analysis yields evidence for host bias (yes), evidence for host bias mixed (yes), network metric, unclear how result should be interpreted in context of structural host specificity (?).
Per cent cases with bias gives percentage of tested epiphytes for which non-random distribution was found.
1Number does not refer to species level but to higher level taxonomic groups.
Numerous statistical methods are associated with this diversity of response variables (Table 1). Occupancy data are typically analysed with a test for independence of variables in a contingency table (like Pearson's χ2 or Fisher's exact test). Alternatively, some authors used binary logistic regression or permutation tests. Data on epiphyte abundance have been analysed with χ2 tests as well—but they also have been analysed, like richness and fitness data, with non-parametric ANOVA-style tests and general linear models with host identity as an explaining variable (these models may also incorporate size and other covariates). Species composition data call for multivariate statistics. Ordination methods like canonical correspondence analysis or cluster analysis have been employed. Resampling is typically used to test for deviations of observed network topology from the expected one—but occupancy, abundance and richness data have also been analysed with these methods.
Pitfalls
Although the research question (‘Are epiphyte species distributed randomly among tree species?’) is simple, rigorously testing for structural host specificity is a difficult task because of the complex nature of species assemblages in the real world. Several critical factors should be taken into account when analysing data on epiphyte distribution to test for host specificity: most important are area and age of the substrate per tree species and clumped distribution patterns of epiphytes that are not caused by host specificity but by dispersal limitation.
Epiphyte occupancy and abundance per tree correlate axiomatically with two variables: tree age and substrate area (Zotz and Vollrath 2003). Longer exposure to epiphytic seed rain increases the probability of (repeated) colonization. Moreover, epiphyte accumulation rate should increase with tree age because young trees only accumulate epiphytes after diaspores successfully crossed the gap between their source host and the focal tree, while older trees will increasingly host reproductive epiphytes whose offspring is most likely to establish on the source tree (Zotz and Vollrath 2003). Similarly self-evident is the correlation with tree size: A larger tree will intercept more diaspores because it has a larger surface area exposed to the seed rain (Benzing 1990). However, the relationship between host size and age and epiphyte load is more complex. Larger trees within a forest stand will offer more diverse microclimatic conditions (Flores-Palacios and García-Franco 2006)—thus large trees may host additional species that do not find appropriate conditions on smaller trees. Certain host traits may change during tree ontogeny which, in turn, may lead to differential ‘life stage biases’. For example, Merwin et al. (2003) found that changes in host quality were related to ontogenetic changes in leaf size and deciduousness. Bark properties often change substantially with tree and branch age (Johansson 1974). The bark of young individuals and branches of trees with otherwise rough bark is often relatively smooth and thin (e.g. Everhart et al. 2009; Ranius et al. 2009). Extrinsic aging effects (bark weathering and the accumulation of non-vascular epiphytes and canopy soil) further change substrate quality (Catling and Lefkovitch 1989). Species-specific growth rates of trees, which are unknown for most field sites, add a complicating component to the study of structural host specificity because differential growth rates lead to different epiphyte loads at comparable tree sizes (Medeiros et al. 1993; Nieder et al. 2000; Hirata et al. 2009) or comparable tree age (Merwin et al. 2003).
Spatial autocorrelation is another issue. Many epiphyte species show clumped distributions (Nieder et al. 2000; Burns and Zotz 2010), which are primarily attributable to dispersal limitation (epiphyte offspring establishes most likely in proximity to the mother plant, i.e. on the same or nearby trees, Trapnell et al. 2004). Similarly, tree species may have a clumped distribution—even in highly diverse lowland rainforest habitats (Valencia et al. 2004).
When sampling design is plot-based or transect-based, tree species are sampled in unequal numbers. Additionally, size distributions of the sampled tree species differ, due to differential maximal sizes, longevity and growth rates but also stochasticity associated with low numbers of sampled trees per species in a typical field study. The limited number of sampled conspecific trees is particularly problematic in combination with clumped epiphyte and host distributions. Given data with such complexities, appropriate null expectations for the number of epiphytic individuals ‘per tree species’ should ideally be determined as functions of substrate area and age offered per tree species. In addition, spatial autocorrelation (clumped distribution patterns) should be considered. Note that the problem of spatial autocorrelation is alleviated when occupancy rather than abundance is used as response variable because in this case only the potentially clumped distribution of tree species (epiphyte diaspores land with a higher probability on neighbouring trees) may be a confounding factor.
Unfortunately, all these complexities and the associated problems in data analysis have been largely ignored in the past. For one, clumped distribution patterns and tree age have, so far, never been accounted for (Table 1). As the age of individual trees in a forest is typically unknown, tree size may be used as a compound proxy for size and age, although this approach is not ideal because growth rates generally differ among species. However, in 43 % of the published tests for structural host specificity (Table 1) only relative frequencies of tree species—but not tree size—were included (some of these studies performed separate analyses to test for a correlation between size and the respective response variable). Ignoring tree size may be acceptable when (i) tree species have comparable size distributions and (ii) large sample sizes preclude stochastic size differences. However, it becomes an issue whenever size distributions differ substantially between species (e.g. when the tree assemblage comprises understory shrubs, treelets and emergents). Researchers used different approaches to account for differences in tree size (Table 1). In some cases the size of the sampled trees or area was standardized. Alternatively, per cent substrate cover was used as response variable instead of numbers of individuals per tree. Finally, tree size was sometimes included as a covariate in statistical models or used to calculate expected occupancy or epiphyte load in contingency tables and permutation tests. Several proxies for tree size (e.g. dbh = diameter at breast height, tree height and canopy volume) were used. Arguably, substrate area is more directly linked to epiphyte load and occupancy than any of these proxies but has been only used in the exceptional case (Benavides et al. 2011; Wyse and Burns 2011).
Empirical Evidence for Host Specificity
Basic host specificity
While host range is of great interest in the case of mistletoes (e.g. Downey 1998), we are not aware of a single study that systematically quantified basic host specificity of vascular epiphytes. However, there are numerous observational accounts of epiphyte species that apparently grow exclusively on one host or several very closely related host species (‘extreme basic specificity’; Table 2).
Table 2.
Studies reporting extreme basic host specificity.
| References | Epiphyte species | Epiphyte family | Endemic | Host species/genus | Host family | Host life form | Habitat | Other trees? |
|---|---|---|---|---|---|---|---|---|
| Copeland (1916) | Stenochlaena areolaris | Blechnaceae | Yes | Pandanus simplex1 | Pandanaceae | Tree | n/a | n/a |
| Cortez (2001) | Trichomanes capillaceum | Hymenophyllaceae | No | Alsophila spp., Cyathea spp., Dicksonia spp. | Cyatheaceae, Dicksoniaceae | Tree fern | n/a | Present |
| da Mota et al. (2009) | Lepanthopsis vellozicola | Orchidaceae | Yes | Vellozia compacta | Velloziaceae | Shrubby monocot | Outcrop | n/a |
| Hernández and Diaz (2000) | Tetramicra malpighiarum | Orchidaceae | Yes | Malpighia sp. | Malpighiaceae | Shrub | Forest | n/a |
| La Croix et al. (1991) | Polystachya dendrobiiflora | Orchidaceae | n/a | Xerophyta spp. | Velloziaceae | Shrubby monocot | Outcrop | n/a |
| Lecoufle (1964) | Cymbidiella falcigera1 | Orchidaceae | Yes | Raphia sp. | Arecaceae | Palm | n/a | n/a |
| Cymbidiella pardalina1 | Orchidaceae | Yes | Platycerium madagascariense | Polypodiaceae | Epiph. herb | n/a | n/a | |
| Li and Dao (2014) | Coelogyne pianmaensis | Orchidaceae | Yes | Tsuga spp. | Pinaceae | Tree | Forest | Present |
| Matias et al. (1996) | Constantia cipoensis | Orchidaceae | Yes | Vellozia piresiana, Vellozia compacta | Velloziaceae | Shrubby monocot | Outcrop | n/a |
| Mayo et al. (2000) | Anthurium bromelicola subsp. bromelicola | Araceae | Yes | Hohenbergia sp. | Bromeliaceae | Terr. herb | Outcrop | n/a |
| Anthurium bromelicola subsp. bahiense | Araceae | Yes | Aechmea sp. | Bromeliaceae | Terr. herb | Coastal | n/a | |
| Mesler (1975) | Ophioglossum palmatum | Ophioglossaceae | No | Sabal palmetto | Arecaceae | Palm | Forest | Present |
| Morris (1968) | Polystachya johnstonii | Orchidaceae | n/a | Xerophyta splendens | Velloziaceae | Shrubby monocot | Outcrop | n/a |
| Porembski (2003) | Polystachya microbambusa | Orchidaceae | No | Afrotrilepis pilosa | Cyperaceae | Shrubby monocot | Outcrop | n/a |
| Polystachya pseudodisa | Orchidaceae | No | Afrotrilepis pilosa | Cyperaceae | Shrubby monocot | Outcrop | n/a | |
| Polystachya odorata var. trilepidis | Orchidaceae | Yes | Afrotrilepis pilosa | Cyperaceae | Shrubby monocot | Outcrop | n/a | |
| Polystachya dolichophylla | Orchidaceae | No | Afrotrilepis pilosa | Cyperaceae | Shrubby monocot | Outcrop | n/a | |
| Pseudolaelia vellozicola | Orchidaceae | n/a | n/a | Velloziaceae | Shrubby monocot | Outcrop | n/a | |
| Schimper (1888) | Trichomanes polypodioides1 | Hymenophyllaceae | No | n/a | n/a | Tree fern | Forest | n/a |
| Zygopetalum sp. | Orchidaceae | n/a | n/a | n/a | Tree fern | n/a | n/a | |
| Sehnem (1977) | Zygopetalum maxillare | Orchidaceae | n/a | n/a | n/a | Tree fern | n/a | n/a |
| Pecluma truncorum | Polypodiaceae | n/a | n/a | n/a | Tree fern | n/a | n/a | |
| Song et al. (2009) | Thrixspermum odoratum | Orchidaceae | Yes | Quercus bawanglingensis | Fagaceae | Tree | Forest | Present |
| Soto Arenas (1994) | Laelia speciosa | Orchidaceae | Yes | Quercus deserticola | Fagaceae | Tree | Forest | Absent |
| Tremblay et al. (1998) | Lepanthes caritensis | Orchidaceae | Yes | Micropholis guianensis | Sapotaceae | Tree | Forest | Present |
| Válka Alves et al. (2008) | Vriesea oligantha | Bromeliaceae | No | Vellozia spp. | Velloziaceae | Shrubby monocot | Outcrop | Present |
| Epidendrum saxatile | Orchidaceae | No | Vellozia spp. | Velloziaceae | Shrubby monocot | Outcrop | Present | |
| Van den Berg et al. (2006) | Leptotes vellozicola | Orchidaceae | Yes | Vellozia sp. | Velloziaceae | Shrubby monocot | Outcrop | n/a |
Plant names follow The Plant List (2013, Version 1.1. Published on the Internet; http://www.theplantlist.org/).
n/a, not available (no information given in publication); Endemic, notes whether epiphyte species has a small distributional range; Other trees?, notes whether other potential hosts are present in the area to which publication refers.
1A different species name was used in the original publication.
In total, we found 28 reports of extreme basic specificity, most of which are species descriptions and/or purely observational (Appendix 1 [see Supporting Information]). Only two studies support their claim with quantitative data (Tremblay et al. 1998; Válka Alves et al. 2008). While a single field observation can prove the inhabitability of a tree species and thus ‘expand’ presumably limited host ranges, non-observations of epiphyte–tree interactions are only weak indicators for basic host specificity (Grenfell and Burns 2009). Thus, besides considering relative bark substrate areas of hosts and spatial autocorrelations, very large sample sizes are crucial to support claims of limited host ranges. Moreover, findings should be corroborated with experiments investigating exclusion mechanisms. To date, no claim of basic host specificity among vascular epiphytes satisfies these requirements.
The suspicion that many claims of extreme host specificity in epiphytes (Table 2) are sampling artefacts, or are only applicable to particular sites, is supported by a number of observations. Most species with allegedly extreme basic host specificity (71 %) are orchids. This is conceivably due to a possible host tree specificity of the symbiotic fungus (see the section on mechanisms), but rarity and localized populations of many orchids (Rogers and Walker 2002) offer alternative explanations. The study of few and small populations of an epiphyte species increases the likelihood that limitation to a single tree species is simply due to stochasticity in combination with host biases. A case in point is Lepanthes caritensis. This very rare orchid was found exclusively on the tree Micropholis guyanensis in the Carite State Forest, Puerto Rico, by Tremblay et al. (1998). However, later it was found on at least three other host species in another forest (Crain and Tremblay 2012). Similarly, while Ophioglossum palmatum may be monospecific in Florida (Mesler 1975), it colonizes other hosts in its distributional range (Ibisch et al. 1995; Cortez 2001). Finally, Válka Alves et al. (2008) report that the Vellozia ‘specialists’ at their study site grow on rocks elsewhere. However, there are cases in which independent observations corroborate a strong degree of basic host specificity. For example, the filmy fern Trichomanes capillaceum seems indeed to be restricted to tree ferns (Schimper 1888; Mickel and Beitel 1988; Cortez 2001; Mehltreter et al. 2005) as originally observed by Schimper (1888).
Porembski (2003) drew attention to rock outcrops in Africa and South America where shrubby or arborescent, mat forming monocots serve as hosts (several Velloziaceae and one Cyperaceae species). Almost half of all claims of extreme basic specificity refer to this type of vegetation (Table 2). Unfortunately, information on the diversity of potential hosts in this vegetation is largely missing (but see Válka Alves et al. 2008), opening the possibility that a lack of alternative hosts, rather than the inability to grow on other substrates, leads to the observed narrow host range. A blatant example where the reported extreme basic specificity should be attributable to the lack of alternative hosts is the case of Laelia speciosa, which occurs in monospecific forests (Soto Arenas 1994).
Interestingly, numerous cases of extreme basic host specificity feature hosts which are not the typical ‘tree’ growth form (Table 2). Notable examples are the mentioned shrubby monocots, tree ferns (five cases) and palms (two cases). There are also reports of an orchid that exclusively grows on an epiphytic fern (Lecoufle 1964) and on the association of an aroid with terrestrial bromeliads (Mayo et al. 2000)—although the aroid rather satisfies the definition of a miniature liana and its two subspecies associate with different bromeliad species.
Structural host specificity
In almost all (89 %) of the 86 analyses that used statistical tests to detect structural host specificity (omitting network analyses because of their often unclear interpretation) authors rejected the null hypothesis of host neutrality (Table 1). Only nine analyses found no significant deviation from null expectations. While almost all analyses that focused on single epiphyte species found significant host biases (exception: Martin et al. 2007) this was not the case in analyses of entire epiphyte assemblages. Deviation from null expectations, i.e. a host bias, was only seen in a subset of the tested epiphyte species (47 ± 33 % SD; n = 11 studies that tested >4 epiphyte species and reported the number of host biases, Table 1). Presumably, studies that focus on few epiphyte species have higher statistical power (due to larger sample sizes per epiphyte species) to detect host specificity and/or the studied epiphyte species were selected based on prior observations that suggested host specificity.
In summary, we are not aware of any attempts to quantify potential basic host specificity in vascular epiphytes. Extreme basic host specificity seems to be an exception possibly restricted to especially demanding habitats, whereas host biases are ubiquitous among vascular epiphytes. However, we identified serious methodological issues (section on investigating epiphyte host specificity). Hence, future studies, which appropriately consider these complexities, might reveal that apparent host biases were, at least in some cases, methodological artefacts.
Mechanisms
Physical bark characteristics
Bark stability, texture and water-holding capacity are frequently hypothesized to be important for the suitability of trees as epiphyte hosts. The long-standing notion that trees with flaking or peeling bark are poor hosts is based on the assumption that such instable substrate hampers the establishment and survival of epiphytes (e.g. Schimper 1888; Went 1940). However, surprisingly few studies investigate this mechanism experimentally and no quantitative evidence of its importance exists. Schlesinger and Marks (1977) quantified ‘bark sloughability’ of branches with comparable diameter. They found that bark of host branches with higher bromeliad cover was more stable. However, the branches were also older and thus the effect of bark stability was possibly confounded by substrate age. Bark stability, just like other bark properties, may depend on tree ontogeny and plant part. For example, the bark of Bursera fagaroides peels with a higher rate on boles and large diameter branches as compared to small diameter branches and twigs (López-Villalobos et al. 2008). Surprisingly, the mortality of seedlings of two Tillandsia species was lower at locations with a higher peeling rate. Oliver (1930) stated that a number of New Zealand's conifers (among them the kauri, Agathis australis) are poor hosts due to their flaking bark. However, the crowns of mature kauris host many epiphytes (Ecroyd 1982), presumably because they offer a more stable substrate than trunks or branches of young trees.
Another common notion links bark roughness with the probability of epiphyte colonization, establishment and survival since rough bark offers a better foothold and thus prevents seeds and plants from being washed off. However, none of several correlative studies provide clear support for a mechanistic link (Migenis and Ackerman 1993; Otero et al. 2007a; Vergara-Torres et al. 2010). To our knowledge, the study of Callaway et al. (2002) is the only one to investigate how bark roughness influences epiphyte colonization in some detail. These authors quantified adherence of Tillandsia usneoides seeds and strands to bark of different host species and found that host biases, bark roughness and strand adherence were correlated. Nevertheless, correlations were weak because two species with relatively rugose bark (both pines) were poor hosts.
The capacity of bark to absorb (‘water-holding’) and temporarily store (‘water-retention’) rain water may increase host quality. Water-holding and retention capacity are functions of bark porosity (Johansson 1974) and thickness (Mehltreter et al. 2005). While Castro-Hernández et al. (1999) found no differences in final germination success on bark pieces and seedling mortality on trees with differential water-holding and retention capacity, several other studies do indicate that water-holding and/or retention capacity might play a role in host biases. For one, higher epiphyte loads of tree ferns could be explained by higher water-retention capacity and older age, as compared to angiosperms (Mehltreter et al. 2005). Similarly, when comparing two Tasmanian tree fern species, the species with higher water-holding capacity hosted significantly more epiphyte species (Roberts et al. 2005). Finally, size-corrected abundance of T. usneoides was positively correlated to bark water-holding and -retention capacities of 10 tree species (Callaway et al. 2002).
Leaf and bark chemistry
Chemical properties of bark and leaves may also play a role in host specificity. Bark pH of tree species varies; different amounts of nutrients leach from leaves upon wetting and some tree species might exudate substances that act as allelochemicals.
In contrast to non-vascular epiphytes, for which bark pH has been strikingly often correlated to host biases (Barkman 1958; Löbel et al. 2006; Cáceres et al. 2007), bark pH has attracted only limited attention as a possible determinant of vascular epiphyte distribution and the few existing studies do not indicate any importance. Pessin (1925) detected no difference in pH of bark of hosts and non-hosts of the fern Polypodium polypodioides—although he noted that two pine species with low bark pH were completely devoid of the fern. The only recent pertinent study (Mehltreter et al. 2005) found no correlation of epiphyte abundance and bark acidity on the lower trunk either.
Epiphytes procure their nutrients either directly from atmospheric sources (dry and wet deposition), from throughfall and stemflow enriched with leaf and bark leachates or from tree and epiphyte litter (litter directly trapped by epiphyte structures and canopy soil). Although the proportions of tree and epiphyte litter in canopy soil are unknown, its chemical properties vary significantly between tree species (Cardelús et al. 2009). Assuming that leaf or bark leachates or tree litter play a significant role for epiphyte nutrition, host specificity may result from tree-specific differences (Benzing and Renfrow 1974). All studies that investigated the link of mineral nutrition and host biases focused on leaf leachates: Schlesinger and Marks (1977) determined the concentrations of minerals leached from foliage of three tree species, in throughfall and in bromeliad tissue in different forest types. Leachates of good hosts of T. usneoides were more enriched in minerals than those of poorer hosts, the throughfall concentrations of several minerals in different forest types correlated with bromeliad abundance, and the bromeliad tissue mineral concentrations reflected the forest types in a PCA. A quarter of a century later, Husk et al. (2004) were unable to replicate these results. This inconsistency may indicate that the host effect is modulated by changes in atmospheric nutrient inputs. A third study (Callaway et al. 2002) found only subtle effects of throughfall on T. usneoides and the fern P. polypodioides. Throughfall collected under good hosts increased the germination of P. polypodioides spores but did not increase epiphyte growth.
Trees might exude substances that are detrimental (allelopathic) to epiphytes. It was, e.g. hypothesized that tree species that exude latex are poor hosts for orchids (Piers 1968). Callaway et al. (2002) found that T. usneoides strands grew better when watered with rainwater as compared with throughfall collected below certain tree species. This contrasts with results from another study, in which strand elongation of mature T. usneoides was not influenced by leaf extracts (Schlesinger and Marks 1977). Allelopathic effects may be more important for earlier life stages. Indeed, several experimental studies found inhibitory effects of bark on germination and seedling performance: The inhibitory effect of different bark extracts on germination of Tillandsia recurvata seeds was negatively correlated with in situ epiphyte loads (Valencia-Díaz et al. 2010). In two older studies, the effect of powdered bark in the cultivation medium on germination and seedling development of the orchid Encyclia tampensis was tested (Frei and Dodson 1972; Frei 1973a). Expectations were based on differential orchid loads on these tree species in Mexico (Frei 1973b) and Florida. While differential inhibitory effects agreed quite well with field observations in Mexico, this was not the case for those from Florida. An important issue in the study of allelopathic effects is the concentration of potentially allelopathic substances. Arguably, the bark extracts used in experimental studies reach concentrations never occurring in situ.
Architecture
Three important aspects of tree architecture are prevailing branch angles, diameter distribution of branches and leaf density. Dense foliage may buffer temperature and vapour pressure fluctuations and decrease the light intensity within canopies (Cardelús and Chazdon 2005). Additionally, it may decrease the amount of throughfall (Park and Cameron 2008)—especially during small rainfall events. Typically, dense foliage is assumed to have a negative effect on host quality. For example, Garth (1964) proposed that the strong interception of rain by the foliage of pines may partly explain why T. usneoides is underrepresented on them. However, the effect of dense foliage may be context-dependent. Gottsberger and Morawetz (1993) argued that in savannah habitats shade ‘favours’ epiphyte growth.
In many tropical forests deciduous and evergreen tree species co-occur, the proportion of deciduous species to evergreen species being correlated to the aridity of the respective site (Condit et al. 2000). During the leafless phase, usually coinciding with the dry season in the tropics, epiphytes experience more extreme microclimates on deciduous trees (Einzmann et al. 2015). The effect of deciduousness may depend on the particular epiphyte species. For xerophytic taxa like cacti (Andrade and Nobel 1997; Cardelús 2007) or some bromeliads (Birge 1911; Bennett 1987; Cardelús 2007) deciduousness may increase host suitability while it may decrease host suitability for more mesic species. Due to this filtering effect epiphyte richness should be lower on deciduous trees in tropical habitats (Einzmann et al. 2015). However, Hirata et al. (2009) found higher epiphyte richness on deciduous than on evergreen trees in a warm-temperate forest.
The prevailing branch inclination can influence the suitability of tree species via various mechanisms: (i) The danger of detachment and falling should be reduced if seeds or plants attach to horizontal as opposed to vertical substrate. (ii) Horizontal branches accumulate more canopy soil than branches with a steeper inclination (Nadkarni and Matelson 1991). An extreme example on how host architecture promotes the amount of accumulated canopy soil is the case of palms with persisting leaf bases. López Acosta (2007) found, on average, >9 kg (dry weight) of accumulated organic substrate per Sabal mexicana individual, a palm species that supported significantly more epiphytes and hemiepiphytes than non-palm trees of similar size (Aguirre et al. 2010). (iii) Branching angles may influence the ratio of stemflow to total precipitation (Park and Cameron 2008). Válka Alves et al. (2008) observed that water poured on trees from above ran down the trunk of two Vellozia species but ‘tended to fall vertically’ in other species being possibly responsible for the high epiphyte load of Vellozia species. Similarly, leaf traits (upward position and folding at night) have also been proposed to increase stemflow and dew formation and thus improve host quality (Wee 1978; Gottsberger and Morawetz 1993).
Conceivably, the relative distribution of branch diameters per tree species is involved in host specificity. Small diameter branches are more susceptible to mechanical damage and thus have higher turnover rates than large diameter branches (Watt et al. 2005). Therefore, tree taxa (like pines) lacking large diameter branches due to their branching pattern may be overall poor hosts (Garth 1964). Similarly, greater wood density could increase branch longevity and thus epiphyte load (Sáyago et al. 2013). Some epiphytes seem to depend on certain substrate diameters (Zimmerman and Olmsted 1992). For example, so-called twig epiphytes are found disproportionately on very small diameter branches (Catling et al. 1986). The underlying mechanism is unclear.
Tree microhabitat
Sometimes the habitat of the host (i.e. its climatic niche) is viewed as a mechanism that causes host specificity. Arguably, it seems rather farfetched to conceive e.g. ‘boreal’ forest trees as poor hosts for ‘tropical’ orchids. However, it is a sensible perspective if tree species grow in distinctive locations within the same forest (e.g. Valencia et al. 2004; Kraft et al. 2008), and thus offer epiphytes specific microclimatic conditions. Annaselvam and Parthasarathy (2001) hypothesized that in their study plot Chionanthus mala-elengi was an overall good host ‘because of its occurrence in riverine areas’. Similarly, Mucunguzi (2008) observed that the ‘most preferred tree species occupied lower slopes and valleys near swamps’ in Kibale National Park, Uganda.
Tree longevity and size at maturity
In the section on the investigation of host specificity, we pointed out that epiphyte occupancy and load generally increase with tree age and size and that ignoring these covariates may result in wrong conclusions on host specificity. However, tree species differ substantially in their longevity (e.g. live expectancy of trees in Central Amazonia ranges between 10 and 1000 years, Laurance et al. 2004) and their size at maturity (e.g. large size differences of mature understory shrubs and canopy trees) and these tree traits potentially influence host quality (e.g. Catling and Lefkovitch 1989; Wolf 1994; Annaselvam and Parthasarathy 2001; Bernal et al. 2005; Válka Alves et al. 2008).
Tree longevity and size at maturity may cause host biases via several mechanisms. First, there are the inherent age/size effects, i.e. accumulation probability increases with larger substrate area and longer substrate exposition. In this case host biases will only be seen if the ‘currencies’ in which epiphyte performance is measured are either occupancy or epiphyte abundance while individual plant fitness parameters do not depend on these effects. Other age and size effects should be observed with all performance currencies. Since exposed microsites may be limited to canopy tree species in forest stands, epiphytes specialized to such conditions have a low performance on understory tree species (Malizia 2003), albeit the same should be true for epiphytes on young individuals of canopy tree species (Zotz and Vollrath 2003). Moreover, canopy soil and other epiphytes may accumulate with time and may change the substrate quality on long-lived tree individuals.
Epiphyte traits
Tree traits define host quality. However, ‘differential’ host specificity requires trait differences between epiphyte species. The candidate traits are numerous. One important set of traits are those that lead to microclimatic specificity. Vertical stratification of epiphyte assemblages is well documented and generally attributed to microclimatic gradients within vegetation (e.g. Zotz 2007). Epiphyte traits related to microclimatic specificity may cause differential host specificity if host species differ consistently in the microclimatic conditions they offer. Xerophytic epiphyte species are underrepresented on understory tree species while mesic species may be underrepresented on deciduous species (Einzmann et al. 2015). Other traits may allow particular epiphyte species to cope with substrate instability. For instance, the ability to entangle whole stems or branches with roots or stolons has been associated with successful establishment on trees with flaking bark (Schimper 1888; Benzing 1978). Fast life cycles and small size at maturity may be prerequisites to grow on host species with many small diameter branches while epiphytes that attain a large size may be restricted to host species that offer strong branches or large branch forks for mechanical support (ter Steege and Cornelissen 1989; Hemp 2001). Finally, diaspore characteristics and dispersal mode may also influence host specificity (Dejean and Olmsted 1997), e.g. via the ability to adhere to bark surfaces or via directed animal dispersal.
The demands of epiphytes for particular growing conditions may change during ontogeny, which may affect their host specificity. If, for example, seedling establishment is averted on a host species, it will be a poor host regardless of whether trees offer suitable growing conditions to later life stages (Went 1940; Dressler 1981). Zhang et al. (2010) found a positive correlation of plant size and height above the ground in Asplenium nidus, although this fern is most common in the understory. They concluded that microsites in the understory are more favourable for establishment, whereas the canopy offers better growth conditions for established individuals. Finally, host specificity may occur at different organizational levels. In this article we are mainly concerned with the species and community level but populations and even genotypes within populations may differ enough in their relevant traits as to show differential host specificity.
Cascading effects
Host traits may not only have direct effects on vascular epiphyte performance but also act indirectly via their effect on other organisms (cascading effects, Thomsen et al. 2010). Associations of non-vascular epiphytes with host species are well established (e.g. Barkman 1958 and references therein, Gauslaa 1985; Palmer 1986; Loppi and Frati 2004). The colonization of trees by non-vascular epiphytes (bryophytes and lichens) may influence substrate suitability for vascular epiphytes and, thus, cause host biases (Pollard 1973; Sanford 1974; Zotz 2002). On the one hand, non-vascular epiphytes may facilitate the establishment of vascular epiphytes by storing water (Tremblay et al. 1998; Watthana and Pedersen 2008), enabling the accumulation of canopy soil (Watthana and Pedersen 2008), helping diaspores to adhere to the substrate (Callaway et al. 2001) and via the leaching of nutrients (Tremblay et al. 1998). On the other hand, they may decrease substrate suitability by competing for space (Zotz and Andrade 2002) or via allelopathic effects (Callaway et al. 2001). Repeatedly, authors have observed correlations between species abundances of non-vascular and vascular epiphytes (Van Oye 1924; Tremblay et al. 1998; Callaway et al. 2001; Zotz and Vollrath 2003; Watthana and Pedersen 2008; Gowland et al. 2011). However, experiments like the ones conducted by Callaway et al. (2001) are needed to assess whether these correlations are caused by cascading effects or are simply due to parallel effects of host traits on both groups.
It is also conceivable that host specificity is mediated by mycorrhizal fungi (Went 1940). Such a cascading effect suggests itself in the case of orchids (Clements 1987; Hietz and Hietz-Seifert 1995a; Gowland et al. 2011, 2013) which depend on the occurrence of symbiotic fungi for successful germination and seedling development (Arditti 1992). Some orchid species show specificity for certain fungal clades (Otero et al. 2002, 2004, 2007b). Moreover, the distribution of orchid mycorrhizal fungi is conceived to be independent of the distribution of orchids and may depend on tree species (see Gowland et al. 2013 and references therein). However, Gowland et al. (2013) could not confirm that the distribution of clades of mycorrhizal fungi (isolated from orchid roots) on host trees fully explains the observed host tree biases of three epiphytic orchid species.
Animals may influence epiphytic distribution patterns (Perry 1978). Zoochorous seeds may land disproportionately often on host species that are attractive to their dispersers due to the fruits or suitable perching sites they offer. This mechanism has already been suggested for hemiepiphytic figs (Guy 1977) and mistletoes (Roxburgh and Nicolson 2005). A special case is the mutualistic association of epiphytes with arboreal ants. For example, Kiew and Anthonysamy (1987) noted that the high epiphyte occupancy of Gluta aptera coincided with a high occupancy by ants. Similarly, Davidson (1988) attributed the biased occurrence of ant garden epiphytes on certain tree species to their extrafloral nectaries and location in disturbed areas (presumably correlated with an increased resource supply rate by phloem-feeding homoptera). Dependence on host tree traits is reduced if epiphytes establish in ant gardens (Benzing 1990). For example, the mediation of ants allows epiphytes to grow on smooth, short-lived giant bamboo culms (Kaufmann et al. 2001). Termites may play a similar role by allowing epiphyte establishment on their carton-runways (Flores-Palacios and Ortiz-Pulido 2005), although epiphytes inhabited their runways less frequently than the nests of arboreal ants (Blüthgen et al. 2001). The presence of arboreal ants might also reduce ‘host quality’: Ants, when living in association with myrmecophytic tree species (e.g. Cecropia spp.), might prune their hosts from establishing epiphyte seedlings (Janzen 1969). Janzen’s suggestion has been tested for vines (Janzen 1974; Fiala et al. 1989) but not yet for epiphytes.
Climate, potential host pool and the spatial scale
Host biases are frequently inconsistent over large areas (Schimper 1888; Sanford 1974 and references therein, Sehgal and Mehra 1984). This phenomenon has been dubbed ‘regional phorophyte specificity’ (Ibisch et al. 1995) and seems to be similarly common among non-vascular epiphytes (Barkman 1958; Patiño and Gonzalez-Mancebo 2011). There are a number of possible reasons. The effects of the tree traits that potentially influence epiphyte performance are likely to be modulated by climate. For example, while a low bark water-retention capacity of a given tree species may render it a poor host in a xeric habitat, the same tree species may be a good host in a mesic habitat. Host traits might also differ geographically. Schimper (1888) observed that densely foliated Mango trees were poor epiphyte hosts on the West Indies while their less densely foliated conspecifics near Rio de Janeiro were good hosts. Moreover, host specificity is expected to depend strongly on the set of locally available tree species. While an epiphyte species may be restricted to a limited number of tree species in a given locality, it may encounter many other tree species with suitable traits within its distributional range—or when extending its distributional range. Thus, it is crucial to bear in mind that findings of host specificity are only valid for the studied location(s).
Although host specificity is usually studied at the species level of both epiphyte and tree, it may also be studied below the species level (Zytynska et al. 2011; Whitham et al. 2012). For example, host shifts within a species may be due to geographic trait shifts of a tree species as in Mango trees. Moreover, genotypic differences between epiphyte populations or even individuals within populations may translate to differential host specificity below the species level.
The degree of host specificity likely correlates with habitat type. Generalist epiphytes are expected to be correlated with habitats with (i) high host diversity or (ii) high climatic variability. Moreover, a dense mat of non-vascular epiphytes arguably buffers the effect of chemical and physical bark properties (Frei 1973b). Thus, host biases should be less pronounced in montane tropical moist forests and lowland temperate rainforests, where bryophyte mats are extremely dense, than in habitats with a high percentage of ‘naked’ bark such as lowland rainforests (Ibisch 1996). Similarly, epiphyte species should show stronger host specificity in habitats where climatic conditions are suboptimal for their performance because the modulating effect of tree traits is stronger under such conditions (Sanford 1974). To summarize, strong host specificity will most likely be associated with exceptionally demanding, aseasonal habitats with a low diversity of potential hosts and a low abundance of non-vascular epiphytes (like rocky outcrop habitats, Table 2).
Synthesis
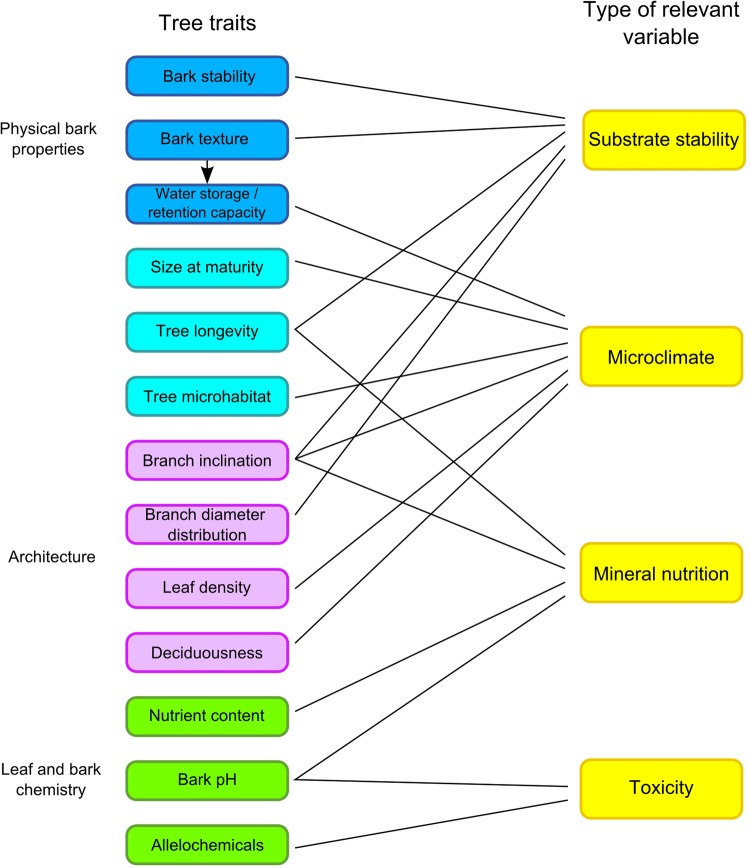
A large number of different tree traits have been invoked to cause epiphyte host specificity and certainly more traits could be added to the list. However, this plethora of traits influences a limited number of variables relevant to epiphyte performance: microclimate (including water availability as well as light, humidity and temperature regime), substrate stability, mineral nutrition and toxicity (Fig. 3). Most hypotheses on the mechanisms underlying host specificity involve microclimate and substrate stability and relatively few involve mineral nutrition and toxicity. Correlations between many of the invoked traits and distributional patterns or individual performance of epiphytes have been reported. However, none of the proposed mechanisms has been thoroughly studied, which makes it impossible at this stage to draw conclusions on their relative importance. Since each tree species features a unique combination of trait values it is vital to study a large number of different traits simultaneously. However, so far studies always focused on a very limited number of traits, typically discussing additional traits only when it comes to explain why a particular tree species did not fit into the expected correlation between the considered traits and host specificity. Arguably, many of the listed traits have weak effects on vascular epiphyte performance and none has a paramount effect. Even more complicating, the effect of tree traits will depend on the functional traits of the focal epiphyte species and will be modulated for given epiphyte–tree pairs by local climate and the tree species pool of the study area.
Figure 3.
Tree traits related to host specificity and their main influences on four types of variables relevant to epiphyte performance.
Outlook
Epiphyte host specificity has received considerable attention as demonstrated by the treatment in >200 publications [see Supporting Information]. Unfortunately, in spite of this large number of studies we are still far from a definite understanding of the importance of host tree identity for the structure and dynamics of vascular epiphytes assemblages. In our view, it seems especially rewarding to pursue the following directions: (i) Investigate whether the general finding of widespread structural specificity is corroborated when all possibly confounding factors are considered. (ii) Once patterns are unambiguously documented, study potential mechanisms in order to determine their relative importance. (iii) Test theoretical predictions about the relative strength of host specificity in different habitats and among different groups of dependent flora.
Since gathering information on tree age and spatial distribution patterns of epiphytes and trees requires a huge effort, we suggest to first explore the influence of these complicating factors in a simulation approach using a range of estimates for the respective parameters. Such an approach could help to decide on minimum sample sizes and appropriate null expectations for the statistical analysis of field data. Moreover, it could be used to assess the error introduced when substrate age is ignored and tree size is used as a proxy for the correlated variables size and age.
As pointed out in the subsection on the investigation of epiphyte host specificity, it is essential to control for tree size, either by standardized sampling or by including it as a covariate in the analysis. Of all size measures, bark surface area should be most closely correlated with epiphyte occupancy and load. The substrate area of a tree species may be determined by summing up the bark surface area over all individuals. Note that we assume for simplicity that substrate quality is independent of substrate type (e.g. trunk and branches) although this is often not true as demonstrated by vertical stratification patterns. Bark surface area can be easily determined if the sampling units are tree trunks only (Wyse and Burns 2011) or if trees lack branches as in tree ferns or palms. Admittedly, it is very labour-intensive to estimate bark surface area of whole trees with a more intricate architecture. A possibility would be to classify tree species by branching type, measure a limited number of trees per class and then model surface area as a function of dbh (see Whittaker and Woodwell 1967). If the response variable is epiphyte abundance per tree, the spatial autocorrelation of epiphytes should be considered when analysing host specificity. Almost two-thirds of the host specificity studies on vascular epiphytes have been performed in tropical or subtropical moist broadleaf forests (Appendix 1 [see Supporting Information]). Unfortunately, the high diversity of epiphytes and trees in these forests makes it very difficult to obtain appropriate sample sizes and renders statistical analyses very susceptible to type II errors. Less ambiguous results may be obtained in low diversity systems (e.g. tropical dry or temperate forests)—although the processes shaping epiphyte assemblages may differ substantially between forest types (Burns and Zotz 2010).
Restricted host ranges seem to be exceptional in vascular epiphytes and it is still unclear whether they exist at all. We suggest using the published reports listed in Table 2 as starting points for rigorous quantitative studies with large sample sizes and corroborating conclusions of host-incompatibility based on field observations with seed inoculation and transplantation experiments.
There are numerous possible mechanisms for host specificity. Some of these have been proposed but have never been tested, for others statistically significant correlations with response variables have been demonstrated. However, the relative importance of these mechanisms is currently unclear.
The suitability of a host species for epiphytes is not caused by its ‘Latin binomial’ (Raffaelli 2007) but rather by the matching of host and epiphyte traits. Thus, shifting the focus from species-associations to correlations of functional traits should advance the understanding of mechanisms and increase the predictive value of studies. Such a functional trait approach would also help to solve the issue of intraspecific tree trait variations due to e.g. ontogenetic changes. Moreover, rare epiphyte and host species that are excluded from analyses of pairwise species-associations for statistical reasons could be considered in correlational analyses of functional traits.
Correlative studies are the first step to identify candidate host traits relevant for host specificity. However, controlled field and laboratory experiments are indispensable to investigate properly the possible effects of bark chemistry and microclimatic variables influenced by host traits. The role of dispersal and of different epiphyte life stages in the determination of host bias patterns should be addressed by seed inoculation and transplantation experiments with plants at different life stages.
The hypotheses regarding the strength of host specificity in different habitat types and of different groups of dependent flora should be tested with systematic comparative studies as the one by Blick and Burns (2009) on epiphyte, liana and mistletoe networks who found stronger host specificity for the antagonistic networks as compared to the commensalistic one. Host specificity has been linked to rarity in epiphytes (Tremblay et al. 1998). A test of the hypothesis that rare species are more host specific than common species would require sampling common and rare species in comparable numbers to ensure comparable statistical certitude for all species—a precondition that is not met by the usual assemblage-based studies.
To conclude, since Schimper's pioneering work, a large body of empirical evidence on host specificity has accumulated and a general pattern of basic host generality and ubiquitous host biases emerges. However, it is now time to place epiphyte host specificity in a broader and more theoretical context. We hope this review inspires new research that helps designing field studies, solving issues regarding the analysis of field data and moving from case studies that document patterns towards a mechanistic understanding of the role of the epiphyte–host interaction for the evolution and ecology of this fascinating group of plants.
Sources of Funding
This work was supported by the Deutsche Forschungsgemeinschaft (Zo 94/5-1).
Contributions by the Authors
G.Z. conceived of the manuscript and collected relevant literature over many years. K.W. carried out the literature review, wrote the manuscript and created the figures. G.M.-L., G.Z. and K.W. contributed ideas and edited the manuscript.
Conflicts of Interest Statement
None declared.
Supporting Information
The following Supporting Information is available in the online version of this article –
Table S1. Lists all studies dealing with host specificity in vascular epiphytes. The methodology of the literature research is described and results based on summary statistics of the table are given.
Acknowledgement
The authors thank Markus Hauck and four anonymous reviewers whose comments helped to improve the manuscript.
Literature Cited
- Ackerman JD, Sabat A, Zimmerman JK. Seedling establishment in an epiphytic orchid: an experimental study of seed limitation. Oecologia. 1996;106:192–198. doi: 10.1007/BF00328598. [DOI] [PubMed] [Google Scholar]
- Addo-Fordjour P, Anning AK, Addo MG, Osei MF. Composition and distribution of vascular epiphytes in a tropical semideciduous forest, Ghana. African Journal of Ecology. 2009;47:767–773. doi: 10.1111/j.1365-2028.2009.01072.x. [DOI] [Google Scholar]
- Aguirre A, Guevara R, García M, López JC. Fate of epiphytes on phorophytes with different architectural characteristics along the perturbation gradient of Sabal mexicana forests in Veracruz, Mexico. Journal of Vegetation Science. 2010;21:6–15. doi: 10.1111/j.1654-1103.2009.01131.x. [DOI] [Google Scholar]
- Andrade JL, Nobel PS. Habitat, CO2 uptake, and growth for the CAM epiphytic cactus Epiphyllum phyllanthus in a Panamanian tropical forest. Journal of Tropical Ecology. 1996;12:291–306. doi: 10.1017/S0266467400009469. [DOI] [Google Scholar]
- Andrade JL, Nobel PS. Microhabitats and water relations of epiphytic cacti and ferns in a lowland neotropical forest. Biotropica. 1997;29:261–270. doi: 10.1111/j.1744-7429.1997.tb00427.x. [DOI] [Google Scholar]
- Annaselvam J, Parthasarathy N. Diversity and distribution of herbaceous vascular epiphytes in a tropical evergreen forest at Varagalaiar, Western Ghats, India. Biodiversity and Conservation. 2001;10:317–329. doi: 10.1023/A:1016670621331. [DOI] [Google Scholar]
- Arditti J. Fundamentals of orchid biology. New York: Wiley-Liss, Inc; 1992. [Google Scholar]
- Aukema JE. Vectors, viscin, and Viscaceae: mistletoes as parasites, mutualists, and resources. Frontiers in Ecology and the Environment. 2003;1:212–219. doi: 10.1890/1540-9295(2003)001[0212:VVAVMA]2.0.CO;2. [DOI] [Google Scholar]
- Azemi H, Laman TG, Budhi S. Distribution and abundance of vascular epiphytes and hemiepiphytic Ficus on dipterocarps in Gunung Palung National Park, West Kalimantan, Indonesia. Tropical Biodiversity. 1996;3:181–192. [Google Scholar]
- Barkman JJ. Phytosociology and ecology of cryptogamic epiphytes. Assen: Van Gorcum & Co; 1958. [Google Scholar]
- Barlow BA, Wiens D. Host-parasite resemblance in Australian mistletoes: the case for cryptic mimicry. Evolution. 1977;31:69–84. doi: 10.2307/2407546. [DOI] [PubMed] [Google Scholar]
- Benavides AM, Vasco A, Duque AJ, Duivenvoorden JF. Association of vascular epiphytes with landscape units and phorophytes in humid lowland forests of Colombian Amazonia. Journal of Tropical Ecology. 2011;27:223–237. doi: 10.1017/S0266467410000726. [DOI] [Google Scholar]
- Bennett BC. A comparison of the spatial distribution of Tillandsia flexuosa and T. pruinosa. Florida Scientist. 1984;47:141–144. [Google Scholar]
- Bennett BC. Spatial distribution of Catopsis and Guzmania (Bromeliaceae) in southern Florida. Bulletin of the Torrey Botanical Club. 1987;114:265–271. doi: 10.2307/2996464. [DOI] [Google Scholar]
- Benzing DH. Germination and early establishment of Tillandsia circinnata Schlecht. (Bromeliaceae) on some of its hosts and other supports in Southern Florida. Selbyana. 1978;5:95–106. [Google Scholar]
- Benzing DH. Vascular epiphytes. General biology and related biota. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Benzing DH, Renfrow A. The nutritional status of Encyclia tampense and Tillandsia circinnata on Taxodium ascendens and the availability of nutrients to epiphytes on this host in South Florida. Bulletin of the Torrey Botanical Club. 1974;101:191–197. doi: 10.2307/2484643. [DOI] [Google Scholar]
- Bernal R, Valverde T, Hernandez-Rosas L. Habitat preference of the epiphyte Tillandsia recurvata (Bromeliaceae) in a semi-desert environment in Central Mexico. Canadian Journal of Botany. 2005;83:1238–1247. doi: 10.1139/b05-076. [DOI] [Google Scholar]
- Birge WI. The anatomy and some biological aspects of “ball moss”, Tillandsia recurvata L. Austin, TX: Bulletin of the University of Texas; 1911. [Google Scholar]
- Bittner J, Trejos-Zelaya J. Analysis of the vascular epiphytes of tree ferns in a montane rain forest in Costa Rica. Revista de Matemática: Teoría y Aplicaciones. 1997;4:63–73. doi: 10.15517/rmta.v4i2.148. [DOI] [Google Scholar]
- Blick R, Burns KC. Network properties of arboreal plants: are epiphytes, mistletoes and lianas structured similarly? Perspectives in Plant Ecology, Evolution and Systematics. 2009;11:41–52. doi: 10.1016/j.ppees.2008.10.002. [DOI] [Google Scholar]
- Blüthgen N, Schmit-Neuerburg V, Engwald S, Barthlott W. Ants as epiphyte gardeners—comparing the nutrient quality of ant and termite substrates in a Venezuelan lowland rain forest. Journal of Tropical Ecology. 2001;17:887–894. doi: 10.1017/S0266467401001651. [DOI] [Google Scholar]
- Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N. Specialization, constraints, and conflicting interests in mutualistic networks. Current Biology. 2007;17:341–346. doi: 10.1016/j.cub.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Boelter CR, Zartman CE, Fonseca CR. Exotic tree monocultures play a limited role in the conservation of Atlantic Forest epiphytes. Biodiversity and Conservation. 2011;20:1255–1272. doi: 10.1007/s10531-011-0026-z. [DOI] [Google Scholar]
- Breier TB. Brazil: Universidade Estadual de Campinas; 2005. O epifitismo vascular em florestas do sudeste do Brasil. PhD Thesis. [Google Scholar]
- Burns KC. Network properties of an epiphyte metacommunity. Journal of Ecology. 2007;95:1142–1151. doi: 10.1111/j.1365-2745.2007.01267.x. [DOI] [Google Scholar]
- Burns KC, Dawson J. Patterns in the diversity and distribution of epiphytes and vines in a New Zealand forest. Austral Ecology. 2005;30:891–899. doi: 10.1111/j.1442-9993.2005.01532.x. [DOI] [Google Scholar]
- Burns KC, Zotz G. A hierarchical framework for investigating epiphyte assemblages: networks, metacommunities and scale. Ecology. 2010;91:377–385. doi: 10.1890/08-2004.1. [DOI] [PubMed] [Google Scholar]
- Cáceres MES, Lücking R, Rambold G. Phorophyte specificity and environmental parameters versus stochasticity as determinants for species composition of corticolous crustose lichen communities in the Atlantic rain forest of northeastern Brazil. Mycological Progress. 2007;6:117–136. doi: 10.1007/s11557-007-0532-2. [DOI] [Google Scholar]
- Callaway RM, Reinhart KO, Tucker SC, Pennings SC. Effects of epiphytic lichens on host preference of the vascular epiphyte Tillandsia usneoides. Oikos. 2001;94:433–441. doi: 10.1034/j.1600-0706.2001.940306.x. [DOI] [Google Scholar]
- Callaway RM, Reinhart KO, Moore GW, Moore DJM, Pennings SC. Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia. 2002;132:221–230. doi: 10.1007/s00442-002-0943-3. [DOI] [PubMed] [Google Scholar]
- Cardelús CL. Vascular epiphyte communities in the inner-crown of Hyeronima alchorneoides and Lecythis ampla at La Selva Biological Station, Costa Rica. Biotropica. 2007;39:171–176. doi: 10.1111/j.1744-7429.2006.00253.x. [DOI] [Google Scholar]
- Cardelús CL, Chazdon RL. Inner-crown microenvironments of two emergent tree species in a lowland wet forest. Biotropica. 2005;37:238–244. doi: 10.1111/j.1744-7429.2005.00032.x. [DOI] [Google Scholar]
- Cardelús C, Mack M, Woods C, DeMarco J, Treseder K. The influence of tree species on canopy soil nutrient status in a tropical lowland wet forest in Costa Rica. Plant and Soil. 2009;318:47–61. doi: 10.1007/s11104-008-9816-9. [DOI] [Google Scholar]
- Castaño-Meneses G, García-Franco JG, Palacios-Vargas JG. Spatial distribution patterns of Tillandsia violacea (Bromeliaceae) and support trees in an altitudinal gradient from a temperate forest in Central Mexico. Selbyana. 2003;24:71–77. [Google Scholar]
- Castro-Hernández JC, Wolf JHD, Garcia-Franco JG, González-Espinosa M. The influence of humidity, nutrients and light on the establishment of the epiphytic bromeliad Tillandsia guatemalensis in the highlands of Chiapas, Mexico. Revísta de Biologia Tropical. 1999;47:763–773. [Google Scholar]
- Catling PM, Lefkovitch LP. Association of vascular epiphytes in a Guatemalan cloud forest. Biotropica. 1989;21:35–40. doi: 10.2307/2388439. [DOI] [Google Scholar]
- Catling PM, Brownell VR, Lefkovitch LP. Epiphytic orchids in a Belizean grapefruit orchard: distribution, colonization, and association. Lindleyana. 1986;1:194–202. [Google Scholar]
- Clavel J, Julliard R, Devictor V. Worldwide decline of specialist species: toward a global functional homogenization? Frontiers in Ecology and the Environment. 2011;9:222–228. doi: 10.1890/080216. [DOI] [Google Scholar]
- Clements MA. Orchid-fungus-host associations of epiphytic orchids. In: Saito K, Tanaka R, editors. Proceedings of the 12th World Orchid Conference. Tokyo: 1987. [Google Scholar]
- Colwell RK, Dunn RR, Harris NC. Coextinction and persistence of dependent species in a changing world. Annual Review of Ecology, Evolution, and Systematics. 2012;43:183–203. doi: 10.1146/annurev-ecolsys-110411-160304. [DOI] [Google Scholar]
- Condit R, Watts K, Bohlman SA, Pérez R, Foster RB, Hubbell SP. Quantifying the deciduousness of tropical forest canopies under varying climates. Journal of Vegetation Science. 2000;11:649–658. doi: 10.2307/3236572. [DOI] [Google Scholar]
- Copeland EB. Natural selection and the dispersal of species. The Philippine Journal of Science. 1916;6:147–171. [Google Scholar]
- Cortez L. Epiphytic pteridophytes found in the Cyatheaceae and Dicksoniaceae from cloud forests of Venezuela. Gayana Botanica. 2001;58:13–23. doi: 10.4067/S0717-66432001000100002. [DOI] [Google Scholar]
- Crain BJ, Tremblay RL. Update on the distribution of Lepanthes caritensis, a rare Puerto Rican endemic orchid. Endangered Species Research. 2012;18:89–94. doi: 10.3354/esr00442. [DOI] [Google Scholar]
- da Mota RC, de Barros F, Stehmann JR. Two new species of Orchidaceae from Brazil: Bulbophyllum carassense and Lepanthopsis vellozicola. Novon. 2009;19:380–387. doi: 10.3417/2007057. [DOI] [Google Scholar]
- Davidson DW. Ecological studies of neotropical ant gardens. Ecology. 1988;69:1138–1152. doi: 10.2307/1941268. [DOI] [Google Scholar]
- de Guaraldo AC, de Boeni BO, Pizo MA. Specialized seed dispersal in epiphytic cacti and convergence with mistletoes. Biotropica. 2013;45:465–473. doi: 10.1111/btp.12041. [DOI] [Google Scholar]
- Dejean A, Olmsted IC. Ecological studies on Aechmea bracteata (Swartz) (Bromeliaceae) Journal of Natural History. 1997;31:1313–1334. doi: 10.1080/00222939700770741. [DOI] [Google Scholar]
- Dejean A, Olmsted IC, Snelling RR. Tree-epiphyte-ant relationships in the low inundated forest of Sian Ka'an Biospere reserve, Quintana Roo, Mexico. Biotropica. 1995;27:57–70. doi: 10.2307/2388903. [DOI] [Google Scholar]
- Desjardins CA, Perfectti F, Bartos JD, Enders LS, Werren JH. The genetic basis of interspecies host preference differences in the model parasitoid Nasonia. Heredity. 2010;104:270–277. doi: 10.1038/hdy.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devictor V, Clavel J, Julliard R, Lavergne S, Mouillot D, Thuiller W, Venail P, Villéger S, Mouquet N. Defining and measuring ecological specialization. Journal of Applied Ecology. 2010;47:15–25. doi: 10.1111/j.1365-2664.2009.01744.x. [DOI] [Google Scholar]
- Diaz Santos F. Orchid preference for host tree genera in a Nicaraguan tropical rain forest. Selbyana. 2000;21:25–29. [Google Scholar]
- Downey PO. An inventory of host species for each aerial mistletoe species (Loranthaceae and Viscaceae) in Australia. Cunninghamia. 1998;5:685–720. [Google Scholar]
- Dressler RL. The orchids. Natural history and classification. Cambridge: Harvard University Press; 1981. [Google Scholar]
- Ecroyd CE. Biological flora of New Zealand. 8. Agathis australis (D. Don) Lindl. (Araucariaceae) kauri. New Zealand Journal of Botany. 1982;20:17–36. doi: 10.1080/0028825X.1982.10426402. [DOI] [Google Scholar]
- Ehrlich PR, Raven PH. Butterflies and plants: a study in coevolution. Evolution. 1964;18:586–608. doi: 10.2307/2406212. [DOI] [Google Scholar]
- Einzmann HJR, Beyschlag J, Hofhansl F, Wanek W, Zotz G. Host tree phenology affects vascular epiphytes at the physiological, demographic and community level. AoB PLANTS. 2015 doi: 10.1093/aobpla/plu073. 7 : plu073; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart SE, Ely JS, Keller HW. Evaluation of tree canopy epiphytes and bark characteristics associated with the presence of corticolous myxomycetes. Botany. 2009;87:509–517. doi: 10.1139/B09-027. [DOI] [Google Scholar]
- Fiala B, Maschwitz U, Pong TY, Helbig AJ. Studies of a South East Asian ant-plant association: protection of Macaranga trees by Crematogaster borneensis. Oecologia. 1989;79:463–470. doi: 10.1007/BF00378662. [DOI] [PubMed] [Google Scholar]
- Flores-Palacios A, García-Franco JG. The relationship between tree size and epiphyte species richness: testing four different hypotheses. Journal of Biogeography. 2006;33:323–330. doi: 10.1111/j.1365-2699.2005.01382.x. [DOI] [Google Scholar]
- Flores-Palacios A, Ortiz-Pulido R. Epiphyte orchid establishment on termite carton trails. Biotropica. 2005;37:457–461. doi: 10.1111/j.1744-7429.2005.00060.x. [DOI] [Google Scholar]
- Fontoura T. Distribution patterns of five Bromeliaceae genera in Atlantic rainforest, Río de Janeiro State, Brazil. Selbyana. 1995;16:79–93. [Google Scholar]
- Frei JK. Effect of bark substrate on germination and early growth of Encyclia tampensis seeds. American Orchid Society Bulletin. 1973a;42:701–708. [Google Scholar]
- Frei JK. Orchid ecology in a cloud forest in the mountains of Oaxaca, Mexico. American Orchid Society Bulletin. 1973b;42:307–314. [Google Scholar]
- Frei JK, Dodson CH. The chemical effect of certain bark substrates on the germination and early growth of epiphytic orchids. Bulletin of the Torrey Botanical Club. 1972;99:301–307. doi: 10.2307/2997072. [DOI] [Google Scholar]
- Futuyma DJ, Moreno G. The evolution of ecological specialization. Annual Review of Ecology and Systematics. 1988;19:207–233. doi: 10.1146/annurev.es.19.110188.001231. [DOI] [Google Scholar]
- García-Franco JG, Peters CM. Patrón espacial y abundancia de Tillandsia spp. a través de un gradiente altitudinal en los altos de Chiapas, México. Brenesia. 1987;27:35–45. [Google Scholar]
- Garrido-Pérez EI, Burnham RJ. The evolution of host-specificity in liana-tree interactions. Puente Biologico. 2010;3:145–157. [Google Scholar]
- Garth RE. The ecology of Spanish moss (Tillandsia usneoides): its growth and distribution. Ecology. 1964;45:470–481. doi: 10.2307/1936100. [DOI] [Google Scholar]
- Gauslaa Y. The ecology of Lobarion pulmonariae and Parmelion caperatae in Quercus dominated forests in southwest Norway. Lichenologist. 1985;17:117–140. doi: 10.1017/S0024282985000184. [DOI] [Google Scholar]
- Gentry AH, Dodson CH. Contribution of non-trees to species richness of a tropical rain forest. Biotropica. 1987;19:149–156. doi: 10.2307/2388737. [DOI] [Google Scholar]
- Gottsberger G, Morawetz W. Development and distribution of the epiphytic flora in an Amazonian savanna in Brazil. Flora. 1993;188:145–151. [Google Scholar]
- Gould SJ, Vrba ES. Exaptation—a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- Gowland KM, Wood J, Clements MA, Nicotra AB. Significant phorophyte (substrate) bias is not explained by fitness benefits in three epiphytic orchid species. American Journal of Botany. 2011;98:197–206. doi: 10.3732/ajb.1000241. [DOI] [PubMed] [Google Scholar]
- Gowland KM, van der Merwe MM, Linde CC, Clements MA, Nicotra AB. The host bias of three epiphytic Aeridinae orchid species is reflected, but not explained, by mycorrhizal fungal associations. American Journal of Botany. 2013;100:764–777. doi: 10.3732/ajb.1200411. [DOI] [PubMed] [Google Scholar]
- Grenfell M, Burns KC. Sampling effects and host ranges in Australian mistletoes. Biotropica. 2009;41:656–658. doi: 10.1111/j.1744-7429.2009.00586.x. [DOI] [Google Scholar]
- Guy PR. Notes on the host species of epiphytic figs (Ficus spp.) on the flood-plain of the Mana Pools Game Reserve, Rhodesia. Kirkia. 1977;10:559–562. [Google Scholar]
- Hawksworth FG, Wiens D. Dwarf mistletoes: biology, pathology, and systematics. Washington, DC: Diane Publishing; 1996. [Google Scholar]
- Hemp A. Life forms and strategies of forest ferns on Mt. Kilimanjaro. In: Gottsberger G, Liede S, editors. Life forms and dynamics in tropical forests. Stuttgart: J. Cramer; 2001. pp. 95–130. [Google Scholar]
- Hernández JA, Diaz MA. A new species of Tetramicra (Orchidaceae) from eastern Cuba. Harvard Papers in Botany. 2000;5:189–192. [Google Scholar]
- Hietz P, Hietz-Seifert U. Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. Journal of Vegetation Science. 1995a;6:487–498. doi: 10.2307/3236347. [DOI] [Google Scholar]
- Hietz P, Hietz-Seifert U. Structure and ecology of epiphyte communities of a cloud forest in central Veracruz, Mexico. Journal of Vegetation Science. 1995b;6:719–728. doi: 10.2307/3236443. [DOI] [Google Scholar]
- Hirata A, Kamijo T, Saito S. Host trait preferences and distribution of vascular epiphytes in a warm-temperate forest. Plant Ecology. 2009;201:247–254. doi: 10.1007/s11258-008-9519-6. [DOI] [Google Scholar]
- Hoffmann A, Fuentes E, Cortés I, Liberona F, Costa V. Tristerix tetrandrus (Loranthaceae) and its host-plants in the Chilean matorral: patterns and mechanisms. Oecologia. 1986;69:202–206. doi: 10.1007/BF00377622. [DOI] [PubMed] [Google Scholar]
- Husk GJ, Weishampel JE, Schlesinger WH. Mineral dynamics in Spanish moss, Tillandsia usneoides L. (Bromeliaceae), from Central Florida, USA. Science of the Total Environment. 2004;321:165–172. doi: 10.1016/j.scitotenv.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ibisch PL. Neotropische Epiphytendiversität—das Beispiel Bolivien. Wiehl: Martina Galunder-Verlag; 1996. [Google Scholar]
- Ibisch PL, Rauer G, Rudolph D. The pantropical epiphyte Ophioglossum palmatum (Ophioglossaceae: Pteridophyta), a new record for Bolivia. Fern Gazette. 1995;15:75–76. [Google Scholar]
- Janzen DH. Allelopathy by myrmecophytes: the ant Azteca as an allelopathic agent of Cecropia. Ecology. 1969;50:147–153. doi: 10.2307/1934677. [DOI] [Google Scholar]
- Janzen DH. Swollen-thorn acacias of Central America. Washington, DC: Smithsonian Institution Press; 1974. [Google Scholar]
- Johansson D. Ecology of vascular epiphytes in West African rain forests. Acta Phytogeographica Suecica. 1974;59:1–129. [Google Scholar]
- Kassen R. The experimental evolution of specialists, generalists, and the maintenance of diversity. Journal of Evolutionary Biology. 2002;15:173–190. doi: 10.1046/j.1420-9101.2002.00377.x. [DOI] [Google Scholar]
- Kaufmann E, Weissflog A, Hashim R, Maschwitz U. Ant-Gardens on the giant bamboo Gigantochloa scortechinii (Poaceae) in West-Malaysia. Insectes Sociaux. 2001;48:125–133. doi: 10.1007/PL00001754. [DOI] [Google Scholar]
- Kiew R, Anthonysamy S. A comparative study of vascular epiphytes in three epiphyte-rich habitats at Ulu Endau, Johore, Malaysia. Malayan Nature Journal. 1987;41:303–315. [Google Scholar]
- Kitching RL. Crafting the pieces of the diversity jigsaw puzzle. Science. 2006;313:1055–1057. doi: 10.1126/science.1131117. [DOI] [PubMed] [Google Scholar]
- Köster N, Nieder J, Barthlott W. Effect of host tree traits on epiphyte diversity in natural and anthropogenic habitats in Ecuador. Biotropica. 2011;43:685–694. doi: 10.1111/j.1744-7429.2011.00759.x. [DOI] [Google Scholar]
- Kraft NJB, Valencia R, Ackerly DD. Functional traits and niche-based tree community assembly in an amazonian forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- Krömer T, Kessler M, Gradstein SR. Vertical stratification of vascular epiphytes in submontane and montane forest of the Bolivian Andes: the importance of the understory. Plant Ecology. 2007;189:261–278. doi: 10.1007/s11258-006-9182-8. [DOI] [Google Scholar]
- La Croix IF, La Croix EAS, La Croix TM. Orchids of Malawi: the epiphytic and terrestrial orchids from South and East Central Africa. Rotterdam: Taylor & Francis; 1991. [Google Scholar]
- Ladwig LM, Meiners SJ. Liana host preference and implications for deciduous forest regeneration. Journal of the Torrey Botanical Society. 2010;137:103–112. doi: 10.3159/09-RA-041.1. [DOI] [Google Scholar]
- Laube S, Zotz G. Neither host-specific nor random: vascular epiphytes on three tree species in a Panamanian lowland forest. Annals of Botany. 2006;97:1103–1114. doi: 10.1093/aob/mcl067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance WF, Nascimento HEM, Laurance SG, Condit R, D'Angelo S, Andrade A. Inferred longevity of Amazonian rainforest trees based on a long-term demographic study. Forest Ecology and Management. 2004;190:131–143. doi: 10.1016/j.foreco.2003.09.011. [DOI] [Google Scholar]
- Lecoufle M. Notes about Cymbidiella. Orchid Review. 1964;72:233–236. [Google Scholar]
- Li R, Dao Z-L. A new species of Coelogyne (Orchidaceae) from western Yunnan, China. Phytotaxa. 2014;162:115–119. doi: 10.11646/phytotaxa.162.2.7. [DOI] [Google Scholar]
- Löbel S, Snäll T, Rydin H. Species richness patterns and metapopulation processes—evidence from epiphyte communities in boreo-nemoral forests. Ecography. 2006;29:169–182. doi: 10.1111/j.2006.0906-7590.04348.x. [DOI] [Google Scholar]
- López Acosta JC. Xalapa, Veracruz, México: Universidad Veracruzana; 2007. Variación ontogénica en la estrategia de defensas antiherbívoro en plantas hemiepífitas: un estudio con Ficus obtusifolia en un palmar biodiverso y amenazado. MSc Thesis. [Google Scholar]
- López-Villalobos A, Flores-Palacios A, Ortiz-Pulido R. The relationship between bark peeling rate and the distribution and mortality of two epiphyte species. Plant Ecology. 2008;198:265–274. doi: 10.1007/s11258-008-9402-5. [DOI] [Google Scholar]
- Loppi S, Frati L. Influence of tree substrate on the diversity of epiphytic lichens: comparison between Tilia platyphyllos and Quercus ilex (Central Italy) Bryologist. 2004;107:340–344. doi: 10.1639/0007-2745(2004)107[0340:IOTSOT]2.0.CO;2. [DOI] [Google Scholar]
- Male TD, Roberts GE. Host associations of the strangler fig Ficus watkinsiana in a subtropical Queensland rain forest. Austral Ecology. 2005;30:229–236. doi: 10.1111/j.1442-9993.2005.01442.x. [DOI] [Google Scholar]
- Malizia A. Host tree preference of vascular epiphytes and climbers in a subtropical montane cloud forest of Northwest Argentina. Selbyana. 2003;24:196–205. [Google Scholar]
- Martin CE, Lin T-C, Hsu C-C, Lin S-H. No effect of host tree species on the physiology of the epiphytic orchid Bulbophyllum japonicum in a subtropical rainforest in Northeastern Taiwan. Taiwan Journal of Forest Science. 2007;22:241–251. [Google Scholar]
- Martínez-Meléndez N, Pérez-Farrera MA, Flores-Palacios A. Vertical stratification and host preference by vascular epiphytes in a Chiapas, Mexico, cloud forest. Revista de Biologia Tropical. 2008;56:2069–2086. [PubMed] [Google Scholar]
- Matias LQ, Braga PIS, Freire AG. Reproductive biology of Constantia cipoensis Porto and Brade (Orchidaceae), an endemic species from the Serra do Cipo, Minas Gerais. Revista Brasileira de Botânica. 1996;19:119–125. [Google Scholar]
- Mayo SJ, Félix LP, Jardim JG, Carvalho AM. Anthurium bromelicola—a remarkable new species from Northeast Brazil. Aroideana. 2000;23:89–99. [Google Scholar]
- Medeiros AC, Loope LL, Anderson SJ. Differential colonization of epiphytes on native (Cibotium spp.) and alien (Cyathea cooperi) tree ferns in a Hawaiian rain forest. Selbyana. 1993;14:71–74. [Google Scholar]
- Medel R. Assessment of parasite-mediated selection in a host-parasite system in plants. Ecology. 2000;81:1554–1564. [Google Scholar]
- Mehltreter K, Flores-Palacios A, García-Franco JG. Host preferences of low-trunk vascular epiphytes in a cloud forest of Veracruz, Mexico. Journal of Tropical Ecology. 2005;21:651–660. doi: 10.1017/S0266467405002683. [DOI] [Google Scholar]
- Merwin MC, Rentmeester SA, Nadkarni NM. The influence of host tree species on the distribution of epiphytic bromeliads in experimental monospecific plantations, La Selva, Costa Rica. Biotropica. 2003;35:37–47. [Google Scholar]
- Mesler MR. The gametophytes of Ophioglossum palmatum L. American Journal of Botany. 1975;62:982–992. doi: 10.2307/2441643. [DOI] [Google Scholar]
- Mickel JT, Beitel JM. Pteridophyte flora of Oaxaca, Mexico. Memoirs of the New York Botanical Garden. 1988;46:412–567. [Google Scholar]
- Migenis LE, Ackerman JD. Orchid-phorophyte relationships in a forest watershed in Puerto Rico. Journal of Tropical Ecology. 1993;9:231–240. doi: 10.1017/S0266467400007227. [DOI] [Google Scholar]
- Moran RC, Russell RV. The occurrence of Trichomanes godmanii (Hymenophyllaceae) on Welfia georgii (Arecaceae) at the La Selva Biological Station, Costa Rica. American Fern Journal. 2004;94:70–76. doi: 10.1640/0002-8444(2004)094[0070:TOOTGH]2.0.CO;2. [DOI] [Google Scholar]
- Moran RC, Klimas S, Carlsen M. Low-trunk epiphytic ferns on tree ferns versus angiosperms in Costa Rica. Biotropica. 2003;35:48–56. [Google Scholar]
- Morris B. The epiphytic orchids of the Shire Highlands, Malawi. Proceedings of the Linnean Society of London. 1968;179:51–66. doi: 10.1111/j.1095-8312.1968.tb01100.x. [DOI] [Google Scholar]
- Mucunguzi P. Diversity and distribution of epiphytic orchids in Kibale National Park, Uganda. Selbyana. 2008;29:217–225. [Google Scholar]
- Muñoz AA, Chacon P, Perez F, Barnert ES, Armesto JJ. Diversity and host tree preferences of vascular epiphytes and vines in a temperate rainforest in southern Chile. Australian Journal of Botany. 2003;51:381–391. doi: 10.1071/BT02070. [DOI] [Google Scholar]
- Nadkarni NM, Matelson TJ. Fine litter dynamics within the tree canopy of a tropical cloud forest. Ecology. 1991;72:2071–2082. doi: 10.2307/1941560. [DOI] [Google Scholar]
- Nieder J, Engwald S, Klawun M, Barthlott W. Spatial distribution of vascular epiphytes (including hemiepiphytes) in a lowland Amazonian rain forest (Surumoni crane plot) of southern Venezuela. Biotropica. 2000;32:385–396. doi: 10.1111/j.1744-7429.2000.tb00485.x. [DOI] [Google Scholar]
- Nieder J, Prosperi J, Michaloud G. Epiphytes and their contribution to canopy diversity. Plant Ecology. 2001;153:51–63. doi: 10.1023/A:1017517119305. [DOI] [Google Scholar]
- Norton DA, Carpenter MA. Mistletoes as parasites: host specificity and speciation. Trends in Ecology and Evolution. 1998;13:101–105. doi: 10.1016/S0169-5347(97)01243-3. [DOI] [PubMed] [Google Scholar]
- Novotny V, Drozd P, Miller SE, Kulfan M, Janda M, Basset Y, Weiblen GD. Why are there so many species of herbivorous insects in tropical rainforests? Science. 2006;313:1115–1118. doi: 10.1126/science.1129237. [DOI] [PubMed] [Google Scholar]
- Okubamichael DY, Griffiths ME, Ward D. Reciprocal transplant experiment suggests host specificity of the mistletoe Agelanthus natalitius in South Africa. Journal of Tropical Ecology. 2013;30:153–163. doi: 10.1017/S0266467413000801. [DOI] [Google Scholar]
- Oliver WRB. New Zealand epiphytes. Journal of Ecology. 1930;18:1–50. doi: 10.2307/2255890. [DOI] [Google Scholar]
- Otero JT, Ackerman JD, Bayman P. Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. American Journal of Botany. 2002;89:1852–1858. doi: 10.3732/ajb.89.11.1852. [DOI] [PubMed] [Google Scholar]
- Otero JT, Ackerman JD, Bayman P. Differences in mycorrhizal preferences between two tropical orchids. Molecular Ecology. 2004;13:2393–2404. doi: 10.1111/j.1365-294X.2004.02223.x. [DOI] [PubMed] [Google Scholar]
- Otero JT, Aragón S, Ackerman JD. Site variation in spatial aggregation and phorophyte preference in Psychilis monensis (Orchidaceae) Biotropica. 2007a;39:227–231. doi: 10.1111/j.1744-7429.2006.00258.x. [DOI] [Google Scholar]
- Otero JT, Flanagan NS, Herre EA, Ackerman JD, Bayman P. Widespread mycorrhizal specificity correlates to mycorrhizal function in the neotropical, epiphytic orchid Ionopsis utricularioides (Orchidaceae) American Journal of Botany. 2007b;94:1944–1950. doi: 10.3732/ajb.94.12.1944. [DOI] [PubMed] [Google Scholar]
- Palmer MW. Pattern in corticolous bryophyte communities of the North-Carolina Piedmont—do mosses see the forest or the trees? Bryologist. 1986;89:59–65. doi: 10.2307/3243078. [DOI] [Google Scholar]
- Park A, Cameron JL. The influence of canopy traits on throughfall and stemflow in five tropical trees growing in a Panamanian plantation. Forest Ecology and Management. 2008;255:1915–1925. doi: 10.1016/j.foreco.2007.12.025. [DOI] [Google Scholar]
- Patiño J, Gonzalez-Mancebo JM. Exploring the effect of host tree identity on epiphyte bryophyte communities in different Canarian subtropical cloud forests. Plant Ecology. 2011;212:433–449. doi: 10.1007/s11258-010-9835-5. [DOI] [Google Scholar]
- Pennings S, Callaway R. Parasitic plants: parallels and contrasts with herbivores. Oecologia. 2002;131:479–489. doi: 10.1007/s00442-002-0923-7. [DOI] [PubMed] [Google Scholar]
- Perry DR. Factors influencing arboreal epiphytic phytosociology in Central America. Biotropica. 1978;10:235–237. doi: 10.2307/2387910. [DOI] [Google Scholar]
- Pessin LJ. An ecological study of the polypody fern Polypodium polypodioides as an epiphyte in Mississippi. Ecology. 1925;6:17–38. doi: 10.2307/1929237. [DOI] [Google Scholar]
- Piazzon M, Larrinaga AR, Santamaria L. Are nested networks more robust to disturbance? A test using epiphyte-tree, comensalistic networks. PLoS ONE. 2011;6:e19637. doi: 10.1371/journal.pone.0019637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piers F. Orchids of East Africa. Lehre: Cramer; 1968. [Google Scholar]
- Poisot T, Bever JD, Nemri A, Thrall PH, Hochberg ME. A conceptual framework for the evolution of ecological specialisation. Ecology Letters. 2011;14:841–851. doi: 10.1111/j.1461-0248.2011.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard G. La opinión de un hombre. Orquidea. 1973;3:184–190. [Google Scholar]
- Porembski S. Epiphytic orchids on arborescent Velloziaceae and Cyperaceae: extremes of phorophyte specialisation. Nordic Journal of Botany. 2003;23:505–512. doi: 10.1111/j.1756-1051.2003.tb00424.x. [DOI] [Google Scholar]
- Poulin R, Krasnov BR, Mouillot D. Host specificity in phylogenetic and geographic space. Trends in Parasitology. 2011;27:355–361. doi: 10.1016/j.pt.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Putz FE. How trees avoid and shed lianas. Biotropica. 1984;16:19–23. doi: 10.2307/2387889. [DOI] [Google Scholar]
- Raffaelli D. Food webs, body size and the curse of the Latin binomial. In: Rooney N, McCann KS, Noakes DLG, editors. From energetics to ecosystems: the dynamics and structure of ecological systems. The Netherlands: Springer; 2007. pp. 53–64. [Google Scholar]
- Ranius T, Niklasson M, Berg N. A comparison of methods for estimating the age of hollow oaks. Ecoscience. 2009;16:167–174. doi: 10.2980/16-2-3200. [DOI] [Google Scholar]
- Ravigné V, Dieckmann U, Olivieri I. Live where you thrive: joint evolution of habitat choice and local adaptation facilitates specialization and promotes diversity. The American Naturalist. 2009;174:E141–E169. doi: 10.1086/605369. [DOI] [PubMed] [Google Scholar]
- Reyes-García C, Griffiths H, Rincón E, Huante P. Niche differentiation in tank and atmospheric epiphytic bromeliads of a seasonally dry forest. Biotropica. 2008;40:168–175. doi: 10.1111/j.1744-7429.2007.00359.x. [DOI] [Google Scholar]
- Roberts NR, Dalton PJ, Jordan GJ. Epiphytic ferns and bryophytes of Tasmanian tree-ferns: a comparison of diversity and composition between two host species. Austral Ecology. 2005;30:146–154. doi: 10.1111/j.1442-9993.2005.01440.x. [DOI] [Google Scholar]
- Rogers G, Walker S. Taxonomic and ecological profiles of rarity in the New Zealand vascular flora. New Zealand Journal of Botany. 2002;40:73–93. doi: 10.1080/0028825X.2002.9512772. [DOI] [Google Scholar]
- Roxburgh L, Nicolson SW. Patterns of host use in two African mistletoes: the importance of mistletoe-host compatibility and avian disperser behaviour. Functional Ecology. 2005;19:865–873. doi: 10.1111/j.1365-2435.2005.01036.x. [DOI] [Google Scholar]
- Sanford WW. The ecology of orchids. In: Withner CL, editor. The orchids. Scientific studies. New York: Wiley; 1974. pp. 1–100. [Google Scholar]
- Sáyago R, Lopezaraiza-Mikel M, Quesada M, Álvarez-Anorve MY, Cascante-Marín A, Bastida JM. Evaluating factors that predict the structure of a commensalistic epiphyte-phorophyte network. Proceedings of the Royal Society B: Biological Sciences. 2013;280:20122821. doi: 10.1098/rspb.2012.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimper AFW. Die epiphytische Vegetation Amerikas. Jena: Gustav Fischer; 1888. [Google Scholar]
- Schlesinger WJ, Marks PL. Mineral cycling and the niche of Spanish moss, Tillandsia usneoides L. American Journal of Botany. 1977;64:1254–1262. doi: 10.2307/2442489. [DOI] [Google Scholar]
- Schnitzer SA, Bongers F. The ecology of lianas and their role in forests. Trends in Ecology and Evolution. 2002;17:223–230. doi: 10.1016/S0169-5347(02)02491-6. [DOI] [Google Scholar]
- Sehgal RN, Mehra PN. Distribution pattern of orchids in Khasi and Jaintia India hills. Indian Journal of Forestry. 1984;7:114–119. [Google Scholar]
- Sehnem A. As filicíneas do Sul do Brasil, sua distribuição geográfica, sua ecologia e suas rotas de imigração. Pesquisas Botânica. 1977;31:1–108. [Google Scholar]
- Silva IA, Ferreira AWC, Lima MIS, Soares JJ. Networks of epiphytic orchids and host trees in Brazilian gallery forests. Journal of Tropical Ecology. 2010;26:127–137. doi: 10.1017/S0266467409990551. [DOI] [Google Scholar]
- Song XQ, Meng QW, Wing YT, Luo YB. Thrixspermum odoratum (Orchidaceae), a new species from Hainan Island, China. Annales Botanici Fennici. 2009;46:595–598. doi: 10.5735/085.046.0617. [DOI] [Google Scholar]
- Soto Arenas MA. Population studies in Mexican orchids. In: Pridgeon A, editor. Proceedings of the 14th world orchid conference. London: HMSO; 1994. pp. 153–160. [Google Scholar]
- Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annual Review of Entomology. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- ter Steege H, Cornelissen JHC. Distribution and ecology of vascular epiphytes in lowland rain forest of Guyana. Biotropica. 1989;21:331–339. doi: 10.2307/2388283. [DOI] [Google Scholar]
- Thomsen MS, Wernberg T, Altieri A, Tuya F, Gulbransen D, McGlathery KJ, Holmer M, Silliman BR. Habitat cascades: the conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integrative and Comparative Biology. 2010;50:158–175. doi: 10.1093/icb/icq042. [DOI] [PubMed] [Google Scholar]
- Todzia C. Growth habits, host tree species, and density of hemiepiphytes on Barro Colorado Island, Panama. Biotropica. 1986;18:22–27. doi: 10.2307/2388357. [DOI] [Google Scholar]
- Trapnell DW, Hamrick JL, Nason JD. Three-dimensional fine-scale genetic structure of the neotropical epiphytic orchid, Laelia rubescens. Molecular Ecology. 2004;13:1111–1118. doi: 10.1111/j.1365-294X.2004.02148.x. [DOI] [PubMed] [Google Scholar]
- Tremblay RL, Zimmerman JK, Lebrón L, Bayman P, Sastre I, Axelrod F, Alers-García J. Host specificity and low reproductive success in the rare endemic Puerto Rican orchid Lepanthes caritensis. Biological Conservation. 1998;85:297–304. doi: 10.1016/S0006-3207(97)00163-8. [DOI] [Google Scholar]
- Valencia R, Foster RB, Villa G, Condit R, Svenning J-C, Hernández C, Romoleroux K, Losos E, Magård E, Balslev H. Tree species distributions and local habitat variation in the Amazon: large forest plot in eastern Ecuador. Journal of Ecology. 2004;92:214–229. doi: 10.1111/j.0022-0477.2004.00876.x. [DOI] [Google Scholar]
- Valencia-Díaz S, Flores-Palacios A, Rodríguez-López V, Ventura-Zapata E, Jiménez-Aparicio AR. Effect of host-bark extracts on seed germination in Tillandsia recurvata, an epiphytic bromeliad. Journal of Tropical Ecology. 2010;26:571–581. doi: 10.1017/S0266467410000374. [DOI] [Google Scholar]
- Válka Alves RJ, Kolbek J, Becker J. Vascular epiphyte vegetation in rocky savannas of southeastern Brazil. Nordic Journal of Botany. 2008;26:101–117. doi: 10.1111/j.0107-055X.2008.00190.x. [DOI] [Google Scholar]
- Van den Berg C, Smidt EC, Marcal S. Leptotes vellozicola: a new species of Orchidaceae from Bahia, Brazil. Neodiversity. 2006;1:1–5. doi: 10.13102/neod.11.1. [DOI] [Google Scholar]
- Van Oye MP. Sur l’écologie des épiphytes a la surface des troncs d'arbres à Java. Revue Génerale de Botanique. 1924;36:12–30,. 68–83. [Google Scholar]
- Vergara-Torres CA, Pacheco-Alvarez MC, Flores-Palacios A. Host preference and host limitation of vascular epiphytes in a tropical dry forest of central Mexico. Journal of Tropical Ecology. 2010;26:563–570. doi: 10.1017/S0266467410000349. [DOI] [Google Scholar]
- Watt MS, Moore JR, McKinlay B. The influence of wind on branch characteristics of Pinus radiata. Trees—Structure and Function. 2005;19:58–65. doi: 10.1007/s00468-004-0363-6. [DOI] [Google Scholar]
- Watthana S, Pedersen HÆ. Phorophyte diversity, substrate requirements and fruit set in Dendrobium scabrilingue Lindl. (Asparagales: Orchidaceae): basic observations for re-introduction experiments. The Natural History Journal of Chulalongkorn University. 2008;8:135–142. [Google Scholar]
- Wee YC. Vascular epiphytes of Singapore's wayside trees. Gardens’ Bulletin, Singapore. 1978;31:114–126. [Google Scholar]
- Went FW. Soziologie der Epiphyten eines tropischen Regenwaldes. Annales du Jardin Botanique de Buitenzorg. 1940;50:1–98. [Google Scholar]
- Whitham TG, Gehring CA, Lamit LJ, Wojtowicz T, Evans LM, Keith AR, Smith DS. Community specificity: life and afterlife effects of genes. Trends in Plant Science. 2012;17:271–281. doi: 10.1016/j.tplants.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Whittaker RH, Woodwell GM. Surface area relations of woody plants and forest communities. American Journal of Botany. 1967;54:931–939. doi: 10.2307/2440715. [DOI] [Google Scholar]
- Wolf JHD. Factors controlling the distribution of vascular and non-vascular epiphytes in the northern Andes. Vegetatio. 1994;112:15–28. doi: 10.1007/BF00045096. [DOI] [Google Scholar]
- Wyse SV, Burns BR. Do host bark traits influence trunk epiphyte communities? New Zealand Journal of Botany. 2011;35:296–301. [Google Scholar]
- Yan Z. Resistance to haustorial development of two mistletoes, Amyema preissii (Miq.) Tieghem and Lysiana exocarpi (Behr.) Tieghem ssp. exocarpi (Loranthaceae), on host and nonhost species. International Journal of Plant Sciences. 1993;154:386–394. doi: 10.1086/297120. [DOI] [Google Scholar]
- Zhang LW, Nurvianto S, Harrison R. Factors affecting the distribution and abundance of Asplenium nidus L. in a tropical lowland rain forest in Peninsular Malaysia. Biotropica. 2010;42:464–469. doi: 10.1111/j.1744-7429.2009.00607.x. [DOI] [Google Scholar]
- Zhou D, Hyde KD. Host-specificity, host-exclusivity, and host-recurrence in saprobic fungi. Mycological Research. 2001;105:1449–1457. doi: 10.1017/S0953756201004713. [DOI] [Google Scholar]
- Zimmerman JK, Olmsted IC. Host tree utilization by vascular epiphytes in a seasonally inundated forest (Tintal) in Mexico. Biotropica. 1992;24:402–407. doi: 10.2307/2388610. [DOI] [Google Scholar]
- Zotz G. Gefässepiphyten in temperaten Wäldern. Bauhinia. 2002;16:13–22. [Google Scholar]
- Zotz G. Johansson revisited: the spatial structure of epiphyte assemblages. Journal of Vegetation Science. 2007;18:123–130. doi: 10.1111/j.1654-1103.2007.tb02522.x. [DOI] [Google Scholar]
- Zotz G. ‘Hemiepiphyte’: a confusing term and its history. Annals of Botany. 2013a;111:1015–1020. doi: 10.1093/aob/mct085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G. The systematic distribution of vascular epiphytes—a critical update. Botanical Journal of the Linnean Society. 2013b;171:453–481. doi: 10.1111/boj.12010. [DOI] [Google Scholar]
- Zotz G, Andrade JL. La ecología y la fisiología de las epífitas y las hemiepífitas. In: Guariguata MR, Kattan GH, editors. Ecología y Conservación de Bosques Neotropicales. San José: Libro Universitario Regional del Instituto Tecnológico de Costa Rica; 2002. pp. 271–296. [Google Scholar]
- Zotz G, Schultz S. The vascular epiphytes of a lowland forest in Panama-species composition and spatial structure. Plant Ecology. 2008;195:131–141. doi: 10.1007/s11258-007-9310-0. [DOI] [Google Scholar]
- Zotz G, Vollrath B. The epiphyte vegetation of the palm Socratea exorrhiza—correlations with tree size, tree age and bryophyte cover. Journal of Tropical Ecology. 2003;19:81–90. doi: 10.1017/S0266467403003092. [DOI] [Google Scholar]
- Zotz G, Mendieta-Leiva G, Wagner K. Vascular epiphytes at the treeline—composition of species assemblages and population biology. Flora. 2014;209:385–390. doi: 10.1016/j.flora.2014.06.001. [DOI] [Google Scholar]
- Zytynska SE, Fay MF, Penney D, Preziosi RF. Genetic variation in a tropical tree species influences the associated epiphytic plant and invertebrate communities in a complex forest ecosystem. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:1329–1336. doi: 10.1098/rstb.2010.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.