Abstract
BACKGROUND:
Pediatric traumatic brain injury (TBI) contributes to impairments in functioning in everyday settings. Evidence suggests that online family problem-solving therapy (FPST) may be effective in reducing adolescent behavioral morbidity. This article examines the efficacy of Counselor-Assisted Problem Solving (CAPS), a form of online FPST in improving long-term functional outcomes of adolescents with TBI relative to Internet resources only.
METHODS:
Children, aged 12 to 17 years, who were hospitalized in the previous 7 months for TBI were enrolled in a multisite, assessor-blinded randomized controlled trial. Consented participants were randomly assigned to CAPS or an Internet resource comparison (IRC) condition. Outcomes were assessed at baseline and at follow-ups 6, 12, and 18 months postbaseline. The Child and Functional Assessment Scale and the Iowa Family Interaction Rating Scale (IFIRS) served as primary outcomes of child and family functioning respectively.
RESULTS:
For the Child and Functional Assessment Scale total, we found a significant group × time interaction, with less impaired functioning for the CAPS group than for the IRC group at the final follow-up. Parent education moderated the efficacy of CAPS on overall rates of impairment and school/work functioning, with the advantage of CAPS over IRC evident at the final follow-up only for participants with less-educated parents. Neither group differences nor group × time interactions were found for the IFIRS.
CONCLUSIONS:
Relatively brief, online treatment shortly after injury may result in long-term improvements in child functioning, particularly among families of lower socioeconomic status. Clinical implementation of CAPS during the initial months postinjury should be considered.
Keywords: brain injury, online, therapy, RCT, child, adolescent
What’s Known on This Subject:
Pediatric traumatic brain injury (TBI) contributes to impairments in functioning across multiple settings. Online family problem-solving therapy may be effective in reducing adolescent behavioral morbidity after TBI. However, less is known regarding maintenance of effects over time.
What This Study Adds:
This large randomized clinical trial in adolescents with TBI is the only study to examine maintenance of treatment effects. Findings reveal that brief, online treatment may result in long-term improvements in child functioning, particularly among families of lower socioeconomic status.
Traumatic brain injury (TBI) is a common cause of morbidity, affecting nearly 750 000 children per year.1 Although mild injuries often resolve quickly,2 more severe TBI contributes to persistent deficits in behavior, social competence, and adaptive skills. Adolescents with TBI may be at particular risk of academic difficulties, substance use, and criminal offending.3,4 Adolescents are at elevated risk of sustaining TBI1 and may be responsive to interventions designed to improve self-regulation and executive function skills5,6 that underlie deficits in everyday functioning.7
Recent reviews highlight the paucity of high-quality trials addressing behavior and attention problems after TBI,8,9 although some notable exceptions exist.10,11 Family problem-solving therapy (FPST) has emerged as a potentially efficacious treatment of reducing behavioral and executive dysfunction after TBI in adolescents.5–7,12–15 Family functioning contributes to recovery after TBI16–19; thus, interventions promoting family problem-solving may improve the child’s behavior and functioning.
A review of 3 published studies of FPST using randomized designs revealed significant improvements in behavior immediately after treatment among participants assigned to family problem-solving relative to those assigned to an Internet resource comparison (IRC).5–7,12,13 Both family socioeconomic status (SES) and the child’s age moderated treatment efficacy. Older children and those from lower-income families showed the greatest improvements in parent-reported behavior problems and executive dysfunction.5,7
Tele-health treatments may benefit adolescents after TBI.5–7,12–15,20 However, meta-analyses comparing online to traditional face-to-face approaches for various psychiatric and medical conditions have yielded mixed conclusions.14,21,22 Although some reviews indicated comparable efficacy of traditional and tele-health interventions,22 others noted smaller effects for online treatments.23 Factors such as greater treatment intensity/duration, therapist involvement, and inclusion of behavioral change methods such as self-monitoring are associated with stronger tele-health intervention effects.21,23,24
Previous studies have been limited by reliance on parent-reported outcomes and lack of longer-term follow-up. The current study addresses these limitations by examining the efficacy of FPST in improving child functioning as assessed by standardized clinical ratings of child functional status. Previous reports from this randomized controlled trial in 132 youth ages 12 to 17 years hospitalized for TBI revealed that immediately after treatment there were fewer behavior problems13 and lower levels of executive dysfunction7 among older adolescents in the treatment versus the comparison condition. We extend this previous work by evaluating long-term effects of problem-solving therapy on child functioning and examining the influence of SES on long-term outcomes.
Methods
Participants
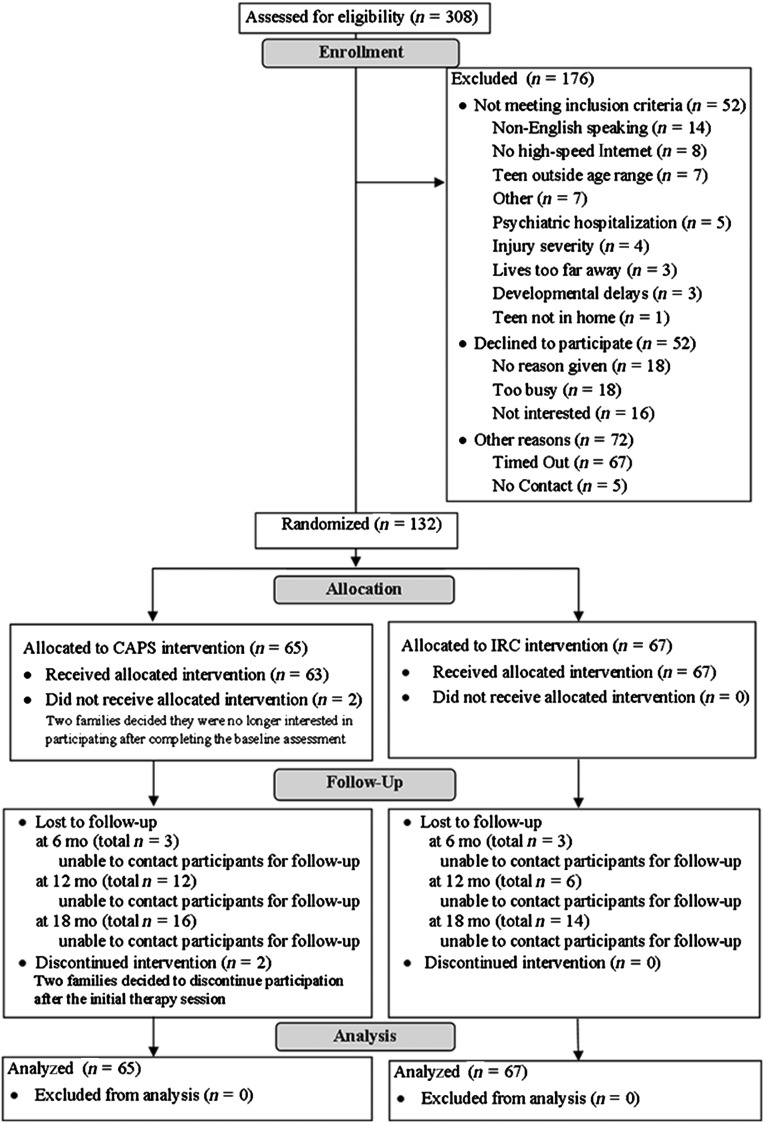
Participants were enrolled at 5 major trauma centers in the central and western regions of the United States. Eligibility criteria included age between 12 and 17 years and hospitalization for a moderate to severe TBI13 within 7 months of study enrollment. Moderate TBI was defined as a Glasgow Coma Scale (GCS) score of 9 to 12 or a GCS score >12 with evidence of neurologic insult on imaging,25 and severe TBI was defined as a GCS score <9.26 We targeted ages 12 to 17 years given the emerging demands for effective, autonomous problem-solving in this age range and the desire to use consistent measures across participants. Additional requirements included English as the primary language, availability of the adolescent to participate (eg, not incarcerated), and family residence within a 3-hour drive of the hospital. Reasons for exclusion included the following: (1) insufficient recovery to participate (eg, minimally responsive state) or unavailability of high-speed Internet access at their address, (2) child or parent hospitalization for psychiatric problems during the year before injury, or (3) premorbid intellectual disability (see Fig 1). The trial was registered with clinicaltrials.gov (assigned identifier NCT00409448) and approved by the institutional review boards of participating institutions.
FIGURE 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart.
Baseline and Follow-up Assessments
Personnel obtained informed consent and completed the baseline assessment at the families’ homes. Families were given a new computer, Web camera, and high-speed Internet access and links to online TBI resources. The structured interview and parent-reported measures were completed at baseline and again immediately after treatment (6 months postbaseline) and at 12 and 18 months postbaseline.20
Participants were randomly assigned to the following groups: (1) Counselor-Assisted Problem Solving (CAPS), a 6-month Web-based, therapist-moderated intervention providing training in problem-solving, communication, and self-regulation, or (2) IRC, a control intervention providing self-guided, Web-based TBI information and resources. Randomization was stratified according to race and gender. A sealed envelope containing group assignment was handed to participants at the visit completion, allowing the coordinator to remain naive to assignment.
Treatment Groups
CAPS Intervention
Four licensed clinical psychologists delivered CAPS. Session objectives and procedures were detailed in a treatment manual. Fidelity was maintained through weekly supervision calls and verified by session checklists.
The initial CAPS treatment sessions were conducted in the families’ homes. During this visit, the psychologist completed a background interview, provided a treatment overview, and identified family goals for problem-solving. Subsequent CAPS sessions were completed online via video conferencing. Each session included a Web-based module that was completed independently and a Skype session with the psychologist to review skills and implement the problem-solving process to address family-identified goals. The teen with TBI and at least 1 parent were required to participate. However, all family members were encouraged to participate.27
IRC
The IRC program consisted of a home page with links to online resources including local, state, and national brain-injury association Web sites and sites about pediatric TBI. Families were instructed to spend at least 1 hour per week on these educational sites and to log the sites that they visited and time spent at each site in a diary provided for that purpose. This diary was collected at treatment completion.
Follow-up Assessments
Follow-up assessments were scheduled at treatment completion (6 months after baseline) and at 12 and 18 months postbaseline. Although it was anticipated that most participants would complete the CAPS treatment by the 6-month follow-up, assessments were scheduled without knowledge of whether the participant had completed treatment.
Measures
Family Socioeconomic and Youth Premorbid Status
Information regarding preinjury learning or psychiatric conditions and sociodemographic information, including primary caregiver educational attainment and parent-reported income, was collected via interview at the baseline assessment. Given the significant association between caregiver education and family income (P < . 01), we chose to use highest educational attainment for the primary caregiver as a proxy variable for family SES.6 Educational attainment was dichotomized into high school education or less versus at least some postsecondary education.
Cognitive Functioning
Processing speed, as measured by the Processing Speed Index of either the Wechsler Intelligence Scale for Children, Fourth Edition,28 or the Wechsler Adult Intelligence Scale, Fourth Edition,29 depending on age, provided a measure of TBI-related cognitive impairment.
Receipt of Other Therapies
The Service Assessment for Children and Adolescents was used to assess the use of mental health services after TBI in outpatient and school settings.30
Everyday Functioning
Functioning in everyday settings was assessed by using the Child and Adolescent Functional Assessment Scale (CAFAS). The CAFAS uses information from structured interviews with key informants (eg, parents) to generate standardized ratings of functioning across domains and settings. The CAFAS was chosen as a primary outcome because it better predicts subsequent service utilization than either behavior checklists or psychiatric diagnoses31 and has been widely used to assess changes in clinical outcomes for youth receiving mental health services.20 The CAFAS generates ratings of functioning in 8 domains: school, home, community, behavior toward others, moods/emotions, self-harmful behaviors, substance abuse, and thinking.32 Functioning in each domain is rated on an ordinal scale ranging from 0 (no impairment) to 30 (severe impairment) in increments of 10. A total score is created by summing domain scores. This total score is a continuous variable with a range of 0 to 240. The CAFAS has established validity and excellent interrater reliability, ranging from 0.74 to 0.99.33 On the basis of the CAFAS manual and previous research, clinically significant change was defined as an improvement of ≥10 points. Total scores of ≤50 were considered to be “unimpaired.” Two research personnel with advanced degrees in psychology or counseling were certified as CAFAS trainers after attending a 2-day training session. Additional raters were trained to achieve interrater reliability >80%, as recommended by the creator of the CAFAS. Ten percent of interviews were taped and jointly rated, yielding an overall interrater reliability of 95%.
Analyses
To compare the groups (CAPS versus IRC) on baseline demographic, injury, and behavioral characteristics and to examine baseline differences between those who completed the study and those who dropped out, t, χ2, and 2-tailed Fisher’s exact tests were used. Table 1 reports baseline characteristics for completers versus those missing ≥1 follow-ups. Caregivers of noncompleters had disproportionately lower income and were more often single than caregivers of completers. Attrition did not differ significantly between the treatment groups. As reported in Table 2, the CAPS and IRC groups were well matched and did not differ significantly on demographic characteristics or injury severity at baseline.
TABLE 1.
Comparison of Cohort and Dropouts: CAPS Study
| Cohort (n = 99) | Dropoutsa (n = 33) | P | |
|---|---|---|---|
| Treatment group, n (%) | 0.13 | ||
| CAPS (n = 65) | 45 (69.2) | 20 (30.8) | |
| IRC (n = 67) | 54 (80.6) | 13 (19.4) | |
| Age at baseline, mean (SD), y | 14.8 (1.8) | 15.1 (1.6) | 0.39 |
| Age at injury, mean (SD), y | 14.5 (1.8) | 14.8 (1.6) | 0.42 |
| Time since injury at baseline, mean (SD), mo | 3.5 (1.8) | 4.1 (1.8) | 0.09 |
| Female gender, n (%) | 34 (34.3) | 13 (36.4) | 0.83 |
| Race/ethnicity, n (%) | 0.12 | ||
| White | 83 (83.9) | 23 (69.7) | |
| Black | 10 (10.1) | 9 (24.2) | |
| Mixed/other | 6 (6.1) | 2 (6.1) | |
| Injury severity, n (%) | 0.12 | ||
| Moderate TBI | 57 (57.6) | 24 (72.7) | |
| Severe TBI | 42 (42.4) | 9 (27.3) | |
| Primary caregiver education, n (%) | 0.19 | ||
| High school or less | 41 (41.4) | 18 (54.6) | |
| More than high school | 58 (58.6) | 15 (45.5) | |
| Income, n (%) | 0.01* | ||
| <$40 000 | 32 (32.3) | 20 (60.6) | |
| $40 000–$89 999 | 37 (37.4) | 8 (24.2) | |
| >$90 000 | 30 (30.3) | 5 (15.2) | |
| Primary caregiver marital status, n (%) | 0.008* | ||
| Married/living with partner | 70 (70.7) | 18 (54.6) | |
| Not married | 29 (9.3) | 15 (45.5) |
N = 132. *Cohort and dropouts differed significantly, P < .05 (χ2 or t test).
Dropouts included those who missed ≥1 of the 4 visits.
TABLE 2.
Comparison of Intervention and Control Groups: CAPS Study
| CAPS (n = 65) | IRC (n = 67) | P | |
|---|---|---|---|
| Age at baseline, mean (SD), y | 14.7 (1.7) | 15.0 (1.8) | 0.33 |
| Age at injury, mean (SD), y | 14.4 (1.7) | 14.7 (1.8) | 0.37 |
| Time since injury at baseline, mean (SD), mo | 3.7 (1.9) | 3.5 (1.7) | 0.48 |
| Female gender, n (%) | 21 (2.3) | 25 (37.3) | 0.55 |
| Race/ethnicity, n (%) | 0.23 | ||
| White | 52 (80) | 45 (80.6) | |
| Black | 7 (10.8) | 1 (16.4) | |
| Mixed/other | 6 (9.2) | 2 (3.0) | |
| TBI severity, n (%) | 0.97 | ||
| Moderate TBI | 40 (61.5) | 41 (61.2) | |
| Severe TBI | 25 (38.5) | 26 (38.8) | |
| Primary caregiver education, n (%) | 0.19 | ||
| High school or less | 26 (40) | 33 (49.3) | |
| More than high school | 39 (60) | 34 (50.8) | |
| Income, n (%) | 0.41 | ||
| <$40 000 | 22 (33.9) | 30 (44.8) | |
| $40 000–$89 999 | 25 (38.5) | 20 (29.9) | |
| >$90 000 | 18 (27.7) | 17 (25.4) | |
| Primary caregiver marital status, n (%) | 0.44 | ||
| Married/living with partner | 44 (67.7) | 41 (61.2) | |
| Not married | 21 (32.2) | 26 (38.8) |
N = 132.
A single mixed-models analysis, with random intercepts and slopes, was conducted to examine the intention-to-treat group differences on the CAFAS total across follow-up. Mixed-models analysis retains participants in the model who are missing data for ≥1 assessments and is thus less affected by attrition. An unstructured covariance structure was chosen on the basis of Akaike’s information criterion. Six post hoc analyses of the CAFAS domain subscales allowed us to elucidate the nature of the effects. The self-harmful behaviors and substance abuse scales were excluded from analyses due to insufficient variability in scores over time. General estimating equation analyses were conducted to examine group differences in the proportion of youth with clinically significant impairment on the CAFAS: no/mild impairment (score range: 0–50) and at risk (score range: >50). Before constructing final models, preliminary analyses were conducted examining the effects of injury severity, child age, gender, premorbid history of learning or behavior difficulties, injury-related impairment as defined by processing speed score on the Wechsler Intelligence Scale for Children, and caregiver education and marital status on the CAFAS total. Final models included the aforementioned covariates and the interaction of treatment group × caregiver education × visit along with the component interaction terms. When a significant interaction was detected, post hoc analyses examined group differences at each time point to examine outcome trajectories by group. All analyses were conducted by using SAS version 9.3 (SAS Institute, Cary, NC).
Results
A total of 132 children were enrolled, with 65 assigned to CAPS and 67 assigned to IRC. Complete follow-up data were available for 75% of the sample, and attrition did not significantly vary by group (see Table 1). At enrollment, 13 children in the CAPS group (20%) and 15 in the IRC group (22.39%) were receiving behavioral treatments (P > .7). Similar rates of receipt of other treatments were reported at visits 2 through 4 (all P > .4).
Parent-reported time spent on the intervention Web sites did not differ by group. Forty-three percent of parents in CAPS versus 48% in IRC spent <30 minutes per week, and 50% of parents in CAPS versus 47% in IRC spent 30 minutes to 2 hours per week. Similarly, 43% of CAPS teens versus 48% of IRC teens reported spending <30 minutes per week on study Web sites. CAPS participants completed an average of 8 sessions (range: 0–13). Within CAPS, the number of sessions completed was unrelated to functioning at visits 2 and 3. However, at visit 4, total sessions completed was positively correlated with CAFAS total (P = .04), suggesting that families with greater problems completed more supplemental sessions.
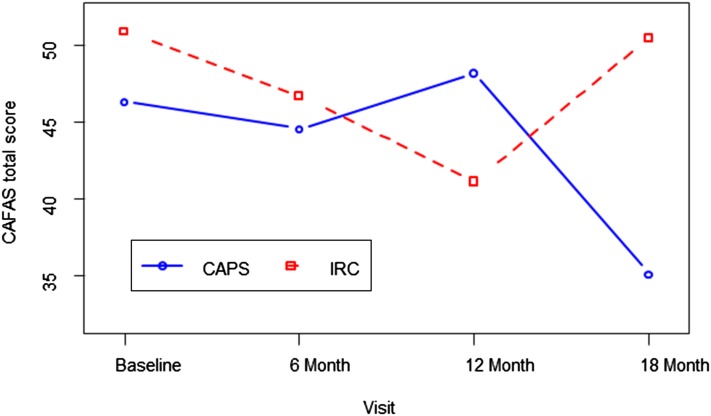
As depicted in Fig 2, there was a significant group × visit interaction for the CAFAS total score (P < .01). Post hoc analyses revealed significantly lower CAFAS total scores (17.88 points) at the final visit in the CAPS versus IRC participants, suggesting that improvements in everyday functioning emerged over time after the intervention. The groups did not differ significantly at other time points.
FIGURE 2.
Average total CAFAS scores over time by group. There was a significant group × visit interaction: F(3, 301) = 4.18, P = .006. Total score for the CAPS group was significantly less than the IRC group at visit 4, P < .05.
General estimating equation analyses examining the proportion of youth who exceeded the CAFAS cutoff for clinical impairment (total >50) revealed a significant group × time × caregiver education interaction (P < .01). As reported in Table 3, at the final visit only, the odds of being impaired in the IRC group were significantly higher than the odds of being impaired in the CAPS group among youth with less educated parents (odds ratio: 6.75; 95% confidence interval: 1.97–23.24).
TABLE 3.
Proportions Above CAFAS Cutoff by Treatment, Parental Education, and Visit
| Group | College | Baseline (Pretreatment) | 6 Months (Posttreatment) | 12-Month Follow-up | 18 Month Follow-up |
|---|---|---|---|---|---|
| CAPS | No | 48.0 | 44.4 | 42.11 | 6.7 |
| CAPS | Yes | 31.6 | 17.1 | 25.0 | 23.1 |
| IRC | No | 48.5 | 35.7 | 41.7 | 72.2* |
| IRC | Yes | 27.3 | 18.2 | 14.3 | 15.4 |
Data are presented as percentages (proportions). CAFAS cutoff: >50 = impaired. *Significantly different from CAPS group with no college education, P < .05.
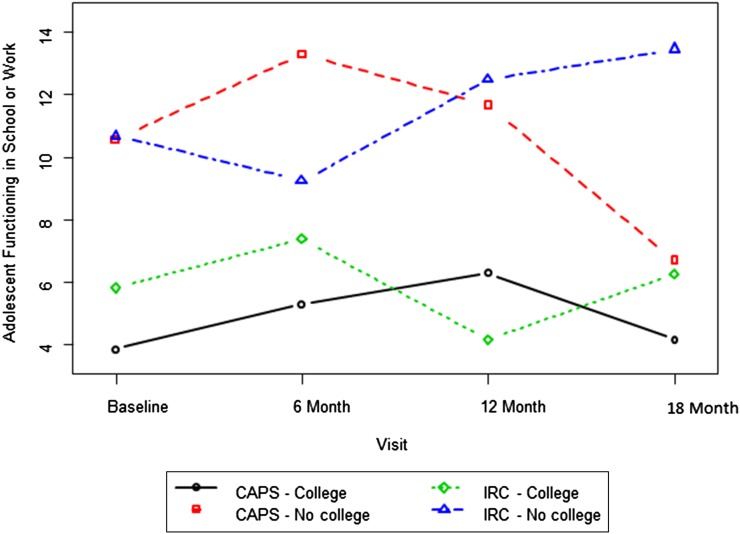
Post hoc analyses to elucidate the nature of the effects revealed significant interactions of treatment group with visit and parent education for the domain of school functioning (Fig 3) (P = .03). The CAPS group had significantly better functioning 12 months posttreatment than the IRC group among adolescents with less educated parents. For the domain of community functioning, there was a significant group × visit interaction (P = .03). Consistent with the pattern of improvements observed for the CAFAS total, the CAPS group was rated as having significantly better functioning in the community than the IRC group at the final assessment (P = .04).
FIGURE 3.
Ratings of functioning at school or work over time by group and primary caregiver education. There was a significant group × time × caregiver education interaction, F(3, 315) = 3.26, P = .02. Post hoc contrasts indicate a significant group difference between the low-education CAPS and IRC groups at visit 4: t(351) = −2.20, P = .03.
Analyses failed to reveal intervention effects on functioning in the home, behavior toward others, moods/emotions, or thinking domains. Parental education was a significant predictor of both behavior toward others and thinking difficulties, with children of less educated parents evidencing poorer functioning (behavior toward others, P = .03; thinking, P = .04). The caregiver’s marital status was a significant predictor of CAFAS total score and subdomains including school and behavior toward others (CAFAS total, P = .03; school, P = .01; behavior toward others, P = .01), with children with married caregivers rated significantly better than those with unmarried caregivers. Premorbid history of learning, attention, or behavior problems was also associated with poorer functioning on the CAFAS total (P = .02), school (P = .03), community (P = .03), and behavior toward others (P = .01) domains. Similarly, processing speed at baseline was a significant predictor of CAFAS total (P < .01), home (P = .02), behavior toward others (P < .01), moods/emotions (P = .02), and thinking (P < .01) domains, with faster processing speed associated with better functioning. The child’s age, gender, and severity of injury were unrelated to CAFAS total or domain scores.
Discussion
This study is the first to our knowledge to show long-term improvements in functioning after online FPST in a large cohort of adolescents with TBI. The CAPS treatment integrated many of the features known to boost the efficacy of tele-health treatment, including therapist involvement, greater intensity/duration, and integration of evidence-based behavioral change strategies.23 Differences in functioning between treatment and control groups did not become evident until a full 12 months after treatment completion, suggesting that the effects of problem-solving therapy delivered soon after injury may successfully generalize to the youth’s functioning in everyday settings over time. Consistent with previous research,5,34 improvements after CAPS were particularly evident among youth from lower SES families.
CAFAS scores are based on behaviors/functioning for the 3-month period preceding the interview. Thus, the posttreatment follow-up assessment reflects functioning for the period while the youth was still participating in treatment. It may take longer for treatment-related improvements in problem-solving and executive functioning8 to translate into improved everyday functioning. Previous research with this cohort showed that executive function skills accounted for significant variance in impairments in everyday functioning on the CAFAS administered before treatment.35 Subsequent analyses revealed significant improvements in executive function skills among older adolescents in the CAPS group, relative to the IRC, immediately after treatment.7 These findings suggest that initial improvements in executive function behaviors may support longer-term improvements in functional outcomes.
Post hoc analyses of specific CAFAS domains indicated greater efficacy of CAPS in improving functioning outside the home (eg, school/work and community). Given that adolescents with TBI are at risk of deficits in school performance36 and may have difficulty sustaining employment37 as they transition into adulthood, improving school and community functioning may be particularly important for long-term life success.
Consistent with previous research, improvements in clinical impairment as well as school/work functioning were moderated by family SES.5,34 Previous research suggests that social disadvantage, including lower family income and lower levels of parental education, is associated with poorer outcomes after TBI.38–40 Disadvantaged youth may lack access to treatment30 and may have greater difficulty adhering to treatment when available.40 The current intervention addressed many barriers to care by delivering care within the home at times convenient for the family. Nonetheless, lower income and unmarried parents were more likely to drop out, underscoring the need to develop strategies for engaging and retaining disadvantaged families in treatment.41
These findings should be interpreted in the context of existing limitations. Youth with TBI were not required to have TBI-related behavior problems or functional impairments, which may have limited our ability to detect significant improvements in functioning (floor effects). Conversely, given that tele-health interventions may be most effective for individuals with less severe impairments, our findings may reflect a greater level of improvement than would be seen among a sample of youth with more severe behavioral difficulties.23 Although both the CAPS and IRC groups received access to online resources regarding TBI, the groups were not equated for therapist attention or intensity. As such, it is not possible to distinguish the specific effects of problem-solving therapy from those associated with therapist attention. Although we sought to minimize attrition effects, differential dropout among unmarried and lower income participants may limit the generalizability of the findings or subtly bias the intent-to-treat analyses. The study was inadequately powered to examine the role of race/ethnicity in attrition and treatment response. The findings from this study are limited to children admitted to the hospital with a TBI who had recovered sufficiently within the first 7 months to participate in study procedures.
In summary, results support the utility of problem-solving therapy in improving functional outcomes after TBI, particularly for youth of lower SES. CAPS may be a useful adjunct to standard follow-up for TBI and could be readily integrated into ongoing care. Further research is needed to determine the optimal timing and intensity of intervention and who is most likely to benefit from more intensive approaches.
Footnotes
Dr Wade conceptualized the original study design, supervised data collection at all participating sites, conceptualized the manuscript and analyses, and drafted the manuscript; Dr Kurowski contributed to the conceptualization of hypotheses and analyses and interpretation of findings and participated in critical review and revision of multiple drafts of the manuscript; Drs Kirkwood, Brown, Stancin, and Taylor coordinated and supervised data collection at 1 site and critically reviewed and revised the manuscript; Dr Zhang performed the statistical analysis and critically reviewed and revised the manuscript; Dr Cassedy conducted the statistical analyses and critically reviewed and revised the manuscript; Dr Nielsen coordinated and supervised the training and administration of the Child and Functional Assessment Scale (CAFAS) and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The National Institute of Mental Health, the Colorado Brain Injury Trust Fund, or the National Institute of Disability and Rehabilitation Research were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Dr Wade (principal investigator) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors further certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on them or on any organization with which they are associated and we certify that all financial and material support for this research (eg, National Institutes of Health, Colorado Brain Injury Trust Fund Research Program, National Institute of Disability and Rehabilitation Research) and work is clearly identified in the title page of the manuscript.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00409448).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by National Institutes of Health grant R01-MH073764 from the National Institute of Mental Health; a grant from the Colorado Traumatic Brain Injury Trust Fund Research Program, Colorado Department of Human Services, Division of Vocational Rehabilitation, Traumatic Brain Injury Program; grant H133G050239 from the National Institute of Disability and Rehabilitation Research, Department of Education; and National Institute of Child Health and Human Development grant 1K23HD074683-01A1. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths—United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1–32 [PubMed]
- 2.Kirkwood MW, Yeates KO, Taylor HG, Randolph C, McCrea M, Anderson VA. Management of pediatric mild traumatic brain injury: a neuropsychological review from injury through recovery. Clin Neuropsychol. 2008;22(5):769–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilie G, Boak A, Adlaf EM, Asbridge M, Cusimano MD. Prevalence and correlates of traumatic brain injuries among adolescents. JAMA. 2013;309(24):2550–2552 [DOI] [PubMed] [Google Scholar]
- 4.Williams WH, Cordan G, Mewse AJ, Tonks J, Burgess CN. Self-reported traumatic brain injury in male young offenders: a risk factor for re-offending, poor mental health and violence? Neuropsychol Rehabil. 2010;20(6):801–812 [DOI] [PubMed] [Google Scholar]
- 5.Wade SL, Carey J, Wolfe CR. The efficacy of an online cognitive-behavioral family intervention in improving child behavior and social competence following pediatric brain injury. Rehabil Psychol. 2006;51(3):179–189 [Google Scholar]
- 6.Wade SL, Walz NC, Carey J, et al. Effect on behavior problems of teen online problem-solving for adolescent traumatic brain injury. Pediatrics. 2011;128(4). Available at: www.pediatrics.org/cgi/content/full/128/4/e947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurowski BG, Wade SL, Kirkwood MW, Brown TM, Stancin T, Taylor HG. Online problem-solving therapy for executive dysfunction after child traumatic brain injury. Pediatrics. 2013;132(1). Available at: www.pediatrics.org/cgi/content/full/132/1/e158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backeljauw B, Kurowski BG. Interventions for attention problems after pediatric traumatic brain injury: what is the evidence? PM R. 2014;6(9):814–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross KA, McMillan T, Kelly T, Sumpter R, Dorris L. Friendship, loneliness and psychosocial functioning in children with traumatic brain injury. Brain Inj. 2011;25(12):1206–1211 [DOI] [PubMed] [Google Scholar]
- 10.van’t Hooft I, Andersson K, Bergman B, Sejersen T, von Wendt L, Bartfai A. Sustained favorable effects of cognitive training in children with acquired brain injuries. NeuroRehabilitation. 2007;22(2):109–116 [PubMed] [Google Scholar]
- 11.Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–1303 [DOI] [PubMed] [Google Scholar]
- 12.Wade SL, Walz NC, Carey J, et al. A randomized trial of teen online problem solving for improving executive function deficits following pediatric traumatic brain injury. J Head Trauma Rehabil. 2010;25(6):409–415 [DOI] [PubMed] [Google Scholar]
- 13.Wade SL, Stancin T, Kirkwood M, Brown TM, McMullen KM, Taylor HG. Counselor-Assisted Problem Solving (CAPS) improves behavioral outcomes in older adolescents with complicated mild to severe TBI. J Head Trauma Rehabil. 2014;29(3):198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy MRT, Coelho C, Turkstra L, et al. Intervention for executive functions after traumatic brain injury: a systematic review, meta-analysis and clinical recommendations. Neuropsychol Rehabil. 2008;18(3):257–299 [DOI] [PubMed] [Google Scholar]
- 15.Gan C, Gargaro J, Brandys C, Gerber G, Boschen K. Family caregivers’ support needs after brain injury: a synthesis of perspectives from caregivers, programs, and researchers. NeuroRehabilitation. 2010;27(1):5–18 [DOI] [PubMed] [Google Scholar]
- 16.Micklewright JL, King TZ, O’Toole K, Henrich C, Floyd FJ. Parental distress, parenting practices, and child adaptive outcomes following traumatic brain injury. J Int Neuropsychol Soc. 2012;18(2):343–350 [DOI] [PubMed] [Google Scholar]
- 17.Max JE, Wilde EA, Bigler ED, et al. Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J Neuropsychiatry Clin Neurosci. 2012;24(4):427–436 [DOI] [PubMed] [Google Scholar]
- 18.Anderson VA, Catroppa C, Dudgeon P, Morse SA, Haritou F, Rosenfeld JV. Understanding predictors of functional recovery and outcome 30 months following early childhood head injury. Neuropsychology. 2006;20(1):42–57 [DOI] [PubMed] [Google Scholar]
- 19.Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI). J Pediatr Psychol. 2008;33(7):707–718 [DOI] [PubMed] [Google Scholar]
- 20.Kurowski BG, Wade SL, Kirkwood MW, Brown TM, Stancin T, Taylor HG. Long-term benefits of an early online problem-solving intervention for executive dysfunction after traumatic brain injury in children: a randomized clinical trial. JAMA Pediatr. 2014;168(6):523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cushing CC, Steele RG. A meta-analytic review of eHealth interventions for pediatric health promoting and maintaining behaviors. J Pediatr Psychol. 2010;35(9):937–949 [DOI] [PubMed] [Google Scholar]
- 22.Andrews G, Cuijpers P, Craske MG, McEvoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS ONE. 2010;5(10):e13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peñate W. About the effectiveness of telehealth procedures in psychological treatments. Int J Clin Health Psychol. 2012;12(3):475–487 [Google Scholar]
- 24.Webb MS, Rodríguez-Esquivel D, Baker EA. Smoking cessation interventions among Hispanics in the United States: a systematic review and mini meta-analysis. Am J Health Promot. 2010;25(2):109–118 [DOI] [PubMed] [Google Scholar]
- 25.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27(3):422–428 [DOI] [PubMed] [Google Scholar]
- 26.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81–84 [DOI] [PubMed] [Google Scholar]
- 27.Wade SL, Karver CL, Taylor HG, et al. Counselor-assisted problem solving improves caregiver efficacy following adolescent brain injury. Rehabil Psychol. 2014;59(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler D. WISC-IV Administration Manual. San Antonio, TX: Pearson Assessment; 2003 [Google Scholar]
- 29.Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition (WAIS-IV). San Antonio, TX: Pearson Education Inc; 2008 [Google Scholar]
- 30.Slomine BS, McCarthy ML, Ding R, et al. CHAT Study Group . Health care utilization and needs after pediatric traumatic brain injury. Pediatrics. 2006;117(4). Available at: www.pediatrics.org/cgi/content/full/117/4/e663 [DOI] [PubMed] [Google Scholar]
- 31.Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24(3):345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodges K, Wong MM, Latessa M. Use of the Child and Adolescent Functional Assessment Scale (CAFAS) as an outcome measure in clinical settings. J Behav Health Serv Res. 1998;25(3):325–336 [DOI] [PubMed] [Google Scholar]
- 33.Xue Y, Hodges K, Wotring J. Predictors of outcome for children with behavior problems served in public mental health. J Clin Child Adolesc Psychol. 2004;33(3):516–523 [DOI] [PubMed]
- 34.Wade SL, Walz NC, Carey J, et al. A randomized trial of teen online problem solving: efficacy in improving caregiver outcomes after brain injury. Health Psychol. 2012;31(6):767–776 [DOI] [PubMed] [Google Scholar]
- 35.Kurowski BG, Taylor HG, Yeates KO, Walz NC, Stancin T, Wade SL. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PMR. 2011;3(9):836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor HG, Wade SL, Stancin T, Yeates KO, Drotar D, Montpetite M. Long-term educational interventions after traumatic brain injury in children. Rehabil Psychol. 2003;48(4):227–236 [Google Scholar]
- 37.Todis B, Glang A. Redefining success: results of a qualitative study of postsecondary transition outcomes for youth with traumatic brain injury. J Head Trauma Rehabil. 2008;23(4):252–263 [DOI] [PubMed] [Google Scholar]
- 38.Max JE, Roberts MA, Koele SL, et al. Cognitive outcome in children and adolescents following severe traumatic brain injury: influence of psychosocial, psychiatric, and injury-related variables. J Int Neuropsychol Soc. 1999;5(1):58–68 [DOI] [PubMed] [Google Scholar]
- 39.Ryan NP, Anderson V, Godfrey C, et al. Predictors of very-long-term sociocognitive function after pediatric traumatic brain injury: evidence for the vulnerability of the immature “social brain”. J Neurotrauma. 2014;31(7):649–657 [DOI] [PubMed] [Google Scholar]
- 40.Hoofien D, Vakil E, Gilboa A, Donovick PJ, Barak O. Comparison of the predictive power of socio-economic variables, severity of injury and age on long-term outcome of traumatic brain injury: sample-specific variables versus factors as predictors. Brain Inj. 2002;16(1):9–27 [DOI] [PubMed] [Google Scholar]
- 41.Blaha RZ, Arnett AB, Kirkwood MW, et al. Factors influencing attrition in a multisite, randomized, clinical trial following traumatic brain injury in adolescence. J Head Trauma Rehabil. Publish Ahead of Print: 10.1097/HTR.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]