Abstract
OBJECTIVE:
To characterize discrepancies between transcutaneous bilirubin (TcB) measurements and total serum bilirubin (TSB) levels among newborns receiving care at multiple nursery sites across the United States.
METHODS:
Medical records were reviewed to obtain data on all TcB measurements collected during two 2-week periods on neonates admitted to participating newborn nurseries. Data on TSB levels obtained within 2 hours of a TcB measurement were also abstracted. TcB – TSB differences and correlations between the values were determined. Data on demographic information for individual newborns and TcB screening practices for each nursery were also collected. Multivariate regression analysis was used to identify characteristics independently associated with the TcB – TSB difference.
RESULTS:
Data on 8319 TcB measurements were collected at 27 nursery sites; 925 TSB levels were matched to a TcB value. The mean TcB – TSB difference was 0.84 ± 1.78 mg/dL, and the correlation between paired measurements was 0.78. In the multivariate analysis, TcB – TSB differences were 0.67 mg/dL higher in African-American newborns than in neonates of other races (P < .001). The TcB – TSB difference also varied significantly based on brand of TcB meter used and hour of age of the infant. For 2.2% of paired measurements, the TcB measurement underestimated the TSB level by ≥3 mg/dL.
CONCLUSIONS:
During routine clinical care, TcB measurement provided a reasonable estimate of TSB levels in healthy newborns. Discrepancies between TcB and TSB levels were increased in African-American newborns and varied based on brand of meter used.
Keywords: jaundice, neonates, transcutaneous bilirubin
What’s Known On This Subject:
In most previous studies, transcutaneous bilirubin measurement has been found to provide an accurate estimate of total serum bilirubin levels. However, most of these studies were conducted in settings that optimized accuracy.
What This Study Adds:
This study provides a “real-world” assessment of the accuracy of transcutaneous bilirubin measurements in multiple clinical settings and identification of sources of discrepancy between transcutaneous and total serum bilirubin measurements.
Prevention of kernicterus in the term or late preterm neonate is a primary focus of newborn care. To promote early detection of significant hyperbilirubinemia, members of the American Academy of Pediatrics Subcommittee on Hyperbilirubinemia recommended that all newborns be screened before discharge with a total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) measurement.1 TcB screening is a potentially attractive modality because it is a quick, noninvasive technique to screen for hyperbilirubinemia.1 It is easy to perform multiple measurements on the same newborn. In addition, rather than waiting for a serum bilirubin test to be performed in a laboratory, the results are virtually instantaneous. Finally, the use of TcB as an initial screen for hyperbilirubinemia, with TSB reserved for neonates with a value above some cutoff value, could potentially lead to substantial cost savings.2
Previously, investigators have found that TcB measurements have correlated well with TSB levels, with correlation coefficients ranging from ∼0.77 to 0.97.3–15 However, in most of these previous studies, a single device was used in 1 hospital, or in a limited number of hospitals, presumably with frequent monitoring of the device’s accuracy and with optimized training of the individuals performing the TcB measurement; all these conditions tend to optimize the accuracy of the measurement.16 The applicability of these results to TcB use in routine clinical settings is unclear. Clinical experience suggests that there is greater and less predictable variability of TcB levels in the “real world” than in the published studies. In a Delphi study conducted by the BORN (Better Outcomes through Research for Newborns) network to determine the research priorities of its members, the utility of TcB measurement as a screening method for jaundice in newborn infants was rated as 1 of the 10 most important topics for investigation.17
For the present study, we conducted a robust assessment of the accuracy of TcB measurements performed as part of routine clinical care. Data were collected from multiple newborn nurseries across the United States on a diverse sample of term and late preterm neonates by using different devices. The primary aims of this analysis were to characterize differences between TcB and TSB levels among newborns undergoing routine clinical care and to identify specific patient and provider characteristics that were associated with this difference.
Methods
A retrospective study was conducted by the BORN network, a network of clinicians providing care to healthy term and late preterm neonates admitted to newborn nurseries located in academic medical centers or community hospitals. The network is a core activity of the Academic Pediatric Association and currently includes 373 members who practice at 82 nurseries located in 35 states.
Nursery-Level Data
Data on jaundice screening processes were first collected from BORN nursery sites by using an electronic survey. The first item on the survey dealt with the type of screening done on all, or virtually all, newborns before discharge from the nursery. Possible responses were as follows: obtain a TSB level on virtually all newborns; obtain a TcB on virtually all newborns; or assess risk factors, visually check infants for jaundice, and obtain a TSB or TcB as indicated. For nurseries that screened all/virtually all newborns with a TcB before discharge, the site representative indicated what brand of TcB device was used (Bilichek [Philips, Monroeville, PA], JM-103 [Draeger Medical, Telford, PA], or both), which types of health care professional typically obtained the TcB level, and what anatomic site was used for the assessment (forehead, chest, multiple, or other). Each site also indicated which laboratory method was used to determine TSB levels (neonatal bilirubin, diazo method, or bilirubin oxidase) at their institution.
Newborn-Level Data
BORN members from nursery sites that screened all/virtually all newborns with a TcB level before discharge were then invited to participate in patient-level data collection. At each participating site, medical records were reviewed on all eligible newborns born during two 2-week periods (4 weeks total). Eligibility criteria included a gestational age at birth ≥35 weeks, admission to the newborn nursery, no evidence of Rh isoimmunization, and at least 1 TcB measurement before discharge. For newborns treated with phototherapy, only pretreatment TcB and TSB data were included. For each eligible newborn, the following data were abstracted from the medical record: gestational age, race and ethnicity, birth weight, type of feeding (exclusively breast milk, breast milk and formula, only formula), and type of delivery. The race and ethnicity of the newborn were collected, if known; otherwise, the race and ethnicity of the mother were recorded. The results of all TcB tests performed before the age of 120 hours on each enrolled newborn were abstracted along with the infant’s age (in hours) and time that the measurement was performed. In addition, data on any TSB measurement performed within 2 hours of a TcB measurement were also collected. TcB and TSB measurements on a study newborn that were obtained within 2 hours of each other were considered paired.
Outcomes and Analysis
The primary study outcome was the TcB – TSB difference between paired values, such that a positive difference indicated that the TcB value was greater than the corresponding TSB level and a negative difference was indicative of the TcB measurement being less than the paired TSB level. In addition to descriptive statistics, the correlation between linked TcB and TSB levels was calculated. Because a TcB measurement is primarily conducted for screening, the worst possible errors occur when the TcB value is substantially less than the actual TSB level. A priori, we decided that clinically relevant underestimations might occur when the TcB level is ≥2 mg/dL or ≥3 mg/dL lower than the matching TSB value. The proportions of differences meeting these criteria were determined. In addition, clinically relevant overestimations of TSB levels (defined as a TcB value that was ≥2 mg/dL or ≥3 mg/dL higher than the corresponding TSB level) could lead to unnecessary blood draws for TSB testing after TcB screening. The proportion of differences meeting these criteria was also calculated.
The associations of several patient characteristics with the TcB – TSB difference, including gestational age, birth weight, race, feeding type, and age in hours, were individually assessed by using regression analyses. Race was classified as white versus all other races and African American versus all other races. The associations between these race variables and the TcB – TSB difference were evaluated by using regression analysis. The association between Hispanic ethnicity and TcB – TSB difference was also assessed. Because Hispanic or Latino was included as a “race” at many of the participating newborn nurseries, specific data on Hispanic ethnicity were missing on a large proportion of enrolled newborns. Analyses were performed assessing the association of Hispanic ethnicity with TcB – TSB difference both after excluding neonates with unknown ethnicity and when classifying these newborns as non-Hispanic ethnicity. There were no qualitative differences in the results using either classification schema for Hispanic ethnicity; in the results presented, children with missing information on Hispanic ethnicity were classified as non-Hispanic.
Because the magnitude of the TcB – TSB difference would tend to vary based on the TSB level, TSB was included in all regression models. To account for variance specifically related to site, each nursery was placed into a quintile based on the number of TcB measurements collected. This variable (nursery size quintile) was also included in all models. Finally, to account for multiple measurements on the same newborn, generalized estimating equation (GEE) techniques were used in all models.
A similar procedure was used to identify associations between nursery screening practices and TcB – TSB differences. For these analyses, specific nursery practices were assigned to each paired measurement based on the nursery in which the values were obtained and the responses provided by the BORN representative at that site. Nursery practices evaluated included the brand of TcB meter used (identified as Bilichek or JM-103), whether TcB measurements were usually performed by a nurse versus other health care professional, anatomic site used for measurements, and laboratory method for TSB levels.
Patient and nursery variables statistically associated with a TcB – TSB difference (P < .05) in bivariate analyses were included in a full model to identify characteristics independently associated with the difference. TSB level and nursery size quintile were included in the model, and GEE techniques were used for analysis.
Logistic regression was used to identify characteristics associated with clinically relevant underestimations of TSB by TcB (ie, TcB levels ≥2 mg/dL or ≥3 mg/dL lower than the matching TSB value). For these models, newborn birth weights were dichotomized as >2500 g or ≤2500 g, age as ≥48 hours versus <48 hours, and gestational age as 35 to 37 6/7 weeks versus ≥38 weeks. Variables were initially assessed individually after accounting for TSB level, nursery size, and multiple observations in the same newborn. Those variables statistically associated with the outcome (defined as an odds ratio [OR] with a 95% confidence interval [95% CI] that did not include 1.0) in bivariate analyses were included in a multivariate model. For all of these analyses, TSB level and nursery size (quintile) were included, and GEE was used to account for multiple measurements on the same child. A similar analytic strategy was used to identify characteristics associated with clinically relevant overestimations of TSB by TcB measurements.
The study was approved by the institutional review boards at the University of Washington and at each of the participating BORN nursery sites. Study data were collected on neonates born between January 2012 and June 2013.
Results
Among the 60 BORN newborn nurseries responding to the bilirubin screening survey, 38 (63.3%) screened all or virtually all newborns with a TcB level before discharge, and 14 (23.3%) screened all or virtually all newborns with a TSB level. At 8 BORN nursery sites (13.3%), TcB or TSB levels were only obtained on infants who had clinically apparent jaundice and/or risk factors. Among the 38 sites at which all or virtually all newborns were screened for jaundice with a TcB measurement before discharge, 27 (71%) contributed data for the study. Overall, data were collected on 8319 TcB measurements in 4994 newborns. A total of 925 TSB levels were linked to TcB measurements (12.1% of all TcB levels). Characteristics of the 769 newborns on whom at least 1 paired TcB and TSB level was obtained are summarized in Table 1. Information was collected on a racially and ethnically diverse sample of newborns: 35.8% of study infants were non-white and 24.5% were of Hispanic ethnicity.
TABLE 1.
Characteristics of the 769 Newborns With at Least 1 Paired TcB/TSB Level
| Characteristic | Value |
|---|---|
| Birth weight, mean ± SD, g | 3333 ± 500 |
| Gestational age, mean ± SD, wk | 39.1 ± 1.4 |
| Vaginal delivery, n (%) | 572 (74.5)a |
| Feeding, n (%) | |
| Exclusively breast | 355 (46.3)a |
| Breast and formula | 318 (41.5) |
| Formula only | 93 (12.1) |
| Race, n (%) | |
| American Indian/Alaska Native | 6 (1.0)a |
| African American | 156 (24.9) |
| Asian | 48 (7.7) |
| Pacific Islander/Native Hawaiian | 3 (0.5) |
| White | 402 (64.2) |
| Multiple races | 10 (1.6) |
| Other | 1 (0.2) |
| Hispanic ethnicity | 188 (24.5)b |
Percentages after missing data excluded. Data missing on delivery type in 1 child, feeding type in 3 children, and race in 143.
Infants with ethnicity recorded as unknown were classified as non-Hispanic. Ethnicity data were missing on 2 infants.
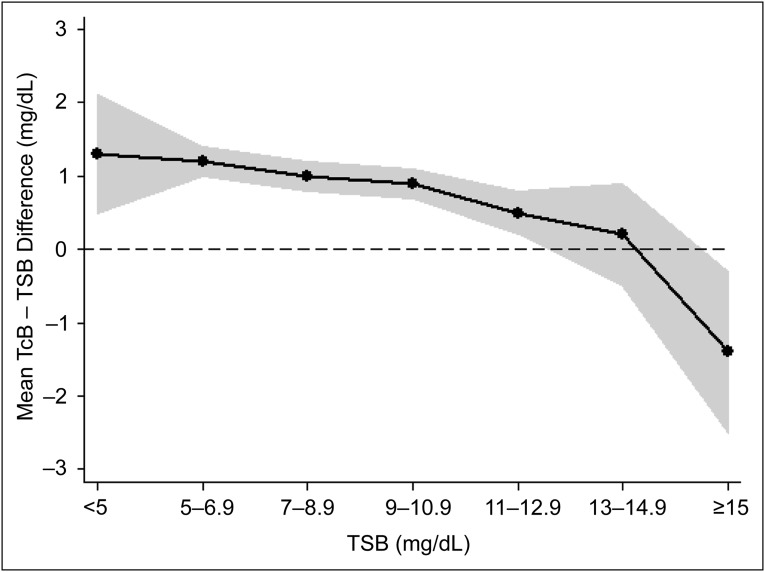
TSB values in study newborns ranged from 1.8 mg/dL to 16.6 mg/dL; 20 TSB values (2.2%) were ≥15 mg/dL. Overall, the mean ± SD TcB – TSB difference for the 925 paired measurements was 0.84 ± 1.78 mg/dL, with differences ranging from –6.9 mg/dL to 8.8 mg/dL. The correlation between paired measurements was 0.78. As shown in Fig 1, the TcB – TSB difference varied based on the TSB level. The mean TcB – TSB difference for the 31 paired measurements when the TSB was <5 mg/dL was 1.3 ± 2.3 mg/dL. The difference became progressively less positive as the TSB level increased, with a mean TcB – TSB difference of –1.4 ± 2.4 mg/dL for the 20 paired measurements when the TSB level was ≥15 mg/dL. There were 52 TcB values (5.6% [95% CI: 4.2–7.3]) that were ≥2 mg/dL lower than the matching TSB level, and 20 TcB values (2.2% [95% CI: 1.3–3.3]) that were ≥3 mg/dL lower than the corresponding TSB level. Conversely, there were 215 TcB values (23.2% [95% CI: 20.6–26.1]) that were 2 mg/dL higher than the TSB level, and 92 TcB values (10.0% [95% CI: 8.1–12.1]) that were ≥3 mg/dL higher than the TSB level. Overall, TcB readings differed from the matched TSB value by a clinically relevant difference in 28.8% (95% CI: 25.9–31.8) or 12.1% (95% CI: 10.1–14.4) of measurements, respectively, defined as a discrepancy (ie, absolute value of difference) of ≥2 mg/dL or ≥3 mg/dL between a TcB and TSB measurement.
FIGURE 1.
Characterization of TcB – TSB difference at variable TSB levels. Data shown are mean values of the difference at different ranges of TSB values. The shaded region represents the 95% CIs around the means.
The associations between clinical characteristics and nursery screening practices with the TcB – TSB difference, when assessed individually, are summarized in Table 2. After controlling for TSB level and nursery site, hour of age, African-American race, white race, and Hispanic ethnicity were significantly associated with TcB – TSB difference. The mean TcB – TSB difference was 1.56 ± 1.46 mg/dL for African-American infants compared with 0.66 ± 1.72 mg/dL for newborns of other races, and 0.64 ± 1.70 mg/dL for white neonates versus 1.33 ± 1.64 mg/dL for non-white infants. Mean differences for Hispanic and non-Hispanic newborns were 0.67 ± 1.67 mg/dL and 0.89 ± 1.81 mg/dL, respectively. The TcB – TSB difference was also significantly associated with the brand of TcB meter used for measurement at the nursery sites and the anatomic location used for the assessment. At nursery sites using the Bilichek meter, the mean TcB – TSB difference was 1.23 ± 1.64 mg/dL compared with 0.28 ± 1.84 mg/dL for nurseries using JM-103.
TABLE 2.
Association Between Individual Patient and Nursery Characteristics and TcB – TSB Difference
| Variable | Coefficient | Pa |
|---|---|---|
| Hour of age | 0.06 | <.001 |
| Gestational age | 0.03 | .45 |
| Birth weight | −0.0002 | .10 |
| TcB meter brand JM-103b | −0.91 | <.001 |
| TcB obtained by nurse | −0.17 | .27 |
| Anatomic site assessed | ||
| Forehead | Ref | |
| Chest | −0.67 | <.001 |
| Both chest and forehead | 0.70 | .004 |
| TSB laboratory method | ||
| Neonatal bilirubin | Ref | |
| Diazo method | 0.16 | .22 |
| Bilirubin oxidase | −0.09 | .82 |
| African-American raceb | 1.05 | <.001 |
| White raceb | −0.79 | <.001 |
| Hispanic ethnicityc | −0.32 | .02 |
Coefficient and P value determined with regression analysis after controlling for TSB level and nursery size (by quintile) and by accounting for multiple measurements in the same newborn.
TcB brand missing for 27 measurements and race missing for 173 measurements.
Infants for whom ethnicity data were recorded as unknown were classified as non-Hispanic. Data on ethnicity missing for 2 measurements.
Characteristics statistically associated with the outcome (ie, TcB – TSB difference), in bivariate analyses were included in the full model. The results of this multivariate analysis are shown in Table 3. Neither white race nor Hispanic ethnicity was independently associated with TcB – TSB differences; the other variables assessed were significant predictors of the difference. The TcB – TSB difference was 0.67 mg/dL higher in African-American infants compared with non–African-American infants and, overall, differences in nurseries that used JM-103 TcB meters were 0.87 mg/dL less than the TcB – TSB difference in nurseries that used Bilichek meters. Even after adjusting for TSB level, the TcB – TSB difference became larger with each hour of advancing age. In the multivariate analysis, TcB – TSB differences were significantly greater in nurseries when both the chest and forehead were used for TcB assessments compared with nurseries in which only the forehead was used. In addition, data on anatomic site of TcB measurements were reanalyzed by using chest as the reference site to directly compare the accuracy of TcB measurements done exclusively on the chest versus use of both chest and forehead (data not shown). In this analysis, use of both chest and forehead for measurements was associated with an increased difference of 0.77 mg/dL compared with those using the chest alone (P = .004).
TABLE 3.
Independent Association Between Patient and Nursery Characteristics and TcB – TSB Difference
| Variable | Coefficienta | Pa |
|---|---|---|
| Hour of age | 0.05 | <.001 |
| TcB meter brand JM-103b | −0.87 | <.001 |
| Anatomic site assessed | ||
| Forehead | Ref | |
| Chest | −0.24 | .24 |
| Both chest and forehead | 0.53 | .04 |
| African-American raceb | 0.67 | .002 |
| White raceb | −0.30 | .11 |
| Hispanic ethnicityc | −0.002 | .99 |
Coefficient and P value determined with regression analysis including all listed variables after controlling for TSB level and nursery size (by quintile) and by accounting for multiple measurements in the same newborn.
TcB brand missing for 27 measurements and race missing for 173 measurements.
Infants for whom ethnicity data were recorded as unknown were classified as non-Hispanic. Data on ethnicity missing for 2 measurements.
The analysis of the association of individual newborn and nursery characteristics with a TcB level that was ≥2 mg/dL lower than or ≥2 mg/dL higher than the corresponding TSB values are summarized in Table 4. In the multivariate analyses, only age <48 hours (OR: 6.76 [95% CI: 2.22–20.56]), JM-103 TcB meter use (OR: 6.05 [95% CI: 1.50–24.31]), and birth weight <2500 g (OR: 8.00 [95% CI: 2.38–26.96]) were independently associated with a TcB level that was ≥2 mg/dL lower than the corresponding TSB value. Conversely, age <48 hours (OR: 0.36 [95% CI: 0.22–0.59]) and JM-103 TcB meter use (OR: 0.45 [95% CI: 0.24–0.84]) were independently associated with a lower chance of a TcB value that was ≥2 mg/dL higher than the matching TSB level; African-American race was independently associated with a higher chance for this outcome (OR: 2.19 [95% CI: 1.09–4.39]).
TABLE 4.
Association Between Individual Patient and Nursery Characteristics and TcB Values That Were ≥2 mg/dL Lower, or ≥2 mg/dL Higher, Than the Matched TSB Level
| Variable | Outcome: TcB ≥2 mg/dL Lower Than TSBa | Outcome: TcB ≥2 mg/dL Higher Than TSBa |
|---|---|---|
| Age <48 h | 3.31 (1.51–7.24) | 0.30 (0.19–0.46) |
| Gestational age ≥38 wk | 1.16 (0.54–2.48) | 1.38 (0.89–2.15) |
| Birth weight <2500 g | 3.68 (1.54–8.79) | 1.05 (0.51–2.15) |
| TcB meter brand JM-103b | 3.25 (1.62–6.25) | 0.44 (0.29–0.65) |
| TcB obtained by nurse | 1.11 (0.52–2.35) | 0.70 (0.45–1.07) |
| Anatomic site assessed | ||
| Forehead | Ref | Ref |
| Chest | 2.27 (1.22–4.24) | 0.50 (0.31–0.81) |
| Both chest and forehead | 0.16 (0.02–1.36) | 2.50 (1.30–4.86) |
| TSB laboratory method | ||
| Neonatal bilirubin | Ref | Ref |
| Diazo method | 0.57 (0.29–1.11) | 1.07 (0.76–1.50) |
| Bilirubin oxidase | 0.49 (0.06–4.20) | 0.32 (0.07–1.48) |
| African-American raceb | 0.13 (0.03–0.56) | 3.09 (2.03–4.70) |
| White raceb | 3.41 (1.36–8.57) | 0.43 (0.29–0.62) |
| Hispanic ethnicityc | 0.92 (0.46–1.85) | 0.47 (0.30–0.71) |
Data are presented as OR (95% CI).
OR and 95% CI after controlling for TSB level and nursery size (by quintile) and by accounting for multiple measurements in the same newborn.
TcB brand missing for 27 measurements and race missing for 173 measurements.
Infants for whom ethnicity data were recorded as unknown were classified as non-Hispanic. Data on ethnicity missing for 2 measurements.
Analyses of the association of newborn and nursery characteristics with a TcB level that was ≥3 mg/dL lower than the matching TSB level were complicated by the low frequency of this outcome (2.2% of paired measurements). In multivariate analysis, age <48 hours (OR: 3.97 [95% CI: 1.12–14.05]) and birth weight <2500 g (OR: 6.68 [95% CI: 1.94–23.05]) were the only characteristics independently associated with this outcome. Conversely, age <48 hours (OR: 0.38 [95% CI: 0.20–0.72]), JM-103 TcB meter use (OR: 0.38 [95% CI: 0.20–0.72]), and Hispanic ethnicity (OR: 0.40 [95% CI: 0.16–0.96]) were independently associated with a TcB measurement that was ≥3 mg/dL higher than the TSB, or an overestimation, in the multivariate analysis.
Discussion
The results of our study indicate that TcB measurement provides a robust estimate of TSB values in healthy newborn infants during their nursery stay. Among a racially and ethnically diverse sample of neonates from multiple nurseries across the United States measured in clinical settings with multiple devices, TcB measurement provided reasonably accurate estimates of TSB values. Overall, the findings in this study suggest that TcB measurements can be used effectively to screen newborn infants for significant hyperbilirubinemia, with TSB measurements reserved for those newborns whose TcB level is above a certain cutoff value.
There are several important caveats to our conclusion that a TcB measurement is an effective screening tool for neonatal jaundice. Among our 925 linked measurements, 2.2% of TcB measurements underestimated the TSB level by ≥3 mg/dL, a difference that was likely clinically relevant in some situations. This rate of clinically relevant underestimation with TcB is higher than that found by Maisels et al,8 who reported that in 849 infants assessed with TcB measurements, an underestimation of TSB by ≥3 mg/dL occurred in only 5 (0.6%). Furthermore, it is likely that among the 7394 TcB measurements in our study that were not linked to a TSB measurement, there were other instances in which the TcB value substantially underestimated the serum bilirubin but were undetected because a TSB level was not obtained. In addition, the risk of a ≥3-mg/dL underestimation of TSB by TcB was significantly increased in newborns with birth weights <2500 g. Because failing to identify a newborn with significant hyperbilirubinemia is the worst outcome of TcB screening, our findings suggest that TcB results be interpreted cautiously in newborns with birth weights <2500 g, particularly in the first 48 hours of life. Significant overestimation of the severity of jaundice by using a TcB measurement may also lead to a clinically relevant change in procedure. We found that 10% of TcB measurements overestimated TSB by at least 3 mg/dL, which would, in many clinical circumstances, result in an unnecessary blood draw for an unneeded TSB level.
A potentially significant issue with the use of TcB screening is that TcB levels provide less accurate estimates of TSB values at higher serum bilirubin levels. As opposed to lower levels in which TcB tended to overestimate TSB levels, we found that at TSB levels ≥15 mg/dL, the corresponding TcB value averaged 1.4 mg/dL lower than the TSB level with substantial variability. Other investigators have reported similar findings. In a study comparing TcB and TSB values among a largely Hispanic population of newborns, Engle et al4 also found that TcB measurements tended to underestimate TSB at higher levels. Similarly, in one of the few assessments of TcB use in newborns after discharge from the nursery, the overall correlation between TcB and TSB levels was 0.77, with increasing variability at higher TSB values; 40% of the 121 newborns in this study had TSB levels ≥15 mg/dL.14 Overall, our results and the results of other studies suggest that TcB screening might be most effective at an age when most TSB levels would be expected to be <15 mg/dL.
Our data suggest that TcB measurements significantly overestimate TSB levels in African-American newborns compared with newborns of other races. Previous investigators have reported similar findings.8,18 We did not detect any independent association with the TcB – TSB difference in Hispanic newborns.
Although we found a difference in the overall accuracy of estimating TSB levels in the 2 brands of TcB meters used in the participating BORN nurseries (Bilichek or JM-103), this result should be interpreted cautiously. The assignment of brand of meter associated with each TcB measurement was based solely on the brand that was reportedly used at each nursery site. Because we did not measure the specific TcB meter used for each measurement linked to a TSB level, there may be some misclassification of TcB brand. There was also no direct comparison (ie, in the same newborn) between the Bilichek and the JM-103 meter. In addition, although TcB measurements in nurseries that reportedly used the JM-103 meter were significantly closer to the actual TSB value than those obtained in nurseries using the Bilichek meter, there was a significantly higher proportion of TcB measurements that underestimated the TSB level by ≥2 mg/dL in nurseries using JM-103 than in those using Bilichek. Overall, it is impossible to conclude that 1 brand of TcB meter is superior to the other based on our data.
We found no significant differences in the accuracy of TcB measurement based on anatomic site used for the assessment (either forehead or chest). However, in nurseries that used both anatomic sites for measurement, TcB – TSB differences were significantly increased. This outcome may suggest that accuracy is maximized when the procedures for TcB measurement are standardized according to site, regardless of the specific site chosen.
Our finding of an independent association between increasing age in a newborn and increased discrepancy between TcB and TSB measurements was unexpected. Yamauchi and Yamauchi19 reported a similar finding in a 1991 study of a TcB device. As with these investigators, we cannot adequately explain this result but speculate that dynamic processes occurring during the first few days of life, such as changes in hemoglobin concentration, may partially account for this phenomenon.20
There are several features to the present study that limit interpretation of the results. Because the decision to obtain TSB levels varied according to site and provider, the differences between TcB and TSB levels that we found may not be representative of all TcB measurements. In addition, information on the race of the infant was missing for 18.7% of TcB measurements; data on missing ethnicity were imputed as non-Hispanic in our primary analyses. Differences in TcB and TSB values associated with specific nursery practice were based on responses to a survey completed before data collection at each site rather than being collected at the time of each TcB measurement. Thus, some of the nursery practices assigned to individual TcB measurements may be misclassified. Finally, we only evaluated the clinical utility of TcB measurement in the newborn nursery settings. Our findings may not be generalizable to the use of TcB in outpatient newborns.
The correlation coefficient between TcB and TSB measurements of 0.78 that we found in our study is at the low end of previously published comparisons.3 This lower correlation is likely related to several unique features of our study such as the collection of data at 27 sites, combining data obtained from multiple brands of TcB meters, and analysis of data that were collected for clinical use rather than specifically for a study in which conditions might be optimized. Overall, we believe that our analyses provide robust estimates of accuracy of TcB measurement and the sources of error that are applicable to routine clinical settings.
Acknowledgments
Study data were collected at the following newborn nursery sites by BORN members: California: Kaiser Permanente Downey Medical Center, T.E. Burgos; University of California San Francisco Children’s Hospital, V. Flaherman. Colorado: Denver Health Medical Center, B. Chambers. Connecticut: Yale–New Haven Children’s Hospital, J. Loyal. Florida: Tampa General Hospital affiliated with the University of South Florida, M. Balakrishnan. Illinois: Rush University Medical Center, C. Drazba; University of Chicago Medical Center, L. Gray. Indiana: Wishard Health Services, K. Szucs. Kentucky: University of Louisville Hospital, L. Wasser. Michigan: University of Michigan Health System Women’s Hospital Birth Center, J. Schiller. Minnesota: University of Minnesota Amplatz Hospital, D. Madlon-Kay. Missouri: SSM St Mary’s Health Center, D. Halloran and J. Wen. North Carolina: Gaston Memorial Hospital, L. Sinai; North Carolina Women’s Hospital, C. Seashore; Women’s Hospital of Greensboro of the Moses Cone Health System, K. Gable. New Jersey: Morristown Memorial Hospital, M. LoFrumento. New York: Flushing Hospital Medical Center, L. Cohen; NYPH, Weill Cornell Medical Center, J. DiPace and B. Spector. Ohio: Cincinnati Children’s Hospital Medical Center, S. Wexelblatt and L. Ward; Firelands Regional Medical Center, T. Williams and K. Duffy. Pennsylvania: Thomas Jefferson University Hospital, E. Chung, G. Emmett, A. Losasso, and E. Ross. Tennessee: Children’s Hospital at Erlanger, A. Church. Texas: University Medical Center in Lubbock, K. Robinson. Virginia: University of Virginia Health System, A. Kellams; Virginia Commonwealth University Health System, L. Meloy. Washington: University of Washington Medical Center, J. Taylor.
Footnotes
Dr Taylor conceptualized and designed the study, collected data at 1 site, analyzed the study data, and drafted the initial manuscript; Drs Burgos, Flaherman, and Chung assisted in the design of the study and the development of data collect forms, each collected study data at 1 site, assisted in the analysis and interpretation of study data, and critically reviewed the manuscript; Drs Simpson, Goyal, and Von Kohorn assisted in the design of the study and development of data collection forms, assisted in interpretation of study data, and critically reviewed the manuscript; Dr Goyal also assisted in the design of the figure shown in the manuscript; and Ms Dhepyasuwan assisted in the design of the study and development of data collection forms, collated data from all study sites, assisted in analysis and interpretation of study data, and critically reviewed the manuscript. All authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr Burgos owns stock in BiliTool, Inc (co-ownership of technological assets only; currently no income derived); the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Academic Pediatric Association. Dr Flaherman is also supported by a National Institutes of Health grant (K23 HD059818) from the National Institute of Child Health and Human Development. Dr Goyal was supported by a BIRCWH K12 Award (5K12HD051953-07), cofunded by the Office of Research on Women’s Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page 364, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2014-3472.
References
- 1.Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124(4):1193–1198 [DOI] [PubMed] [Google Scholar]
- 2.Maisels MJ, Kring E. Transcutaneous bilirubinometry decreases the need for serum bilirubin measurements and saves money. Pediatrics. 1997;99(4):599–601 [DOI] [PubMed] [Google Scholar]
- 3.NICE, National Institute for Health and Clinical Excellence. Recognition and treatment of neonatal jaundice. Available at: http://guidance.nice.org.uk/CG98. Accessed March 1, 2013
- 4.Engle WD, Jackson GL, Sendelbach D, Manning D, Frawley WH. Assessment of a transcutaneous device in the evaluation of neonatal hyperbilirubinemia in a primarily Hispanic population. Pediatrics. 2002;110(1 pt 1):61–67 [DOI] [PubMed] [Google Scholar]
- 5.Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106(2). Available at: www.pediatrics.org/cgi/content/full/106/2/e17 [DOI] [PubMed] [Google Scholar]
- 6.Ebbesen F, Rasmussen LM, Wimberley PD. A new transcutaneous bilirubinometer, BiliCheck, used in the neonatal intensive care unit and the maternity ward. Acta Paediatr. 2002;91(2):203–211 [DOI] [PubMed] [Google Scholar]
- 7.Samanta S, Tan M, Kissack C, Nayak S, Chittick R, Yoxall CW. The value of Bilicheck as a screening tool for neonatal jaundice in term and near-term babies. Acta Paediatr. 2004;93(11):1486–1490 [DOI] [PubMed] [Google Scholar]
- 8.Maisels MJ, Ostrea EM, Jr,, Touch S, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics. 2004;113(6):1628–1635 [DOI] [PubMed] [Google Scholar]
- 9.Sanpavat S, Nuchprayoon I. Noninvasive transcutaneous bilirubin as a screening test to identify the need for serum bilirubin assessment. J Med Assoc Thai. 2004;87(10):1193–1198 [PubMed] [Google Scholar]
- 10.Sanpavat S, Nuchprayoon I. Comparison of two transcutaneous bilirubinometers—Minolta AirShields Jaundice Meter JM103 and Spectrx Bilicheck—in Thai neonates. Southeast Asian J Trop Med Public Health. 2005;36(6):1533–1537 [PubMed] [Google Scholar]
- 11.Rubaltelli FF, Gourley GR, Loskamp N, et al. Transcutaneous bilirubin measurement: a multicenter evaluation of a new device. Pediatrics. 2001;107(6):1264–1271 [DOI] [PubMed] [Google Scholar]
- 12.Boo NY, Ishak S. Prediction of severe hyperbilirubinaemia using the Bilicheck transcutaneous bilirubinometer. J Paediatr Child Health. 2007;43(4):297–302 [DOI] [PubMed] [Google Scholar]
- 13.Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113(6):1636–1641 [DOI] [PubMed] [Google Scholar]
- 14.Engle WD, Jackson GL, Stehel EK, Sendelbach DM, Manning MD. Evaluation of a transcutaneous jaundice meter following hospital discharge in term and near-term neonates. J Perinatol. 2005;25(7):486–490 [DOI] [PubMed] [Google Scholar]
- 15.Grohmann K, Roser M, Rolinski B, et al. Bilirubin measurement for neonates: comparison of 9 frequently used methods. Pediatrics. 2006;117(4):1174–1183 [DOI] [PubMed] [Google Scholar]
- 16.Maisels MJ. Historical perspectives: transcutaneous bilirubinometry. Neoreviews. 2006;7(5):e217–e225 [Google Scholar]
- 17.Simpson E, Goyal NK, Dhepyasuwan N, et al. Prioritizing a research agenda: a Delphi study of the Better Outcomes through Research for Newborns (BORN) network. Hosp Pediatr. 2014;4(4):195–202 [DOI] [PubMed] [Google Scholar]
- 18.Wainer S, Rabi Y, Parmar SM, Allegro D, Lyon M. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98(12):1909–1915 [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi Y, Yamanouchi I. Transcutaneous bilirubinometry: effect of postnatal age. Acta Paediatr Jpn. 1991;33(5):663–667 [DOI] [PubMed] [Google Scholar]
- 20.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123(2). Available at: www.pediatrics.org/cgi/content/full/123/2/e333 [DOI] [PubMed] [Google Scholar]