Abstract
BACKGROUND AND OBJECTIVES:
Examination of regional care patterns in antenatal corticosteroid use (ACU) rates may be salient for the development of targeted interventions. Our objective was to assess network-level variation using California perinatal care regions as a proxy. We hypothesized that (1) significant variation in ACU exists within and between California perinatal care regions, and (2) lower performing regions exhibit greater NICU-level variability in ACU than higher performing regions.
METHODS:
We undertook cross-sectional analysis of 33 610 very low birth weight infants cared for at 120 hospitals in 11 California perinatal care regions from 2005 to 2011. We computed risk-adjusted median ACU rates and interquartile ranges (IQR) for each perinatal care region. The degree of variation was assessed using hierarchical multivariate regression analysis with NICU as a random effect and region as a fixed effect.
RESULTS:
From 2005 to 2011, mean ACU rates across California increased from 82% to 87.9%. Regional median (IQR) ACU rates ranged from 68.4% (24.3) to 92.9% (4.8). We found significant variation in ACU rates among regions (P < .0001). Compared with Level IV NICUs, care in a lower level of care was a strongly significant predictor of lower odds of receiving antenatal corticosteroids in a multilevel model (Level III, 0.65 [0.45–0.95]; Level II, 0.39 [0.24–0.64]; P < .001). Regions with lower performance in ACU exhibited greater variability in performance.
CONCLUSIONS:
We found significant variation in ACU rates among California perinatal regions. Regional quality improvement approaches may offer a new avenue to spread best practice.
Keywords: antenatal corticosteroids, neonatal intensive care, quality of care, variation
What’s Known on This Subject:
Application of antenatal corticosteroids to mothers before delivery is highly beneficial to very low birth weight infants. Yet despite widespread quality improvement efforts, many eligible infants fail to receive this therapy.
What This Study Adds:
We demonstrate improvement in antenatal corticosteroid use during the study period. However, significant regional variation persists, which network-level quality improvement efforts might help eliminate.
In the United States, ∼60 000 very low birth weight (VLBW, <1500 g) infants are born annually. These infants require prolonged stays in the NICU and frequently ongoing subspecialty care throughout childhood.1 Improving the care of preterm infants is important to reduce adverse consequences to patients, families, and society. Administration of antenatal corticosteroids, ideally provided 48 hours before delivery, is safe for mother and infant and effective in preventing death, as well as potentially devastating short- and long-term complications of infants born prematurely between 24 and 34 weeks’ gestation.2,3 If all mothers who are eligible for antenatal steroids were fully treated in the United States, >1000 newborn lives might be saved, and >3000 cases of respiratory distress syndrome could be avoided. Such an improvement would represent a reduction of 5% in neonatal mortality rates.4 Recent research estimates the number of newborn lives saved through full treatment with antenatal steroids might be as high as 500 000 worldwide.5
Antenatal corticosteroid use (ACU) increased from 24% to 72% from 1991 to 1999,6 but the pace of adoption has since slowed, increasing only to 77% by 2009.7,8 In 1998, a collaborative quality improvement effort sponsored by the California Perinatal Quality Care Collaborative (CPQCC) was successful in improving ACU rates from 76% to 86% among 25 participating NICUs.9 However, collaborative quality improvement efforts only reach a small proportion of the state’s 140 NICUs and those that volunteer to participate are not a representative sample. For all NICUs to improve their ACU rates, a new approach to quality improvement is needed.
With the advent of the Affordable Care Act and the increasing formation of Accountable Care Organizations (ACOs), the traditional fee-for-service payments will be replaced by various global payment arrangements.10,11 Under global payment arrangements, provider ACOs are at financial risk with respect to their quality of care delivery, providing an impetus to monitor and optimize quality of care across the delivery network. This situation provides an impetus for perinatal centers to monitor and optimize quality of care delivery across their delivery network. Therefore, network-level quality improvement may provide an intriguing opportunity to achieve excellence in care delivery. Variation in quality across networks that is not explained by clinical risk may signal an opportunity for network-level quality improvement. In this study, we used California Perinatal Regions as a proxy for “network,” given that each perinatal region contains 1 or 2 perinatal centers and their referral NICUs. The hypotheses tested in this study were that:
Significant variation in ACU exists within and between California perinatal care regions, and
Lower performing regions exhibit greater NICU-level variability in ACU than higher performing regions.
The rationale for the first hypothesis was that the existence of variation at the regional level might reasonably be expanded to the network level. The second hypothesis is based on the implications of variability on quality of care delivery, arguing that regions with less variability exhibit more reliable care.12,13
Methods
Overview
Data for this observational study came from the CPQCC, whose membership consists of >130 hospitals, including all of the state’s perinatal centers. The CPQCC maintains a real-time, risk-adjusted perinatal data system, using standardized data collection procedures to limit measurement bias. Medical record data abstracted locally are transmitted to CPQCC, where they are subjected to logic and completeness checks, including normalized range checks, and alerts for missing data fields. In the event of inconsistencies, data are reconciled against the medical record.
Sample
Our study sample included infants who had a birth weight of <1500 g and a gestational age between 24 0/7 and 32 6/7 weeks born at a CPQCC member hospital between January 1, 2005 and December 31, 2011. The gestational age limit is congruent with inclusion criteria into the CPQCC small infant database and in accordance with other measures of quality endorsed by the National Quality Forum.14 Using standard CPQCC definitions, we excluded patients who died within 12 hours of admission, and NICUs with <30 eligible infants during the study period. We used multiyear analysis because of the small number of VLBW infants cared for at some of the institutions and to explore longitudinal trends in ACU.
Measures
Outcome Variables – ACU Rate
We calculated the proportion of infants born according to the selection criteria who had received antenatal corticosteroids. Responsibility for this therapy was attributed to the hospital of birth.
Explanatory Variables
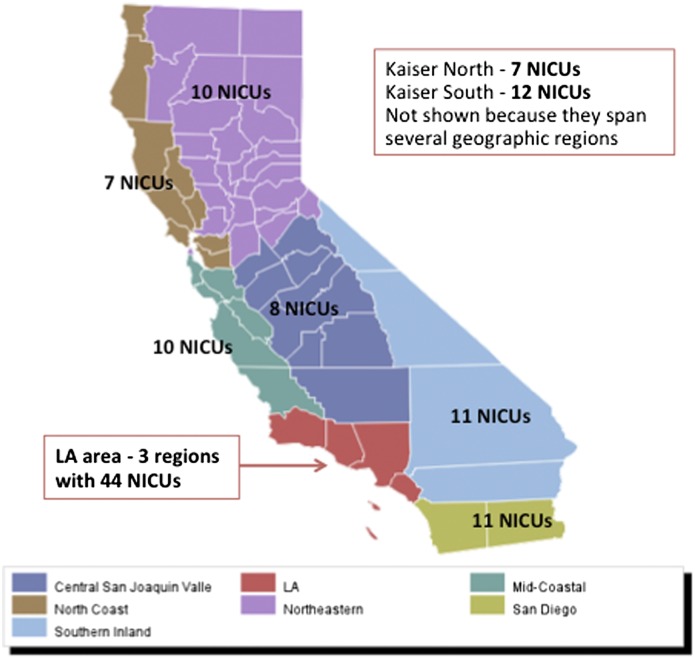
We applied CPQCC standard operational definitions for all explanatory variables. Perinatal region was designated according to the California Department of Public Health (http://www.cdph.ca.gov/programs/rppc/Pages/default.aspx). Figure 1 shows the regions and the number of CPQCC NICUs in each. Two of the regions are defined by membership in a large integrated health care system. Other explanatory variables were selected based on the existing literature.8 We examined pregnancy-related and hospital-level variables. Pregnancy-related variables included maternal age at delivery (<20, 20 to 29, 30 to 39, >39 years), fetal distress (y/n), maternal hypertension (y/n), prolonged rupture of membranes (y/n), any prenatal care (y/n), multiple gestation (y/n), delivery mode (vaginal versus cesarean delivery), infant gender (male or female), and race (non-Hispanic black, Hispanic, non-Hispanic white, Asian/Pacific Islander, Native American, other). NICU levels were self-designated according to the classification described by the Academy of Pediatrics.15 In addition, we examined hospital ownership (government, not-for-profit, for-profit, other), teaching hospital (y/n), and urban (urban versus rural).
FIGURE 1.
California perinatal care regions as defined by the California Department of Public Health.
Statistical Analyses
We conducted descriptive cross-sectional and longitudinal analyses of simple proportions, means, medians, and ranges to examine our patient sample and trends in ACU over the study period.
Hypothesis 1: Significant Variation in ACU Exists Within and Between California Perinatal Care Regions
For this hypothesis, we computed summary statistics for each outcome and explanatory variable. We then calculated proportions of ACU for each perinatal care region and used a χ2 test to assess whether the rates differed across the regions. This analysis was performed at the level of the individual. Initially we tested a candidate set of pregnancy-related and hospital characteristics based on the literature8 for an association with ACU. We then constructed hierarchical logistic regression models in which patients are nested within NICUs and NICUs are nested within perinatal regions.
We developed 2 different models to test whether the regional differences in ACU rates were explained by differences in patient mix or care practices. For all models we designated region as a fixed effect as the variable of interest. For ease of interpretation we chose the region with highest rate of ACU usage as the referent group. To account for the repeated measures of NICU over time we included a random effect for NICU. Model 1 tested for differences in patient mix and used logistic regression to calculate the adjusted odds of maternal characteristics and ACU. Model 2 tested for differences in practices. Building on model 1, we added hospital level variables and NICU as a random effect. Given the longitudinal nature of the data, we also included year of birth as a covariate in the models.
Hypothesis 2: Lower Performing Regions Exhibit Greater NICU-Level Variability in ACU Than Higher Performing Regions
To examine whether higher performance correlated with more reliable care delivery, we plotted the relation between the median ACU rate and its interquartile range (IQR) for each region. We then divided NICUs into high performing and low performing regions based on their mean adjusted regional ACU rates over the entire study period. We used a 1-sided Wilcoxon Sum of Ranks test to assess whether the IQRs between the high and low performing groups were significantly different. All analyses were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC).
Human Subjects Compliance
CPQCC data are collected for quality improvement and meet the criteria for de-identified data. The dataset is then further de-identified with respect to hospital for use as a research dataset. This study was approved by the CPQCC and the Stanford institutional review board.
Results
Characteristics of Infants and NICUs
A total of 33 610 VLBW infants cared for at 120 California NICUs in 11 perinatal care regions met the inclusion criteria for the study. A total of 5460 (16.2%) eligible VLBW infants did not receive antenatal steroids. Regional median (IQR) ACU rates ranged from 68.4% (24.3) to 92.9% (4.8).
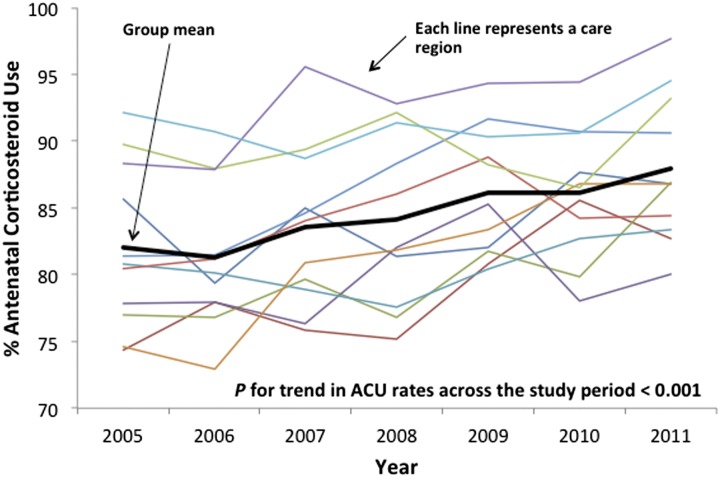
From 2005 to 20011, mean ACU rates across California increased from 82% to 87.9%. Figure 2 shows a significant positive time trend in ACU across the study period by care region (P < .001).
FIGURE 2.
Crude ACU rates by region of care over the study period. Group mean shows rising ACU over the study period (P < .001) but substantial variation across regions.
Hypothesis 1: Significant Variation in ACU Exists Within and Between California Perinatal Care Regions
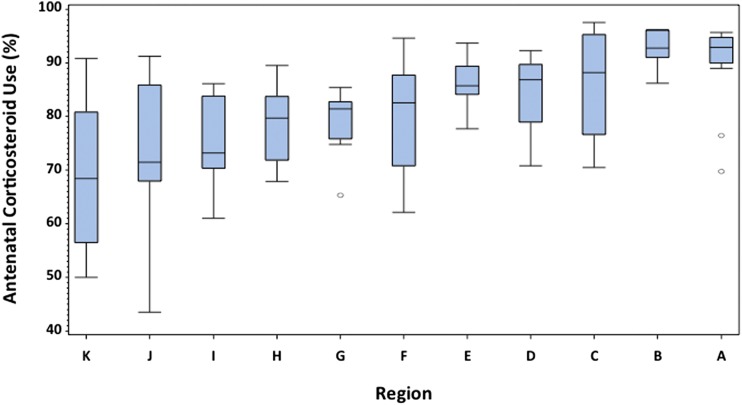
Figure 3 displays the significant variation in unadjusted ACU rates by perinatal care region (P < .001). Variation is evident both between and within regions. Table 1 gives the characteristics of the study sample according to whether patients received or failed to receive antenatal steroids (see Supplemental Appendix for a regional breakdown of infant and hospital characteristics). Region of care was a significant predictor of ACU after controlling for NICU site and individual and hospital characteristics (P < .001). After adjusting for individual characteristics (model 1) regions B and C were no longer statistically different from the referent region A. All other regions had lower odds of ACU. Patients in region K had only one-fifth the odds of receiving ACU. Additional adjustment for organizational factors did not substantially change this assessment (model 2).
FIGURE 3.
Crude ACU rates by region of care. Each box and whisker plot shows the median trend line, the IQR, and 95% CI. Region of care remained a significant predictor of ACU after adjusting for NICU, maternal, and institutional variables (P < .001).
TABLE 1.
Unadjusted and Adjusted Sample Characteristics by Antenatal Corticosteroid Use
| Characteristics | P value | Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|---|---|
| ANS | No ANS | Crude | Individual Factors | Individual and Organizational Factors | ||||
| N = 28150 (%) | N = 5460 (%) | OR (95% CI) | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Region | ||||||||
| A | 1872 (7) | 145 (3) | ref | ref | ref | ref | ref | ref |
| B | 2510 (9) | 242 (4) | 0.80 (0.65–1.00) | * | 0.80 (0.49–1.33) | 0.48 | 0.81 (0.48–1.34) | 0.48 |
| C | 2519 (9) | 292 (5) | 0.67 (0.54–0.82) | ** | 0.61 (0.35–1.07) | 0.15 | 0.53 (0.30–0.92) | * |
| D | 2171 (8) | 329 (6) | 0.51 (0.42–0.63) | ** | 0.43 (0.26–0.72) | ** | 0.40 (0.23–0.68) | ** |
| E | 1841 (7) | 342 (6) | 0.42 (0.34–0.51) | ** | 0.43 (0.26–0.73) | ** | 0.47 (0.27–0.82) | * |
| F | 2549 (9) | 488 (9) | 0.40 (0.33–0.49) | ** | 0.24 (0.15–0.40) | ** | 0.30 (0.17–0.52) | ** |
| G | 5532 (20) | 1299 (24) | 0.33 (0.28–0.39) | ** | 0.34 (0.22–0.53) | ** | 0.31 (0.19–0.48) | ** |
| H | 2589 (9) | 629 (12) | 0.32 (0.26–0.39) | ** | 0.32 (0.19–0.53) | ** | 0.38 (0.22–0.66) | ** |
| I | 2276 (8) | 570 (10) | 0.31 (0.26–0.37) | ** | 0.27 (0.16–0.46) | ** | 0.30 (0.17–0.51) | ** |
| J | 2175 (8) | 556 (10) | 0.30 (0.25–0.37) | ** | 0.32 (0.19–0.53) | ** | 0.38 (0.22–0.66) | ** |
| K | 2116 (8) | 568 (10) | 0.29 (0.24–0.35) | ** | 0.23 (0.14–0.38) | ** | 0.21 (0.12–0.35) | ** |
| Individual Factors | ||||||||
| Prenatal care | 27552 (98) | 4960 (91) | 4.85 (4.27–5.52) | ** | 3.92 (3.45–4.43) | ** | 3.91 (3.44–4.44) | ** |
| PROM | 5518 (20) | 327 (6) | 3.85 (3.43–4.32) | ** | 4.49 (4.06–4.98) | ** | 4.63 (4.15–5.16) | ** |
| Hypertension | 7657 (28) | 1041 (19) | 1.59 (1.47–1.70) | ** | 1.86 (1.74–2.00) | ** | 1.86 (1.73–1.99) | ** |
| Maternal diabetes | 3071 (11) | 426 (8) | 1.44 (1.30–1.60) | ** | 1.10 (1.00–1.21) | 0.09 | 1.08 (0.98–1.20) | 0.18 |
| Multiples | 8443 (30) | 1189 (22) | 1.54 (1.44–1.65) | ** | 1.49 (1.39–1.59) | ** | 1.50 (1.40–1.61) | ** |
| Cesarean birth | 21239 (75) | 3859 (71) | 1.27 (1.20–1.36) | ** | 1.06 (1.00–1.14) | 0.12 | 1.05 (0.98–1.13) | 0.22 |
| Fetal distress | 6216 (23) | 1387 (26) | 0.83 (0.78–0.89) | ** | 0.83 (0.78–0.89) | ** | 0.84 (0.79–0.90) | ** |
| Maternal age, y | ||||||||
| <20 | 2271 (8) | 677 (12) | 0.70 (0.64–0.78) | ** | 0.85 (0.78–0.93) | ** | 0.86 (0.79–0.95) | ** |
| 20 to 29 | 11370 (40) | 2389 (44) | ref | ref | ref | ref | ref | ref |
| 30 to 39 | 12544 (45) | 2076 (38) | 1.27 (1.19–1.35) | ** | 1.05 (0.99–1.11) | 0.19 | 1.06 (0.99–1.12) | 0.15 |
| ≥40 | 1945 (7) | 309 (6) | 1.32 (1.16–1.50) | ** | 0.98 (0.87–1.10) | 0.75 | 0.96 (0.85–1.09) | 0.61 |
| Race and Ethnicity | ||||||||
| White | 8410 (31) | 1392 (26) | ref | ref | ref | ref | ref | ref |
| Asiana | 3114 (11) | 516 (10) | 1.00 (0.90–1.11) | 0.98 | 0.97 (0.87–1.07) | 0.59 | 0.98 (0.88–1.09) | 0.71 |
| Black | 3487 (13) | 708 (13) | 0.82 (0.74–0.90) | ** | 0.89 (0.80–0.98) | * | 0.89 (0.81–0.98) | 0.05 |
| Hispanic | 12340 (45) | 2699 (51) | 0.76 (0.71–0.81) | ** | 0.93 (0.87–1.00) | 0.11 | 0.94 (0.87–1.01) | 0.15 |
| Organizational Factors | ||||||||
| Level of Care as defined by AAP | ||||||||
| Level IV | 6742 (24) | 837 (16) | ref | ref | ref | ref | ref | ref |
| Level III | 19438 (71) | 4079 (76) | 0.59 (0.55–0.64) | ** | 0.57 (0.44–0.75) | ** | 0.59 (0.42–0.81) | ** |
| Level II | 1362 (5) | 460 (9) | 0.37 (0.32–0.42) | ** | 0.39 (0.27–0.56) | ** | 0.40 (0.26–0.62) | ** |
| Teaching hospital | 5695 (22) | 936 (18) | 1.25 (1.16–1.35) | ** | 1.59 (1.22–2.08) | ** | 1.16 (0.81–1.66) | 0.49 |
| Urban location | 27215 (97) | 5197 (95) | 1.47 (1.28–1.70) | ** | 1.54 (1.04–2.28) | 0.05 | 1.42 (0.92–2.20) | 0.19 |
| Hospital ownership | ||||||||
| Not for profit | 19978 (73) | 3908 (74) | ref | ref | ref | ref | ref | ref |
| For profit | 3101 (11) | 641 (12) | 0.95 (0.86–1.04) | 0.24 | 0.89 (0.65–1.22) | 0.53 | 1.06 (0.78–1.45) | 0.74 |
| Government | 4160 (15) | 725 (14) | 1.12 (1.03–1.22) | ** | 1.45 (1.08–1.93) | * | 1.17 (0.81–1.67) | 0.48 |
P < .05; **P < .01. Model 1, adjusted for region, year, fetal distress, maternal diabetes, maternal hypertension, PROM, maternal age, multiple gestation, race/ethnicity, prenatal care, and random effect for NICU. Model 2, Model 1 + level of care, hospital ownership, teaching hospital, and random effect for NICU. Individual variables may not add up to 33 610 owing to assignment to “other” categories or missingness, not shown here for parsimony. Columns may not add to 100 owing to rounding. PROM, prolonged rupture of membranes; ref, reference group.
Includes Pacific Islanders.
Among the individual level factors, receipt of prenatal care, prolonged rupture of membranes, maternal hypertension, and multiple gestation increased the odds of ACU, likely owing to greater interaction with the medical system before birth. Fetal distress reduced the likelihood of ACU, likely because of late presentation for care or the need for urgent delivery. Compared with whites, black mothers were less likely to receive antenatal corticosteroids. There was also a trend for lower ACU in Hispanics.
With regard to hospital-level characteristics, only level of care was associated with higher ACU after controlling for individual and other organizational factors. Compared with birth in a Level IV facility, the odds of ACU in Level IIIs were one-third lower and in Level IIs even less. Hospital ownership, urban location, and teaching status were not significantly associated with ACU in multivariate analyses.
Hypothesis 2: Lower Performing Regions Exhibit Greater NICU-Level Variability in ACU Than Higher Performing Regions
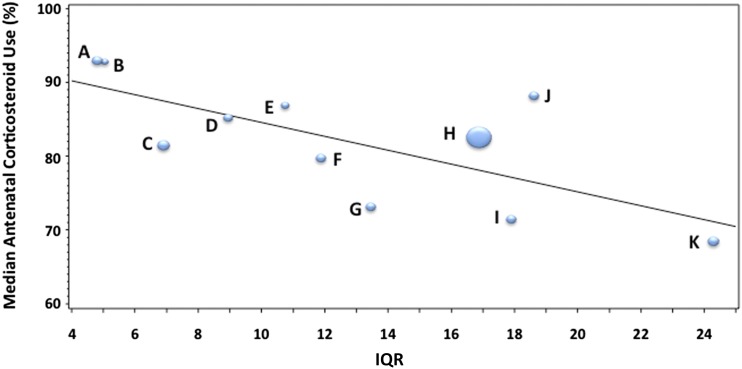
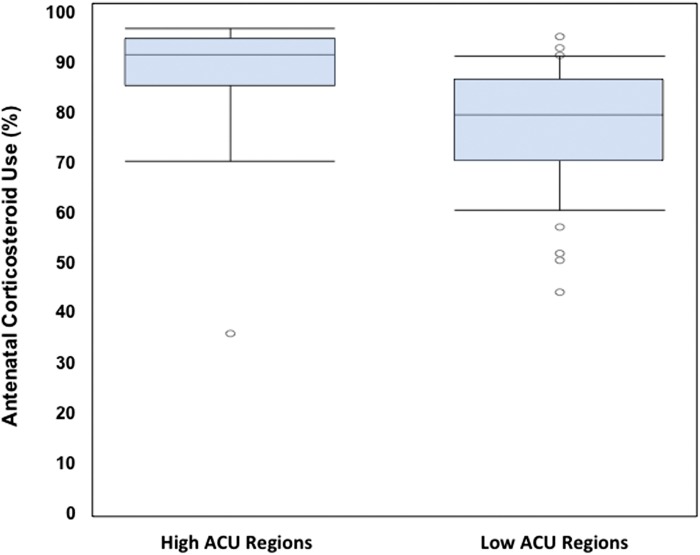
Figure 4 shows a bubble plot of each region’s median ACU rates and IQR. The size of the bubble refers to the relative size of the region. There is a significant trend toward a higher IQR, (ie, more variability in care delivery in lower performing regions). Figure 5 exhibits a boxplot of ACU among regions whose performance was above and below the mean for the combined study period. High ACU regions have a smaller IQR and 95% confidence interval (CI) (ie, less variability). However, even within the high ACU regions, there are low performing NICUs, and even within the low ACU regions there are high performing NICUs. A 1-sided Wilcoxon test showed a trend for significance (P = .11); however, sample sizes in the groups were very small (4 and 7, respectively).
FIGURE 4.
Each bubble represents a region. The sizes of the bubbles are proportionate to the number of VLBW infants in each region. The lower median ACU by region, the greater the IQR of care across NICUs in that region.
FIGURE 5.
Box and whisker plot with a median and IQR, along with 95% CI and outliers. High ACU regions have a smaller IQR and 95% CI (ie, less variability). However, even within the high ACU regions there are low performing NICUs, and even within the low ACU regions there are high performing NICUs.
Discussion
In this study, we examined population-based data on ACU across California perinatal care regions. We posed 2 hypotheses. First, that significant variation exists between and within California perinatal care regions. Second, that lower performing regions exhibit greater variability in ACU. We confirmed both hypotheses. With regard to the first hypothesis, although ACU has been increasing over the study period, significant variation persists across perinatal care regions. Mean ACU rates varied from 69.3% to 92.3% across regions. NICU was a significant independent risk factor of ACU in multivariate models. Black, and to a lesser extent, Hispanic mothers were less likely to receive antenatal corticosteroids, even after adjusting for maternal characteristics, comorbidities, and receipt of prenatal care. With regard to the second hypothesis, we showed greater NICU-level variability in ACU across low performing regions. This finding reaffirms a central tenet of quality science: reducing variation raises quality. Overall, our findings imply that network-level quality improvement may be an attractive new strategy to promote ACU.
The previous literature on ACU has focused on its variation at the hospital-level. For example, Lee et al examined ACU among 20 488 admissions to 17 tertiary level NICUs in Canada between 1996 to 1997 and found that antenatal corticosteroids were administered to 58% of mothers, ranging from 23% to 76%.16 Wirtschafter et al showed improvements in ACU after a CPQCC Quality Collaborative among 25 NICUs with the median rates increasing from 80% to 87.8%.9 Howell et al noted that 20% of infants in 3 NICUs in New York City failed to receive antenatal corticosteroids.17 Lee et al found similar results across 128 NICUs in California.8 Alleman et al demonstrated wide differences among the 16 centers of the Neonatal Research Network (median, 92%; range, 47% to 99% among infants >24 weeks’ gestational age).18 Our finding of variation in ACU at the level of care regions moves beyond quality as a hospital-based to a group-based phenomenon, implying that regional collaboration can be used to extend gains in ACU over and above strategies targeting individual hospitals.
Quality improvement science has stressed that reduction in variation is key to better quality since Walter Shewhart developed statistical process control nearly 100 years ago.19 William Edwards Deming extended this concept for use to management, sparking the revolution in Japan’s industrial output after World War II.12 By showing that higher performing regions exhibited less variation in ACU rates, this study corroborates this central tenet. Although some reduction in variation among high performing regions may be attributable to a ceiling effect, we speculate that other factors, including greater outreach by the referral center or care standardization across the region helped reduce variation. Of course, other factors, such as unmeasured differences in population characteristics may also account for this observation. Nevertheless, standardized care pathways for the use of antenatal corticosteroids implemented across groups of hospitals may be key to further uptake.
This group-level phenomenon may also be driving the rising ACU rates across the study period, validating the sustained efforts to spread this therapy via quality collaboratives over recent years. For example, in California, the CPQCC developed a toolkit and orchestrated large-scale collaborative quality improvement efforts.9 Soll et al found a similar rise in ACU from 2000 to 2009 among 355 806 VLBW infants in the Vermont Oxford Network, where rates increased from 71.7% to 76.8%. Nevertheless, as about 1 in 5 eligible infants continue to fail to receive antenatal corticosteroids, extending opportunities for group-level improvement using a network-level approach may offer further opportunities for improvement.
Network-wide implementation of standardized approaches to ACU may further improve the quality of care delivery for VLBW infants. Current statewide collaborative quality improvement initiatives attract only about one-third of all NICUs in California. The same NICUs tend to participate in consecutive initiatives, limiting the spread of quality improvement capacity. Interest in network-level quality improvement may also be promoted by population-based payments to ACOs, which will require organizations to optimize care across their delivery network. Our finding that infants in Level II and III NICUs were significantly less likely to receive antenatal corticosteroids further supports a network-based approach, but also highlights that implementation of such an approach will require adaptation of current implementation formats to suit the smaller bandwidth for quality improvement of community NICUs.
This study has several limitations. We used perinatal care regions as a proxy for care networks because we did not have network-level data. Although regions and networks may not overlap fully, our finding of significant variation across regions suggests that a network-level study might yield similar results. In addition, in light of our observational study design, associations of ACU with other variables should be interpreted as hypothesis generating, not as causal. It is possible that unobserved confounding factors, such as poverty or access to transportation, reduce the timeliness with which mothers present to obstetric care when in labor. Moreover, our data capture 7 years of data but we only used a recent designation of American Academy of Pediatrics level for the NICUs. Although overall changes in NICU designation are uncommon, some NICUs may have changed over the study period. However, most NICUs change to a higher level of care, which would bias our results toward the null. Finally, although California is the most populous state in the United States and is geographically and socially diverse, it is unknown whether our data are generalizable, owing to state-specific differences in the organization and regulatory overview of health care delivery to sick newborns.
Conclusions
We found significant positive trends in ACU across California during the study period, but persistent variation across perinatal care regions. Routine measurement of quality of care delivery that extends to the regional- or network-level combined with network-level quality improvement initiatives may offer a new approach to extend these gains.
Supplementary Material
Footnotes
Dr Profit acquired funding for this study, conceptualized and designed the study, selected data for inclusion in analyses, assisted with interpretation of the results, and drafted the initial manuscript; he had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Drs Goldstein, Tamaresis, and Lee helped conceptualize and design the study, assisted with interpretation of the results, and revised the manuscript; Ms Kan assisted with design and conduct of the analysis, interpreted the results, and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a Child Research Institute at Stanford University (Principal Investigator, Dr Profit). Dr Lee’s contribution is supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development K23HD068400 (Principal Investigator, Dr Lee). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Behrman RE, Stith Butler A, editors. Washington, DC: National Academies Press; 2007 [PubMed] [Google Scholar]
- 2.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994 [see comments]. Am J Obstet Gynecol. 1995;173(1):322–335 [DOI] [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006; (3):CD004454. [DOI] [PubMed] [Google Scholar]
- 4.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2007 period linked birth/infant death data set. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2011;59:1–30 [PubMed] [Google Scholar]
- 5.Mwansa-Kambafwile J, Cousens S, Hansen T, Lawn JE. Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth. Int J Epidemiol. 2010;39(suppl 1):i122–i133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horbar JD, Badger GJ, Carpenter JH, et al. Members of the Vermont Oxford Network . Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. 2002;110(1 pt 1):143–151 [DOI] [PubMed] [Google Scholar]
- 7.Soll RF, Edwards EM, Badger GJ, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics. 2013;132(2):222–228 [DOI] [PubMed] [Google Scholar]
- 8.Lee HC, Lyndon A, Blumenfeld YJ, Dudley RA, Gould JB. Antenatal steroid administration for premature neonates in California. Obstet Gynecol. 2011;117(3):603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirtschafter DD, Danielsen BH, Main EK, et al. California Perinatal Quality Care Collaborative . Promoting antenatal steroid use for fetal maturation: results from the California Perinatal Quality Care Collaborative. J Pediatr. 2006;148(5):606–612 [DOI] [PubMed] [Google Scholar]
- 10.Homer CJ, Patel KK. Accountable care organizations in pediatrics: irrelevant or a game changer for children? JAMA Pediatr. 2013;167(6):507–508 [DOI] [PubMed] [Google Scholar]
- 11.Profit J, Wise PH, Lee HC. Consequences of the Affordable Care Act for sick newborns [published online ahead of print October 13, 2014]. Pediatrics doi:10.1542/peds.2008-1536 10.1542/peds.2008-1536 [DOI] [PubMed]
- 12.Horbar JD, Gould JB, Lee HC, Profit J. Evaluating and Improving the Quality and Safety of Neonatal Care. In: Fanaroff AA, Martin RJ, Walsh MC, eds. Neonatal-Perinatal Medicine. 10th ed. St Louis, MO: Mosby; 2014 [Google Scholar]
- 13.James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185–1191 [DOI] [PubMed] [Google Scholar]
- 14.National Quality Forum. Perinatal and Reproductive Health Endorsement Maintenance: Technical Report. Washington, DC: 2012
- 15.American Academy of Pediatrics Committee on Fetus And Newborn . Levels of neonatal care. Pediatrics. 2012;130(3):587–597 [DOI] [PubMed] [Google Scholar]
- 16.Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network: 1996–1997. Pediatrics. 2000;106(5):1070–1079 [DOI] [PubMed] [Google Scholar]
- 17.Howell EA, Stone J, Kleinman LC, Inamdar S, Matseoane S, Chassin MR. Approaching NIH guideline recommended care for maternal-infant health: clinical failures to use recommended antenatal corticosteroids. Matern Child Health J. 2010;14(3):430–436 [DOI] [PubMed] [Google Scholar]
- 18.Alleman BW, Bell EF, Li L, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Individual and center-level factors affecting mortality among extremely low birth weight infants. Pediatrics. 2013;132(1). Available at: www.pediatrics.org/cgi/content/full/132/1/e175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best M, Neuhauser D, Walter A. Shewhart, 1924, and the Hawthorne factory. Qual Saf Health Care. 2006;15(2):142–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.