Abstract
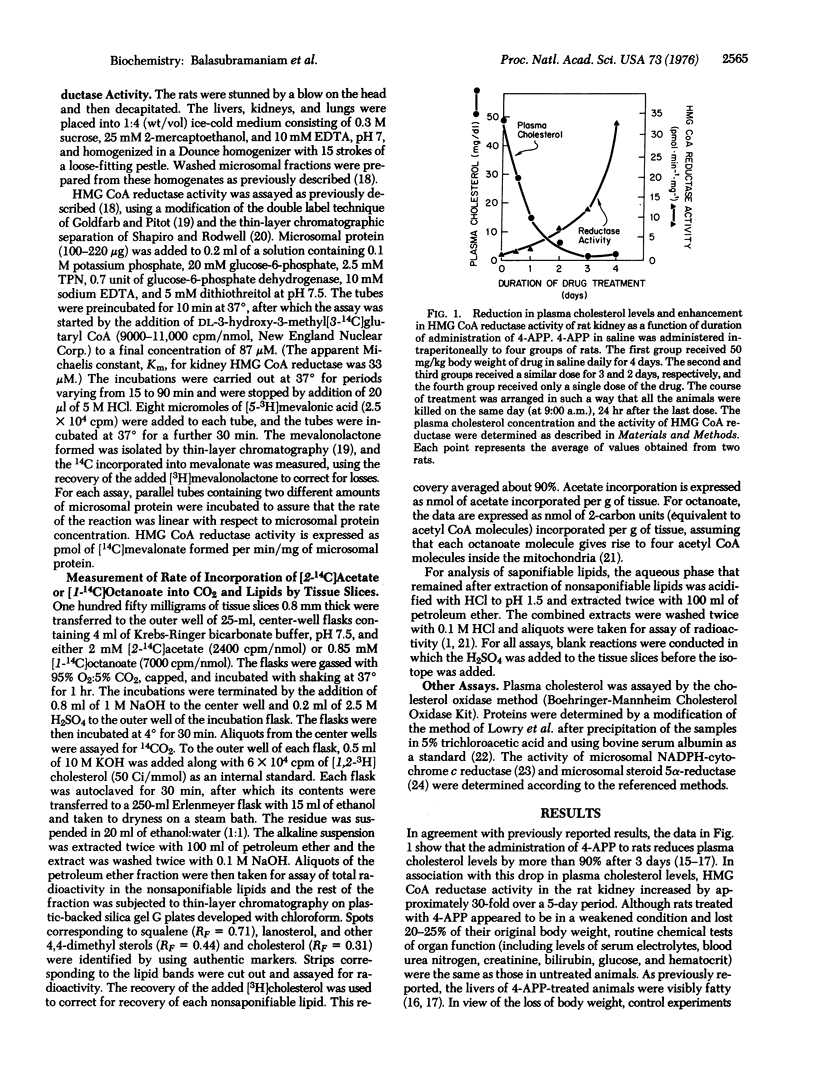
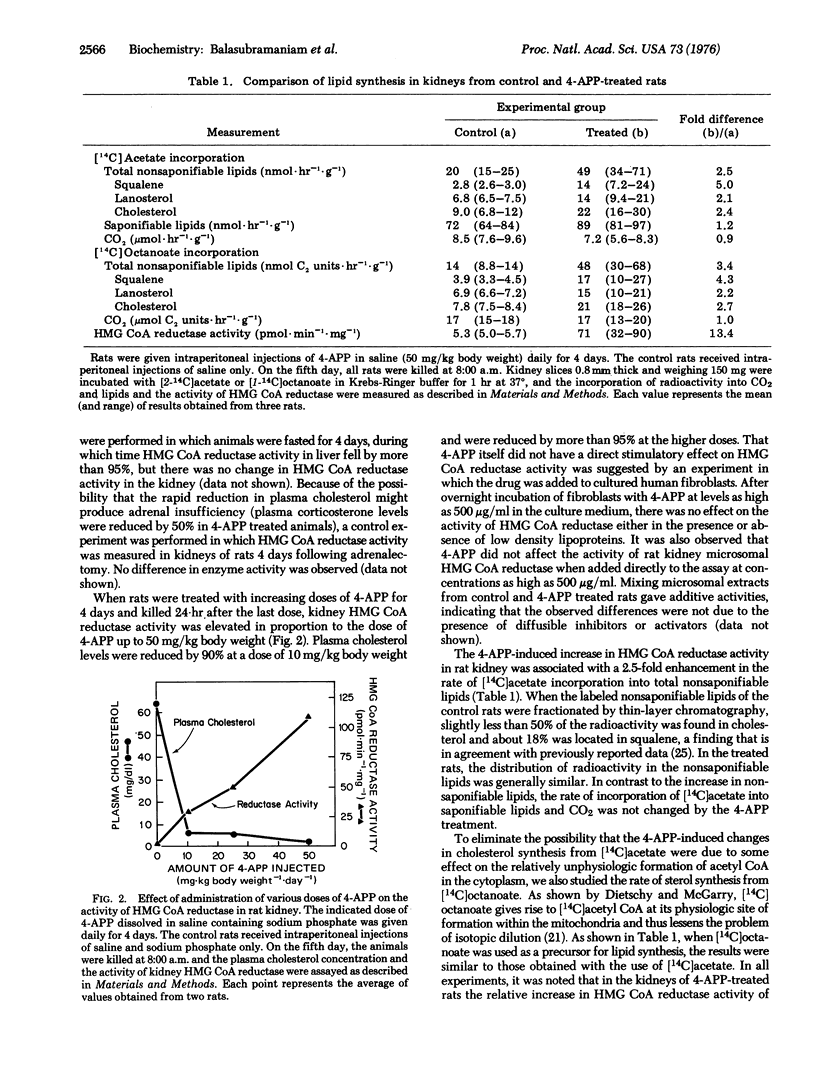
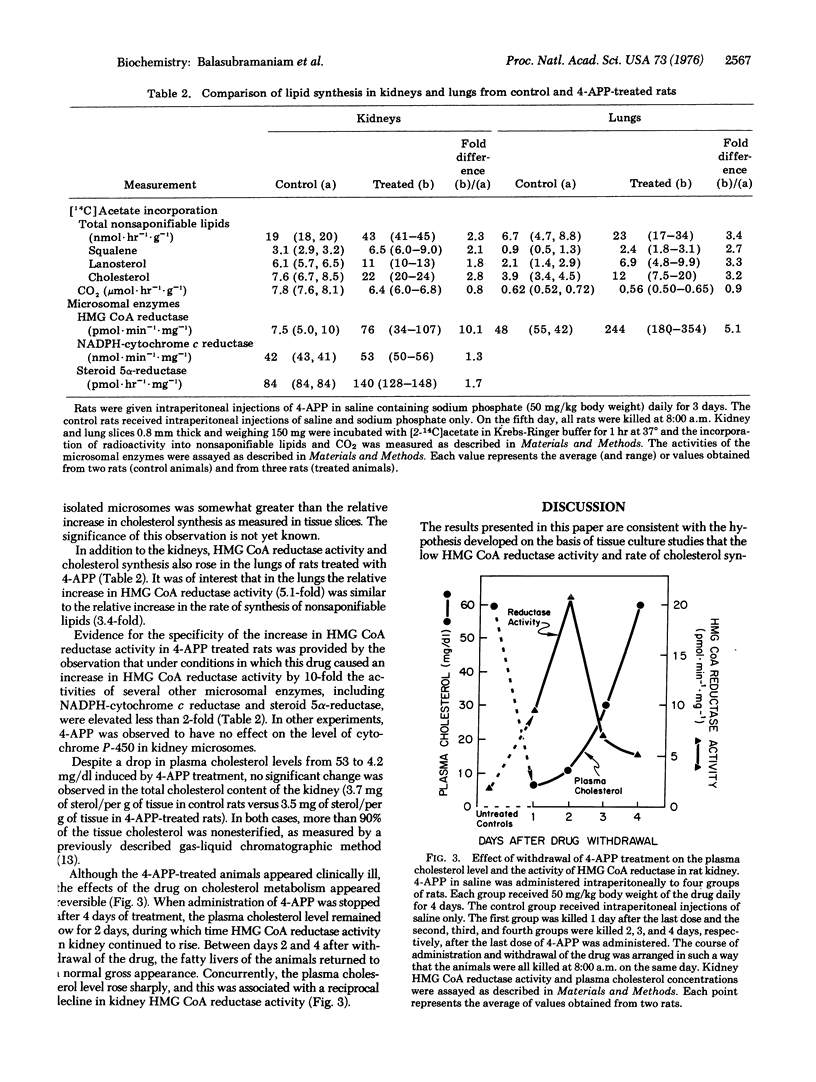
The adenine analogue 4-aminopyrazolopyrimidine has been reported previously to reduce the hepatic secretion of plasma lipoproteins in rats, thereby lowering the plasma cholesterol level. In the current studies, reduction of the plasma cholesterol level by 90% in rats through the administration of aminopyrazolopyrimidine was found to be associated with a 5- to 30-fold increase in the activity of 3-hydroxy-3-methylglutaryl-coenzyme A reductase [mevalonate:NADP+ oxidoreductase (CoA-acylating), EC1.1.1.34] in kidney and lung. In both tissues, the enhanced activity of this microsomal enzyme was associated with a 3-fold elevation in the rate of cholesterol synthesis from either [14C]acetate or [14C]octanoate. Comparable increases were not observed in the activities of several other microsomal enzymes or in the rates of [14C]acetate incorporation into saponifiable lipids or CO2. When administration of 4-aminopyrazolopyrimidine was terminated, plasma cholesterol levels rose and 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity declined in the kidney in a reciprocal manner. These data are consistent with the hypothesis that the low levels of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity and cholesterol synthesis that are normally observed in certain nonhepatic tissues of the rat are due to an active form of feedback regulation mediated by cholesterol carried in plasma lipoproteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Brannan P. G., Bohmfalk H. A., Brunschede G. Y., Dana S. E., Helgeson J., Goldstein J. L. Use of mutant fibroblasts in the analysis of the regulation of cholesterol metabolism in human cells. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):425–436. doi: 10.1002/jcp.1040850409. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Dietschy J. M., Siperstein M. D. 3-Hydroxy-3-methylglutaryl coenzyme A reductase. Solubilization and purification of a cold-sensitive microsomal enzyme. J Biol Chem. 1973 Jul 10;248(13):4731–4738. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., McGarry J. D. Limitations of acetate as a substrate for measuring cholesterol synthesis in liver. J Biol Chem. 1974 Jan 10;249(1):52–58. [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967 Mar;8(2):97–104. [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. J Clin Invest. 1968 Jan;47(1):166–174. doi: 10.1172/JCI105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. I. N Engl J Med. 1970 May 14;282(20):1128–1138. doi: 10.1056/NEJM197005142822005. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Edmond J., Seager J., Popják G. Abnormal induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in leukocytes from subjects with heterozygous familial hypercholesterolemia. J Biol Chem. 1975 Mar 25;250(6):2045–2055. [PubMed] [Google Scholar]
- Goldfarb S., Pitot H. C. Stimulatory effect of dietary lipid and cholestyramine on hepatic HMG CoA reductase. J Lipid Res. 1972 Nov;13(6):797–801. [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Lipoprotein receptors, cholesterol metabolism, and atherosclerosis. Arch Pathol. 1975 Apr;99(4):181–184. [PubMed] [Google Scholar]
- HENDERSON J. F. STUDIES ON FATTY LIVER INDUCTION BY 4-AMINOPYRAZOLOPYRIMIDINE. J Lipid Res. 1963 Jan;4:68–74. [PubMed] [Google Scholar]
- Kayden H. J., Hatam L., Beratis N. G. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and the esterification of cholesterol in human long term lymphoid cell lines. Biochemistry. 1976 Feb 10;15(3):521–528. doi: 10.1021/bi00648a011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mjos O. D., Faergeman O., Hamilton R. L., Havel R. J. Characterization of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest. 1975 Sep;56(3):603–615. doi: 10.1172/JCI108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P., Siperstein M. D. Mevalonate metabolism by renal tissue in vitro. J Lipid Res. 1974 Jan;15(1):20–25. [PubMed] [Google Scholar]
- Shapiro D. J., Rodwell V. W. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol synthesis. J Biol Chem. 1971 May 25;246(10):3210–3216. [PubMed] [Google Scholar]
- Shiff T. S., Roheim P. S., Eder H. A. Effects of high sucrose diets and 4-aminopyrazolopyrimidine on serum lipids and lipoproteins in the rat. J Lipid Res. 1971 Sep;12(5):596–603. [PubMed] [Google Scholar]
- Swann A., Wiley M. H., Siperstein M. D. Tissue distribution of cholesterol feedback control in the guinea pig. J Lipid Res. 1975 Sep;16(5):360–366. [PubMed] [Google Scholar]
- Williams C. D., Avigan J. In vitro effects of serum proteins and lipids on lipid synthesis in human skin fibroblasts and leukocytes grown in culture. Biochim Biophys Acta. 1972 Mar 23;260(3):413–423. doi: 10.1016/0005-2760(72)90056-2. [DOI] [PubMed] [Google Scholar]



