Abstract
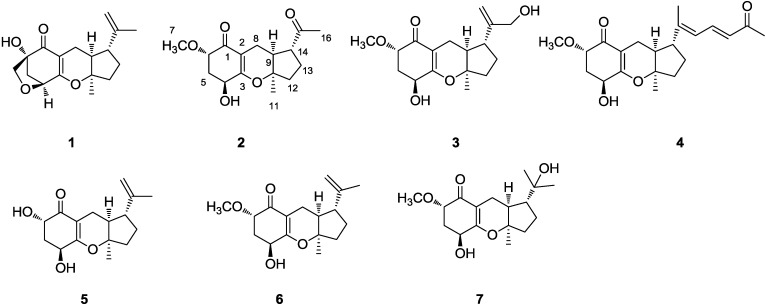
Four new meroterpenoids (2–5), along with three known analogues (1, 6, and 7) were isolated from mangrove plant Acanthus ilicifolius derived endophytic fungus Aspergillus flavipes. The structures of these compounds were elucidated by NMR and MS analysis, the configurations were assigned by CD data, and the stereochemistry of 1 was confirmed by X-ray crystallography analysis. A possible biogenetic pathway of compounds 1–7 was also proposed. All compounds were evaluated for antibacterial and cytotoxic activities.
Keywords: meroterpenoid, Aspergillus flavipes, endophytic fungus
1. Introduction
Marine-derived fungi have recently become a hotspot of new and bioactive metabolites in the marine environment [1]. The mangrove plant Acanthus ilicifolius growing in tropical and subtropical intertidal habitats is a rich source of new natural products. The genus Aspergillus is one of the major contributors to the secondary metabolites of fungal origin. Cytotoxic cytochalasins have been isolated from endophytic fungus Aspergillus flavipes associated with A. ilicifolius. Cytochalasins have the ability to bind to actin filaments and block polymerization and the elongation of actin [2]. Spiroquinazolines [3], cerebrosides [4], isobenzofurans [5,6], cytochalasins [2,7,8,9], and butyrolactones [10] have been isolated from the culture of A. flavipes. They showed antibiotic [9,11,12], cytotoxic [7], substance-p inhibitory [3], peptide deformylase inhibitory [5,6], and HIV-1 integrase inhibitory activities [8].
In the course of our study on marine derived microbes, several active metabolites were isolated [13,14,15,16]. In our previous study, two aromatic butyrolactones were reported from the same fungus, A. flavipes [17]. In a continuing study, four new and three known meroterpenoids were isolated from the same fungus. Herein, the isolation, structural elucidation, biological activities, and plausible biogenetic pathway of compounds 1–7 are reported (Figure 1).
Figure 1.
The chemical structures of compounds 1–7.
2. Results and Discussion
2.1. Structure Elucidation
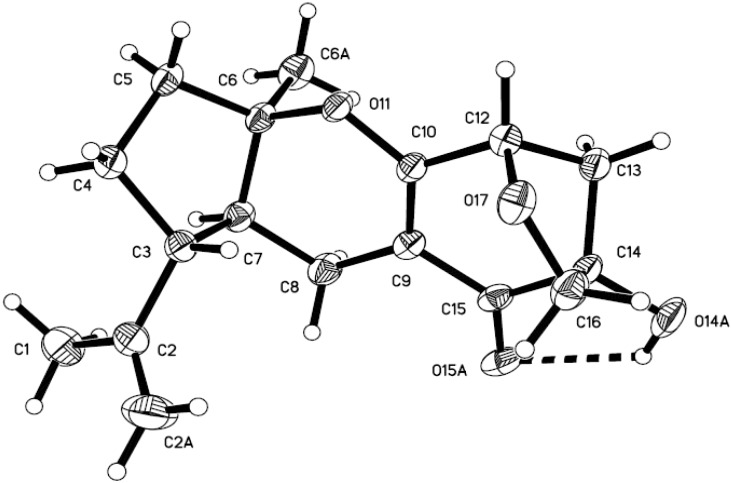
Compound 1 was isolated as colorless crystals. The molecular formula of 1 was established as C17H22O4 according to MS and NMR data. The NMR and optical rotation data were in agreement with those previously published, guignardone A [18]; the absolute configuration was confirmed by X-ray crystallography (Figure 2).
Figure 2.
The X-ray structure of compound 1.
Compound 2 was obtained as yellow oil. Its molecular formula was determined to be C16H22O5 by HRESIMS [M + Na]+ 317.1363 (calcd for 317.1359) and [M + H]+ 295.1544 (calcd for 295.1540). The 1H and 13C NMR spectra of 2 were similar to guignardone H (6) [19], with the only obvious difference being the presence of a ketone group (IR νmax 1608, δC 212.0) instead of a terminal double bond and the presence of a methyl ketone singlet downfield replacing the original vinyl methyl singlet observed in guignardone H. A small coupling constant of 3.0 Hz between H-6 and H-5 placed H-6 in a pseudoequatorial orientation of the cyclohexanone ring. Hence, H-6 was deduced to be β-oriented. Since the specific rotation and CD spectrum of compound 2 has the same sign as guignardone A (1) [18], and on biogenetic grounds, compound 2 was tentatively assigned as having the same absolute configuration as guignardone A.
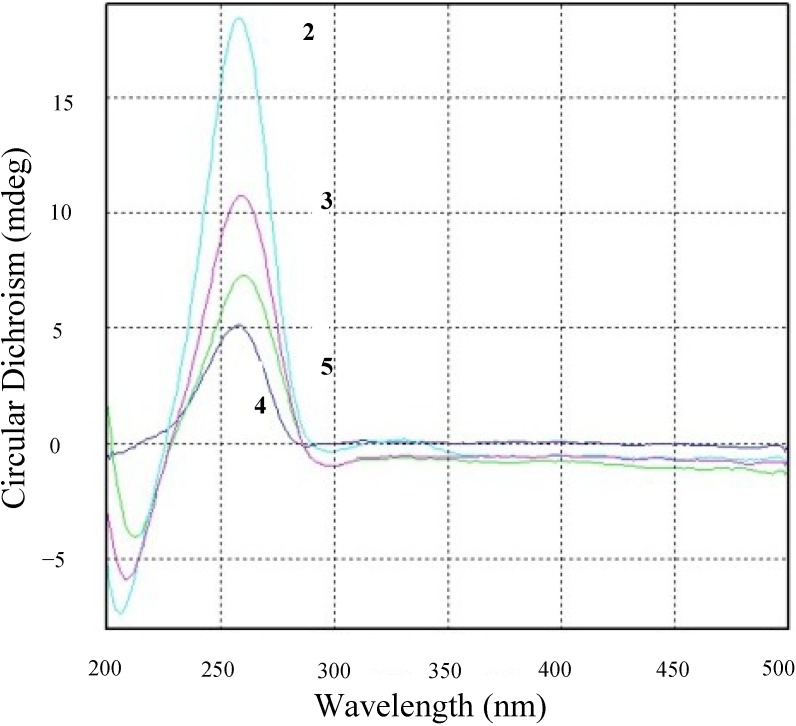
Compound 3 was obtained as a colorless oil. Its molecular formula was determined as C17H24O5 by HRESIMS [M + Na]+ 331.1516 (calcd for 331.1521) and [M + H]+ 309.1697 (calcd for 309.1702). IR absorptions implied the presence of hydroxyl group. The 1H and 13C NMR spectra of 3 were similar to guignardone H (6) [19], with the only obvious difference being the presence of a methyl group replacing a hydroxymethylene group (δC 65.3). A small coupling constants of 3.0 Hz between H-6 and H-5 indicated β-oriented of H-6. The configuration of 3 was tentatively established as same as that of 2 for the biogenetic pathway consideration. The optical rotation exhibited the same sign with 2, and the CD spectrum of 3 showed the same profile with 2 (Figure 3), and thus absolute stereochemistry of 3 was the same as 2, and named guignardone K (3).
Figure 3.
Circular Dichroism profiles of compounds 2–5.
Compound 4 was isolated as a yellow oil. Its molecular formula was determined as C21H28O5 by HRESIMS [M + Na]+ 383.1835 (calcd for 383.1834) and [M + H]+ 361.2012 (calcd for 361.2015). IR absorptions implied the presence of a hydroxyl, a ketone, and a conjugated double bond groups. The 13C NMR and DEPT spectra (Table 1) revealed 21 carbon signals, including four methyl (δC 14.4, 22.2, 27.7, and 58.5), four methylene (δC 16.2, 26.9, 34.4, and 37.7), seven methine (one oxygenated), and six quaternary carbon (including two α, β-unsaturated carbonyl and two oxygenated). Comparison of the 1H NMR and 13C NMR spectroscopic data with guignardone H (6) [19] revealed that 4 had the same tricyclic moiety as 6, but different side chain. The geometry of the disubstituted double bond (C-18) was determined to be E on the basis of the large coupling constants of the respective protons (J = 15.0 Hz). The presence of a methyl ketone group (δH 2.27, s; δC 198.7 and 27.7) at the end of the side chain of 4, which was confirmed by the HMBC correlations, the side chain was connected to the tricyclic moiety at C-5 in the ring. The absolute configuration of 4 was assumed to be the same as that of 2, and named guignardone L (4).
Table 1.
1H and 13C NMR data of compounds 2–5 (500/125 MHz).
| 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | |
| 1 | 198.6 s | 194.9 s | 194.9 s | 198.0 s | ||||
| 2 | 106.2 s | 105.7 s | 105.7 s | 104.2 s | ||||
| 3 | 171.9 s | 166.7 s | 167.8 s | 169.0 s | ||||
| 4 | 4.54 brt 5.0 | 66.4 d | 4.28 brt 5.0 | 65.8 d | 4.26 t 5.0 | 65.7 d | 4.55 t 5.5 | 65.4 d |
| 5 | 1.90 m | 37.4 t | 2.30 m | 34.5 t | 1.96 m | 34.4 t | 2.12 m | 38.2 t |
| 2.64 m | 2.41 m | 2.40 m | 2.28 m | |||||
| 6 | 3.88 dd 5.0, 3.0 | 79.5 d | 3.75 dd, 5.0, 3.0 | 79.2 d | 3.73 dd 5.0, 3.0 | 79.2 d | 4.09 dd 5.5, 3.0 | 69.4 d |
| 7 | 3.53 s | 58.5 q | 3.47 s | 58.4 q | 3.50 s | 58.5 q | ||
| 8 | 2.28 m | 18.6 t | 2.35 m | 16.3 t | 2.31 m | 16.2 t | 2.26 m | 16.4 t |
| 2.19 m | ||||||||
| 9 | 2.46 m | 42.8 d | 2.12 m | 43.8 d | 2.06 m | 43.5 d | 1.96 m | 43.3 d |
| 10 | 89.6 s | 87.6 s | 87.5 s | 89.0 s | ||||
| 11 | 1.36 s | 22.4 q | 1.35 s | 22.4 q | 1.36 s | 22.2 q | 1.35 s | 23.1 q |
| 12 | 1.90 m | 38.7 t | 1.88 m | 37.3 t | 2.00 m | 37.7 t | 1.89 m | 37.6 t |
| 2.22 m | 2.15 m | 2.25 m | 2.15 m | |||||
| 13 | 1.82 m | 26.1 t | 2.06 m | 28.4 t | 1.84 m | 26.9 t | 1.59 m | 26.9 t |
| 2.20 m | 1.57 m | 2.30 m | 1.95 m | |||||
| 14 | 2.68 m | 55.4 d | 2.27 m | 44.8 d | 2.37 m | 51.4 d | 2.16 m | 49.1 d |
| 15 | 212.0 s | 149.7 s | 150.2 s | 145.3 s | ||||
| 16 | 4.88 s | 109.9 t | 1.85 s | 14.4 q | 4.74 s | 111.5 t | ||
| 5.10 s | 4.63 s | |||||||
| 17 | 2.16, s | 29.2 q | 4.11 d 1.5 | 65.3 d | 5.94 d 10.0 | 124.7 d | 1.67 s | 19.2 q |
| 18 | 7.41 dd 15.0, 10.0 | 138.8 d | ||||||
| 19 | 6.09 d 15.0 | 129.3 d | ||||||
| 20 | 198.7 s | |||||||
| 21 | 2.27 s | 27.7 q | ||||||
Compound 5 was isolated as a yellow oil. Its molecular formula was determined as C16H23O4 by HRESIMS [M + Na]+ 301.1407 and [M + H]+ 279.1590. Comparison of the 1H NMR and 13C NMR spectroscopic data with guignardone G [19], revealed that 5 had the same structure as guignardone G, but different stereochemistry at C-6. H-6 was deduced to be β-oriented by a small coupling constant of 3.0 Hz between H- 6 and H-5. The configuration of 5 was established as same as that of 2, and named guignardone M (5).
Compounds 6 and 7 were identified as guignardones H [19] and I [19,20], respectively, by comparison of the 1H and 13C NMR with those reported data.
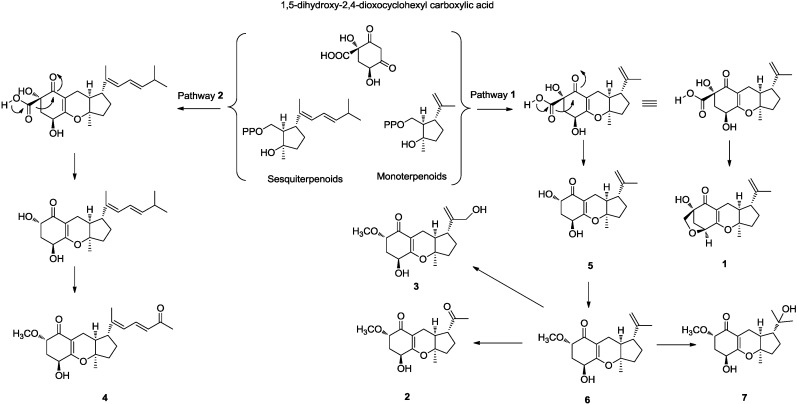
2.2. Plausible Biosynthetic Pathway
As compounds 1–7 were co-metabolites, we propose that they are on the same biosynthetic pathway. A possible biogenetic pathway of compounds 1–7 was proposed as shown in Figure 4. As suggested in pathways 1 and 2, we hypothesized the alkylation of precursor 1,5-dihydroxy-2,4-dioxocyclohexyl carboxylic acid by monoterpenoids or sesquiterpenoids to give the intermediates. Further modification leads to the generation of compounds 1–7.
Figure 4.
Plausible biosynthetic pathway of compounds 1–7.
2.3. Discussion
All isolated compounds were evaluated for antibacterial and cytotoxic activities; none of them are active. The previously reported compounds that have similar molecular architectures with meroterpenoids 1–7, showed antibacterial [19], cytotoxic [2,21,22], antimicrobial [22], and phytotoxic [23,24,25] activities. The structural differences of compounds 1–7 mainly occur in the isoprenoid chain, this may be the reason for their lack of activities. Tricycloalternarene-type meroterpenoids have been isolated from fungi Alternaria citri [26], A. alternata Ly83 [27,28], A. alternate [23,24,29,30], A. tenuissima [31], A. tenuissima SY-P-07 [32], Guignardia bidwellii PSU-G11 [21], G. Mangiferae [18,33,34], Septoria spp. [25], Ulocladium sp. [22], and Pycnoporus sanguineus [20]. Tricycloalternarene-type metabolites appear to be characteristic of the genera Alternaria and Guignardia, and thus may be good chemotaxonomic markers for these two genera. This is first time to isolate this class of compounds from fungus A. flavipes.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured using a Anton Paar MCP-500 Polarimeter. Ultraviolet (UV) spectra were measured in acetone on a Shimadzu UV-2600 Spectrophotometer. Infrared spectra (IR) were recorded on a Shimadzu IR Affinity-1FT-IR Spectrometer. One-dimensional and two-dimensional NMR spectra were recorded on a 500 MHz Bruker FTNMR Spectrometer using TMS as an internal standard. ESIMS and HRESIMS were measured with Bruker maXis impact and amaZon speed Mass Spectrometers. Column chromatography was performed on silica gel (90–150 μm, Qingdao Marine Chemical Company, Qingdao, China), Sephadex LH-20 (40–70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden). Column chromatography was performed with silica gel (100–200 mesh; 300–400 mesh; Jiangyou Silica Gel Development, Inc., Yantai, China) and Sephadex LH-20. Thin layer chromatography (TLC, 0.1–0.2 mm or 0.3–0.4 mm) was carried out with precoated silica gel plates (GF-254, Jiangyou Silica Gel Development, Inc., Yantai, China). TLC spots were visualized under UV light and heating after spray by 5% H2SO4 in EtOH.
3.2. Fungal Material
The endophytic fungus A. flavipes AIL8 was isolated from the inner leaves of mangrove plant A. ilicifolius collected at Daya Bay, Shenzhen, China, in 2011. This fungus was deposited at 4 °C on potato dextrose agar (PDA) slants in CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China.
The strain stored on PDA slants was inoculated and cultured on PDA agar plates for 7 days. Seed medium (potato 200 g, dextrose 20 g, distilled water 1000 mL) in 100 mL × 10 Erlenmeyer flasks was inoculated with fungus and incubated at 25 °C for 48 h on a rotating shaker (180 rpm). Production medium of solid rice in 1000 mL flasks (rice 200 g, distilled water 210 mL) was inoculated with seed solution (10 mL) one for one. Flasks were incubated at 25 °C under still culture and fermented for 40 days, cultures from 30 flasks were harvested for the isolation of substances. Fungal identification was carried out by using the method previously reported [13].
3.3. Extraction and Isolation
After fermentation, culture medium containing the mycelium was inactivated by acetone and extracted with ethyl acetate, and evaporated (40 °C) to remove EtOAc. About 180 g residue was obtained, the residue was dissolved and using the similar procedure as previously described [35], and got 99.0 g residue to chromatograph on silica gel and eluted with solutions gradients, petroleum ether (60–90 °C)/acetone (10:0, 9:1, 8:2, 7:3, 6:4, 5:5), chloroform/methanol (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 0:1), to yield 9 fractions (Frs. 1–9). Frs. 2 chromatographed on a Sephadex LH-20 column using CHCl3/MeOH (1:1), to produce 4 fractions (F2-1–F2-4), was further separated by silica gel column chromatography and eluted with (petroleum ether/acetone, 15:1), to get 4 fractions (F2-1–F2-4). F2-2 was applied to silica gel eluted with a gradient of (petroleum ether/acetone, 18:1) to give 1 (50 mg). Frs. 3 chromatographed on a Sephadex LH-20 column using CHCl3/MeOH (1:1), to produce 7 fractions F3-1–F3-7. F3-2 on a silica gel column (petroleum ether/acetone, 10:1) to produce 6 fractions, the second fraction was chromatographed on silica gel (petroleum ether/acetone, 15:1) to give 6 (11.3 mg). Frs. 4 chromatographed on a Sephadex LH-20 column using CHCl3/MeOH (1:1), to produce 5 fractions (F4-1–F4-5), F4-1- F4-3 was chromatographed on a silica gel column (petroleum ether/acetone, 10:1) to produce compounds 2 (6.6 mg), 3 (7.1 mg), and 7 (5.6 mg). Frs. 5 chromatographed on a Sephadex LH-20 column using CHCl3/MeOH (1:1), to produce 3 fractions (F5-1–F5-3), F5-2 was further separation on a silica gel column (petroleum ether/acetone, 8:1) to give compounds 4 (7.7 mg) and 5 (9.4 mg).
Guignardone J (2). Yellow oil; +35 (c 0.66, acetone); UV (MeOH) λmax: 325 nm (3.25); IR (KBr) νmax 2924, 1608, 1165, 1076 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 317.1363 [M + Na]+ (calcd for C16H22O5Na, 317.1365); HRESIMS [M + H]+ 295.1544 (calcd for C16H23O5, 295.1545).
Guignardone K (3). Colorless oil; +100 (c 0.7, acetone); UV (MeOH) λmax: 327 (2.89), 306 nm (1.43); IR (KBr) νmax 3390, 2927, 1284, 1076 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 331.1516 [M + Na]+ (calcd for C17H24O5Na, 331.1521), m/z 309.1697 [M + H]+ (calcd for C17H25O5, 309.1702).
Guignardone L (4). Yellow oil; +50 (c 0.5, acetone); UV (MeOH) λmax: 327 (3.05), 306 nm (1.56); IR (KBr) νmax 3417, 1612 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 383.1835 [M + Na]+ (calcd for C21H28O5 , 383.1834), [M + H]+ 361.2012 (calcd for C21H29O5, 361.2015).
Guignardone M (5). Yellow oil; +60 (c 0.5, acetone); UV (MeOH) λmax: 360 (3.18), 324 nm (2.04); IR (KBr) νmax 3352, 1608, 1141, 1060 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS [M + Na]+ 301.1407 (calcd. for C16H22O4Na, 301.1416), [M + H]+ 279.1590 (calcd for C16H23O4, 279.1596).
3.4. X-ray Crystallographic Analysis of Guignarenone A (1)
Colorless crystal of C17H22O4, M = 290.35, monoclinic, C2, a = 19.5512(2) Å, b = 5.60488(7) Å, c = 14.50891(19) Å, V = 1512.61(3) Å3, Z = 4, Dcalc = 1.275 g/cm3, F(000) = 624, crystal size: 0.43 × 0.38 × 0.29 mm3, independent reflections: 2659 [R(int) = 0.0504], final R indicates [I > 2σ(I)]: R1 = 0.0402, wR2 = 0.1018. A total of 16,279 unique reflections were collected using a CrysAlis PRO CCD area detector diffractometer with graphite monochromated Cu Kα radiation (λ = 1.54178Å) at 150 K, Absorption corrections were done by semiempirical from equivalents. The structure was solved using direct methods (SHELXL-97) and refined with Full-matrix least-squares on F2, 1 restraint and 195 variable parameters.
3.5. Antimicrobial Activity
Antibacterial activity was tested on strains of Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Bacillus thuringiensis, Candida albicans, and Micrococcus luteus. MIC values of the compounds were determined by the modified 0.5 Mcfarland standard method. Two-fold dilutions of the compounds in the range of (40–0.31 mg/mL) were prepared in 0.5% MeOH. Compounds and positive control (Penicillin) were similarly diluted in 0.5% MeOH to generate a series of concentration, ranging from 40 to 0.31 mg/mL per well. The turbidity of the bacterial suspensions was measured at 600 nm, and adjusted with a medium to match the 0.5 McFarland standard (105–106 colony forming units/mL). Subsequently, 180 μL of bacterial culture was inoculated into each well, and the test solutions (20 μL) were added to 96-well plates. Finally, the plates were incubated at 36 °C for 24 h, and the MIC values were determined in triplicates and re-examined at appropriate times. To ensure that these vehicles had significant effect on the bacterial growth, each of the bacterial species was additionally cultured in a blank solution containing LB broth media at concentrations equivalent to those of test solutions [36].
3.6. Cytotoxicity
Proliferation of PC-3 cells was evaluated by using a cell counting kit (CCK-8, Ddojindo, Japan) following the manufacturer’s protocol. Cells were routinely grown and maintained in mediums RPMI or DMEM with 10% FBS and with 1% penicillin/streptomycin. All cell lines were incubated in a Thermo/Forma Scientific CO2 Water Jacketed Incubator with 5% CO2 in air at 37 °C. Cell viability assay was determined by the CCK8 assay. Three-fold dilutions of the compounds in the range of (10–5.08 × 105 μM) were prepared in DMSO. Cells were seeded at a density of 400–800 cells/ well in 96 well plates and treated with various concentration of compounds, positive control (Trichostatin A and Taxol) or solvent control. After 72 h incubation, CCk8 reagent was added, and absorbance was measured at 450 nm using Envision 2104 multi-label Reader (Perkin Elmer, USA). Dose response curves were plotted to determine the IC50 values using Prism 5.0 (GraphPad Software Inc., San Diego CA, USA). The inhibitory rate was calculated using the formula: (ODcontrol cells − ODtreated cells)/ODcontrol cells × 100% [37,38].
4. Conclusions
Four new meroterpenoids, along with three known analogues, were isolated from marine-derived endophytic fungus A. flavipes AIL8 derived from the mangrove plant Acanthus ilicifolius. A possible biogenetic pathway of compounds 1–7 was proposed. All compounds were evaluated for antibacterial activity and cytotoxicity; none of them are active. Comparison with those reported tricycloalternarenes, the structural differences mainly occur in the isoprenoid side chains, this maybe the reason for their lack of antibacterial and cytotoxic activities.
Acknowledgments
This work was supported financially by the National Key Basic Research Program of China (973)’s Project (2011CB915503), the National High Technology Research and Development Program (863 Program, 2012AA092104), the National Natural Science Foundation of China (Nos. 31270402, 20902094, 41176148, and 21002110), the Strategic Leading Science and Technology Project of Chinese Academy of Sciences (XDA11030403), and Guangdong Marine Economic Development and Innovation of Regional Demonstration Project (GD2012-D01-001).
Supplementary Information
Electronic supplementary information (ESI) available: 1D and 2D NMR, and HRESIMS spectral data of compounds 1–7.
Author Contributions
Z.-Q. Bai contribute to extraction, isolation and identification; X.-P. Lin contribute to the isolation of the fungi; J.-F. Wang, X. Zhou, J. Liu, B. Yang, X. Yang, S.-R. Liao, and L. Wang contribute to structure elucidation and NMR analysis; Y. Liu was the project leader organizing and guiding the experiments and manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2014;31:160–258. doi: 10.1039/c3np70117d. [DOI] [PubMed] [Google Scholar]
- 2.Lin Z.-J., Zhang G.-J., Zhu T.-J., Liu R., Wei H.-J., Gu Q.-Q. Bioactive cytochalasins from Aspergillus flavipes, an endophytic fungus associated with the mangrove plant Acanthus ilicifolius. Helv. Chim. Acta. 2009;92:1538–1544. doi: 10.1002/hlca.200800455. [DOI] [Google Scholar]
- 3.Barrow C.J., Sun H.H. Spiroquinazoline, a novel substance-p inhibitor with a new carbon skeleton, isolated from Aspergillus flavipes. J. Nat. Prod. 1994;57:471–476. doi: 10.1021/np50106a005. [DOI] [PubMed] [Google Scholar]
- 4.Jiang T., Li T., Li J., Fu H.Z., Pei Y.H., Lin W.H. Cerebroside analogues from marine-derived fungus Aspergillus flavipes. J. Asian Nat. Prod. Res. 2004;6:249–257. doi: 10.1080/1028602031000147384. [DOI] [PubMed] [Google Scholar]
- 5.Kwon Y.-J., Sohn M.-J., Kim C.-J., Koshino H., Kim W.-G. Flavimycins A and B, dimeric 1,3-dihydroisobenzofurans with peptide deformylase inhibitory activity from Aspergillus flavipes. J. Nat. Prod. 2012;75:271–274. doi: 10.1021/np200720v. [DOI] [PubMed] [Google Scholar]
- 6.Kwon Y.-J., Zheng C.-J., Kim W.-G. Isolation and identification of FR198248, a hydroxylated 1,3-dihydroisobenzofuran, from Aspergillus flavipes as an inhibitor of peptide deformylase. Biosci. Biotechnol. Biochem. 2010;74:390–393. doi: 10.1271/bbb.90565. [DOI] [PubMed] [Google Scholar]
- 7.Zhou G.X., Wijeratne E.M.K., Bigelow D., Pierson L.S., VanEtten H.D., Gunatilaka A.A.L., Aspochalasins I.J.K. Three new cytotoxic cytochalasans of Aspergillus flavipes from the rhizosphere of Ericameria laricifolia of the Sonoran Desert. J. Nat. Prod. 2004;67:328–332. doi: 10.1021/np030353m. [DOI] [PubMed] [Google Scholar]
- 8.Rochfort S., Ford J., Ovenden S., Wan S.S., George S., Wildman H., Tait R.M., Meurer-Grimes B., Cox S., Coates J., et al. A novel aspochalasin with HIV-1 integrase inhibitory activity from Aspergillus flavipes. J. Antibiot. 2005;58:279–283. doi: 10.1038/ja.2005.34. [DOI] [PubMed] [Google Scholar]
- 9.Kohno J., Nonaka N., Nishio M., Ohnuki T., Kawano K., Okuda T., Komatsubara S. TMC-169, a new antibiotic of the aspochalasin group produced by Aspergillus flavipes. J. Antibiot. 1999;52:575–577. doi: 10.7164/antibiotics.52.575. [DOI] [PubMed] [Google Scholar]
- 10.Nagia M.M., El-Metwally M.M., Shaaban M., El-Zalabani S.M., Hanna A.G. Four butyrolactones and diverse bioactive secondary metabolites from terrestrial Aspergillus flavipes MM2: Isolation and structure determination. Org. Med. Chem. Lett. 2012;2:9. doi: 10.1186/2191-2858-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casinovi C.G., Grandoli G., Mercanti R., Oddo N., Olivieri R., Tonolo A. A new antibiotic produced by a strain of Aspergillus flavipes. Tetrahedron Lett. 1968;27:3175–3178. doi: 10.1016/S0040-4039(00)89581-7. [DOI] [PubMed] [Google Scholar]
- 12.Findlay J.A., Radics L. Flavipucine (3′-isovaleryl-6-methylpyridine-3-spiro-2′-oxiran-2(1H),-4(3H)-dione), an antibiotic from Aspergillus flavipes. J. Chem. Soc. Perkin. 1. 1972;16:2071–2074. doi: 10.1039/p19720002071. [DOI] [PubMed] [Google Scholar]
- 13.Lin X., Zhou X., Wang F., Liu K., Yang B., Yang X., Peng Y., Liu J., Ren Z., Liu Y. A new cytotoxic sesquiterpene quinone produced by Penicillium sp. F00120 isolated from a deep sea sediment sample. Mar. Drugs. 2012;10:106–115. doi: 10.3390/md10010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X., Lin X., Ma W., Fang W., Chen Z., Yang B., Liu Y. A new aromatic amine from fungus Pestalotiopsis vaccinii. Phytochem. Lett. 2014;7:35–37. doi: 10.1016/j.phytol.2013.09.010. [DOI] [Google Scholar]
- 15.Yang B., Dong J., Lin X., Zhou X., Zhang Y., Liu Y. New prenylated indole alkaloids from fungus Penicillium sp. derived of mangrove soil sample. Tetrahedron. 2014;70:3859–3863. doi: 10.1016/j.tet.2014.04.043. [DOI] [Google Scholar]
- 16.Fredimoses M., Zhou X., Lin X., Tian X., Ai W., Wang J., Liao S., Liu J., Yang B., Yang X., et al. New prenylxanthones from the deep-sea derived fungus Emericella sp. SCSIO 05240. Mar. Drugs. 2014;12:3190–3202. doi: 10.3390/md12063190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai Z.-Q., Lin X., Wang Y., Wang J., Zhou X., Yang B., Liu J., Yang X., Wang Y., Liu Y. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia. 2014;95:194–202. doi: 10.1016/j.fitote.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Yuan W.H., Liu M., Jiang N., Guo Z.K., Ma J., Zhang J., Song Y.C., Tan R.X. Guignardones A-C: Three Meroterpenes from Guignardia mangiferae. Eur. J. Org. Chem. 2010;33:6348–6353. doi: 10.1002/ejoc.201000916. [DOI] [Google Scholar]
- 19.Mei W.L., Zheng B., Zhao Y.X., Zhong H.M., Chen X.L.W., Zeng Y.B., Dong W.H., Huang J.L., Proksch P., Dai H.F. Meroterpenes from endophytic fungus A1 of mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs. 2012;10:1993–2001. doi: 10.3390/md10091993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molinar E., Rios N., Spadafora C., Arnold A.E., Coley P.D., Kursar T.A., Gerwick W.H., Cubilla-Rios L. Coibanoles, a new class of meroterpenoids produced by Pycnoporus sanguineus. Tetrahedron Lett. 2012;53:919–922. doi: 10.1016/j.tetlet.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommart U., Rukachaisirikul V., Trisuwan K., Tadpetch K., Phongpaichit S., Preedanon S., Sakayaroj J. Tricycloalternarene derivatives from the endophytic fungus Guignardia bidwellii PSU-G11. Phytochemistry Lett. 2012;5:139–143. doi: 10.1016/j.phytol.2011.11.010. [DOI] [Google Scholar]
- 22.Wang Q.-X., Bao L., Yang X.-L., Guo H., Ren B., Guo L.-D., Song F.-H., Wang W.-Z., Liu H.-W., Zhang L.-X. Tricycloalternarenes F-H: Three new mixed terpenoids produced by an endolichenic fungus Ulocladium sp. using OSMAC method. Fitoterapia. 2013;85:8–13. doi: 10.1016/j.fitote.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Liebermann B., Elliger R., Gunther W., Ihn W., Gallander H. Tricycloalternarenes produced by Alternaria alternata related to ACTG-toxins. Phytochemistry. 1997;46:297–303. doi: 10.1016/S0031-9422(97)00278-1. [DOI] [Google Scholar]
- 24.Liebermann B., Nussbaum R.P., Gunther W. Bicycloalternarenes produced by the phytopathogenic fungus Alternaria alternata. Phytochemistry. 2000;55:987–992. doi: 10.1016/S0031-9422(00)00268-5. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara F., Uzawa J., Esumi Y., Suzuki M., Yoshida S., Strobel G., Steiner J.L.R., Clardy J. Phytotoxins from the Septoria spp. plant pathogenic fungus on leafy spurge. Biosci. Biotechnol. Biochem. 1998;62:638–642. doi: 10.1271/bbb.62.638. [DOI] [PubMed] [Google Scholar]
- 26.Kono Y., Gardner J.M., Suzuki Y., Takeuchi S. Studies on host-selective toxins produced by a pathotype of Alternaria citri causing brown spot disease of mandarins. Agric. Biol. Chem. 1986;50:1597–1606. doi: 10.1271/bbb1961.50.1597. [DOI] [Google Scholar]
- 27.Yuan L., Zhao P.-J., Ma J., Li G.-H., Shen Y.-M. Tricycloalternarenes A-E: Five new mixed terpenoids from the endophytic fungal strain Alternaria alternata Ly83. Helv. Chim. Acta. 2008;91:1588–1594. doi: 10.1002/hlca.200890172. [DOI] [Google Scholar]
- 28.Qiao L.-R., Yuan L., Gao J.-M., Zhao P.-J., Kang Q.-J., Shen Y.-M. Tricycloalternarene derivatives produced by an endophyte Alternaria alternata isolated from Maytenus hookeri. J. Basic Microbiol. 2007;47:340–343. doi: 10.1002/jobm.200610306. [DOI] [PubMed] [Google Scholar]
- 29.Liebermann B., Nussbaum R.P., Gunther W., Teuscher J.M. Biosynthesis of the bicycloalternarenes, mixed terpenoids of Alternaria alternata. Phytochemistry. 2001;56:551–557. doi: 10.1016/S0031-9422(00)00459-3. [DOI] [PubMed] [Google Scholar]
- 30.Nussbaum R.P., Gunther W., Heinze S., Liebermann B. New tricycloalternarenes produced by the phytopathogenic fungus Alternaria alternata. Phytochemistry. 1999;52:593–599. doi: 10.1016/S0031-9422(99)00275-7. [DOI] [PubMed] [Google Scholar]
- 31.Sun H., Gao S., Li X., Li C., Wang B. Chemical constituents of marine mangrove-derived endophytic fungus Alternaria tenuissima EN-192. Chin. J. Oceanol. Limnol. 2013;31:464–470. doi: 10.1007/s00343-013-2106-2. [DOI] [Google Scholar]
- 32.Li D.-M., Zhang Y.-H., Ji H.-X., Wu X., Pei Y.-H., Bai J. Tricycloalternarene derivatives from endophytic fungus Alternaria tenuissima SY-P-07. Nat. Prod. Res. 2013;27:1877–1881. doi: 10.1080/14786419.2013.771352. [DOI] [PubMed] [Google Scholar]
- 33.Guimaraes D.O., Lopes N.P., Pupo M.T. Meroterpenes isolated from the endophytic fungus Guignardia mangiferae. Phytochemistry Lett. 2012;5:519–523. doi: 10.1016/j.phytol.2012.05.004. [DOI] [Google Scholar]
- 34.Liang F., Li D., Chen Y., Tao M., Zhang W., Zhang D. Secondary metabolites of endophytic Guignardia mangiferae from Smilax glabra and their antitumor activities. Chin. Tradit. Herb. Drugs. 2012;43:856–860. [Google Scholar]
- 35.Kjer J., Debbab A., Aly A.H., Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010;5:479–490. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- 36.Pugachev M.V., Shtyrlin N.V., Sapozhnikov S.V., Sysoeva L.P., Iksanova A.G., Nikitina E.V., Musin R.Z., Lodochnikova O.A., Berdnikov E.A., Shtyrlin Y.G. Bis-phosphonium salts of pyridoxine: The relationship between structure and antibacterial activity. Biorg. Med. Chem. 2013;21:7330–7342. doi: 10.1016/j.bmc.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., Shen J., Wu W.K.K., Yu X., Liang J., Qiu G., Liu J. Leptin induces cyclin D1 expression and proliferation of human nucleus pulposus Cells via JAK/STAT, PI3K/Akt and MEK/ERK pathways. PLoS One. 2012;7:e53176. doi: 10.1371/journal.pone.0053176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Wang X., Li J.-P., Yang Y., Ding J., Meng L.-H. A pharmacological model reveals biased dependency on PI3K isoforms for tumor cell growth. Acta Pharmacol. Sin. 2013;34:1201–1207. doi: 10.1038/aps.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]