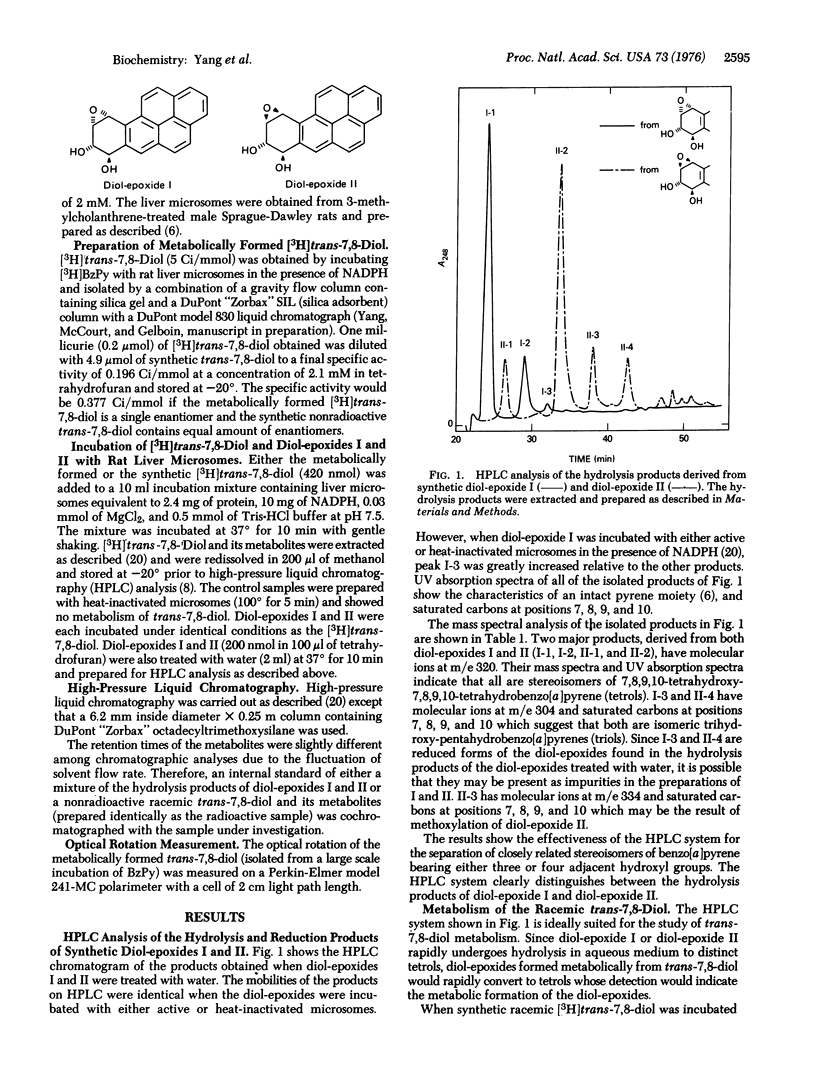
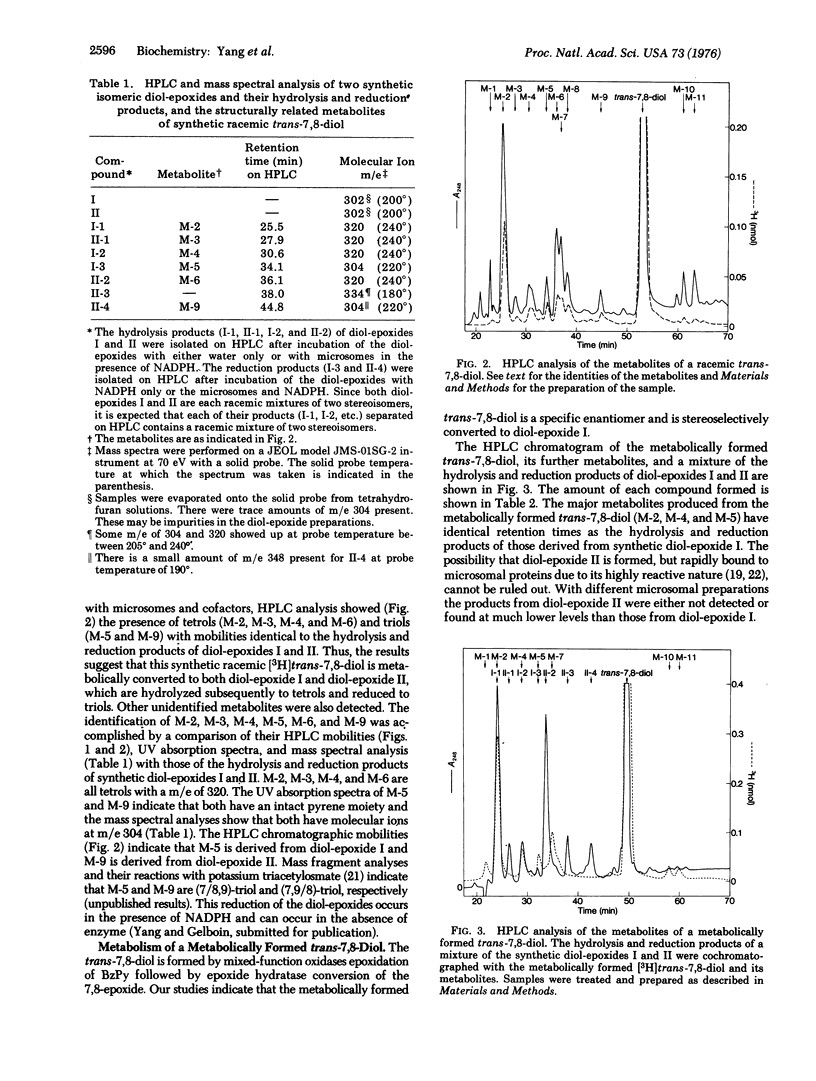
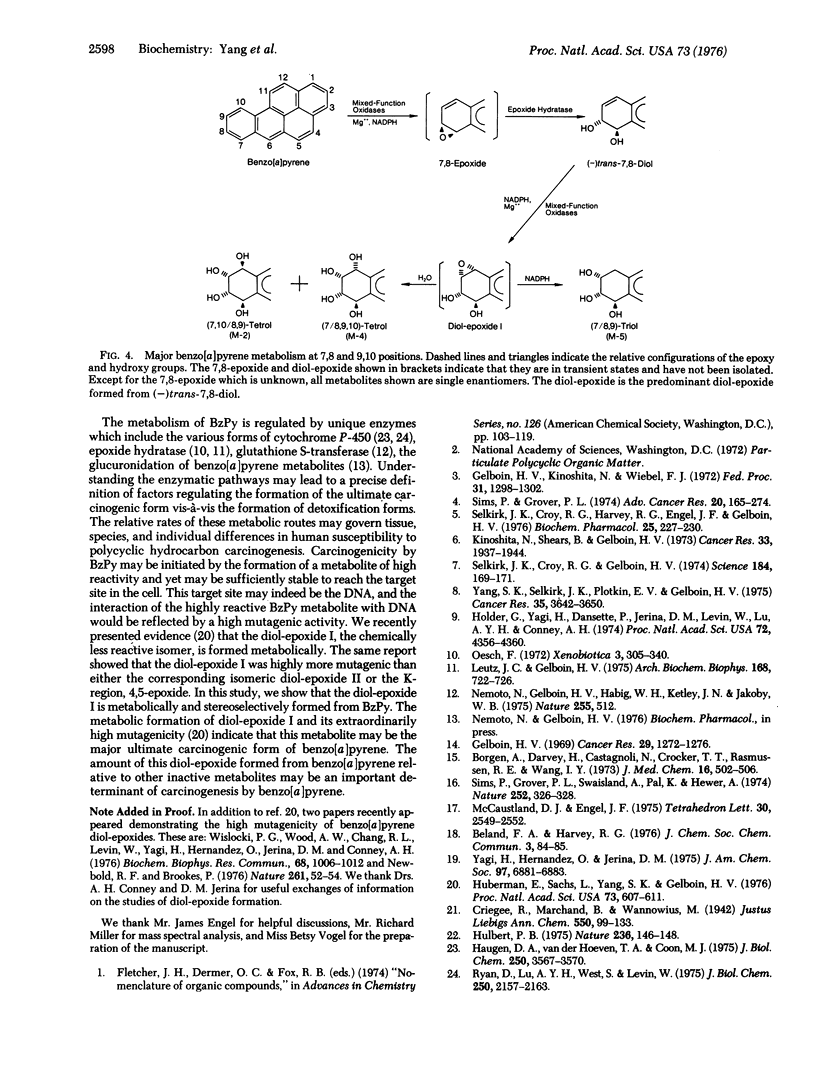
Abstract
Benzo(a)pyrene is metabolically and stereospecifically converted by mixed-function oxidases of rat liver microsomes and epoxide hydratase (glycol hydro-lyase (epoxide-forming), EC 4.2.1.63)to the single enantiomer (-)r-7,t-8-dihydroxy-7,8-dihydrobenzol (A) pyrene. This enantiomer is further metabolized stereoselectively by the mixed-function oxidases to predominantly the diol-epoxide, r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzol(a)pyrene in which the 7-hydroxyl and the 9,10-epoxide are trans. Other unidentified metabolites are also formed from the r-7,t-8-dihydroxy-7,8-dihydrobenzo(a)pyrene. Racemic r-7,t-8-dihydroxy-7,8-dihydrobenzo(a)-pyrene is converted metabolically to both r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene and r-7,t-8-dihydroxy-c-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. The diol-epoxides are unstable in aqueous medium, and their identification and characterization as r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene and r-7,t-8-dihydroxy-c-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene were accomplished by the identity of their tetrahydroxytetrahydrobenzo(a)pyrenes hydrolysis products with those of the authentic synthetic compounds with respect to mobility on high-pressure liquid chromatography and mass and ultraviolet absorption spectral analysis. The diol-epoxides were also reduced in the presence of NADPH to distinct trihydroxypentahydrobenzo(a)pyrenes. Since the synthetic racemic r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene is very highly mutagenic in mammalian cells, we suggest that it is the metabolically formed diol-epoxide that may be an ultimate carcinogenic form of benzo(a)pyrene.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgen A., Darvey H., Castagnoli N., Crocker T. T., Rasmussen R. E., Wang I. Y. Metabolic conversion of benzo(a)pyrene by Syrian hamster liver microsomes and binding of metabolites to deoxyribonucleic acid. J Med Chem. 1973 May;16(5):502–506. doi: 10.1021/jm00263a020. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Selkirk J. K., Harvey R. G., Engel J. F., Gelboin H. V. Separation of ten benzo(a)pyrene phenols by recycle high pressure liquid chromatography and identification of four phenols as metabolites. Biochem Pharmacol. 1976 Jan 15;25(2):227–230. doi: 10.1016/0006-2952(76)90303-8. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. A microsome-dependent binding of benzo[a]pyrene to DNA. Cancer Res. 1969 Jun;29(6):1272–1276. [PubMed] [Google Scholar]
- Gelboin H. V., Kinoshita N., Wiebel F. J. Microsomal hydroxylases: induction and role in polycyclic hydrocarbon carcinogenesis and toxicity. Fed Proc. 1972 Jul-Aug;31(4):1298–1309. [PubMed] [Google Scholar]
- Haugen D. A., van der Hoeven T. A., Coon M. J. Purified liver microsomal cytochrome P-450. Separation and characterization of multiple forms. J Biol Chem. 1975 May 10;250(9):3567–3570. [PubMed] [Google Scholar]
- Holder G., Yagi H., Dansette P., Jerina D. M., Levin W., Lu A. Y., Conney A. H. Effects of inducers and epoxide hydrase on the metabolism of benzo(a)pyrene by liver microsomes and a reconstituted system: analysis by high pressure liquid chromatography. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4356–4360. doi: 10.1073/pnas.71.11.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Sachs L., Yang S. K., Gelboin V. Identification of mutagenic metabolites of benzo(a)pyrene in mammalian cells. Proc Natl Acad Sci U S A. 1976 Feb;73(2):607–611. doi: 10.1073/pnas.73.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert P. B. Carbonium ion as ultimate carcinogen of polycyclic aromatic hydrocarbons. Nature. 1975 Jul 10;256(5513):146–148. doi: 10.1038/256146a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Shears B., Gelboin H. V. K-region and non-K-region metabolism of benzo(a)pyrene by rat liver microsomes. Cancer Res. 1973 Aug;33(8):1937–1944. [PubMed] [Google Scholar]
- Leutz J. C., Gelboin H. V. Benzo(a)pyrene-4,5-oxide hydratase: assay, properties, and induction. Arch Biochem Biophys. 1975 Jun;168(2):722–725. doi: 10.1016/0003-9861(75)90307-0. [DOI] [PubMed] [Google Scholar]
- Nemoto N., Gelboin H. V., Habig W. H., Ketley J. N., Jakoby W. B. K region benzo (alpha) pyrene-4,5-oxide is conjugated by homogeneous gluthathione S-transferases. Nature. 1975 Jun 5;255(5508):512–512. doi: 10.1038/255512a0. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Brookes P. Exceptional mutagenicity of a benzo(a)pyrene diol epoxide in cultured mammalian cells. Nature. 1976 May 6;261(5555):52–54. doi: 10.1038/261052a0. [DOI] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Ryan D., Lu A. Y., West S., Levin W. Multiple forms of cytochrome P-450 in phenobarbital- and 3-methylcholanthrene-treated rats. Separation and spectral properties. J Biol Chem. 1975 Mar 25;250(6):2157–2163. [PubMed] [Google Scholar]
- Selkirk J. K., Croy R. G., Gelboin H. V. Benzo(a)pyrene metabolites: efficient and rapid separation by high-pressure liquid chromatography. Science. 1974 Apr 12;184(4133):169–171. doi: 10.1126/science.184.4133.169. [DOI] [PubMed] [Google Scholar]
- Sims P., Grover P. L. Epoxides in polycyclic aromatic hydrocarbon metabolism and carcinogenesis. Adv Cancer Res. 1974;20:165–274. doi: 10.1016/s0065-230x(08)60111-6. [DOI] [PubMed] [Google Scholar]
- Sims P., Grover P. L., Swaisland A., Pal K., Hewer A. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature. 1974 Nov 22;252(5481):326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- Wislocki P. G., Wood A. W., Chang R. L., Levin W., Yagi H., Hernandez O., Jerina D. M., Conney A. H. High mutagenicity and toxicity of a diol epoxide derived from benzo(a)pyrene. Biochem Biophys Res Commun. 1976 Feb 9;68(3):1006–1012. doi: 10.1016/0006-291x(76)91246-8. [DOI] [PubMed] [Google Scholar]
- Yagi H., Hernandez O., Jerina D. M. Letter: Synthesis of (+/-)-7 beta,8alpha-dihydroxy-9 beta,10beta-epoxy-7,8,-9,10-tetrahydrobenzo(a)pyrene, a potential metabolite of the carcinogen benzo(a)pyrene with stereochemistry related to the antileukemic triptolides. J Am Chem Soc. 1975 Nov 12;97(23):6881–6883. doi: 10.1021/ja00856a057. [DOI] [PubMed] [Google Scholar]
- Yang S. K., Selkirk J. K., Plotkin E. V., Gelboin H. V. Kinetic analysis of the metabolism of benzo(a)pyrene to phenols, dihydrodiols, and quinones by high-pressure chromatography compared to analysis by aryl hydrocarbon hydroxylase assay, and the effect of enzyme induction. Cancer Res. 1975 Dec;35(12):3642–3650. [PubMed] [Google Scholar]



