Abstract
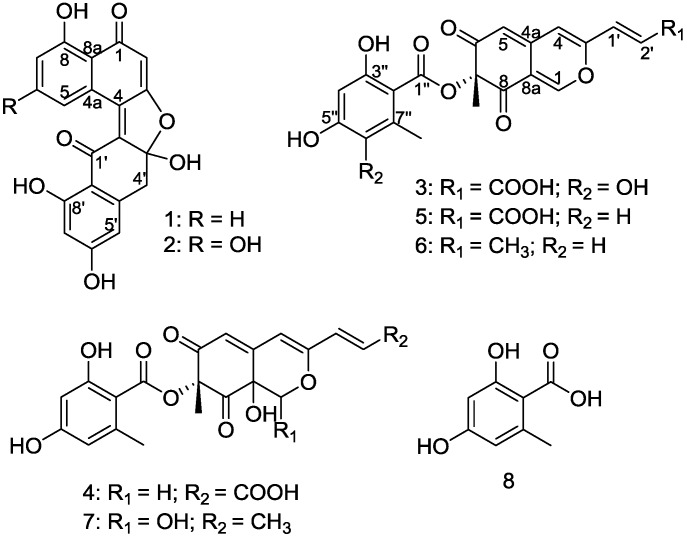
Racemic dinaphthalenone derivatives, (±)-asperlone A (1) and (±)-asperlone B (2), and two new azaphilones, 6″-hydroxy-(R)-mitorubrinic acid (3) and purpurquinone D (4), along with four known compounds, (−)-mitorubrinic acid (5), (−)-mitorubrin (6), purpurquinone A (7) and orsellinic acid (8), were isolated from the cultures of Aspergillus sp. 16-5C. The structures were elucidated using comprehensive spectroscopic methods, including 1D and 2D NMR spectra and the structures of 1 further confirmed by single-crystal X-ray diffraction analysis, while the absolute configuration of 3 and 4 were determined by comparing their optical rotation and CD with those of the literature, respectively. Compounds 1, 2 and 6 exhibited potent inhibitory effects against Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB) with IC50 values of 4.24 ± 0.41, 4.32 ± 0.60 and 3.99 ± 0.34 μM, respectively.
Keywords: marine fungi, Aspergillus sp., asperlones, azaphilones, MptpB inhibitor
1. Introduction
Tuberculosis (TB) ranks as the second leading cause of death from an infectious disease worldwide; an estimated 9.0 million people developed TB and 1.5 million died from the disease in 2013, according to the WHO [1]. In recent years, extensively drug-resistant TB (DR-TB), multidrug-resistant TB (MDR-TB) and HIV-associated TB have made clinical treatment even more difficult and complex. New chemotherapeutic approaches and unusual anti-infective agents are in urgent need, especially those applying to new targets and based on different mechanisms. Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB) is secreted by the microbe and manipulates host signal transduction pathways, which has proven to be an essential virulence factor when M. tuberculosis hosts macrophages [2,3,4,5,6]. Increased research reveals that it exhibits unique and multiple activities against immune responses [7,8,9,10,11,12,13]. Therefore, finding new inhibitors of MptpB could be a promising strategy against M. tuberculosis infection and conducive to the treatment of TB.
As part of our ongoing investigation on unusual biological activity compounds from mangrove endophytic fungi collected from the South China Sea [14,15,16,17,18,19,20], a mangrove endophytic fungus, named Aspergillus sp. 16-5C, attracted our attention. During the course of our investigation on the chemical constituents from the fungus, four new compounds, (±)-asperlones A (1) and B (2), 6″-hydroxy-(R)-mitorubrinic acid (3) and purpurquinone D (4), together with four known compounds, (−)-mitorubrinic acid (5), (−)-mitorubrin (6), purpurquinone A (7) and orsellinic acid (8) (Scheme 1), were isolated. In this report, we describe the isolation, structural elucidation, biosynthetic pathways and biological activity of the metabolites.
Scheme 1.
Chemical structures of compounds (1)–(8).
2. Results and Discussion
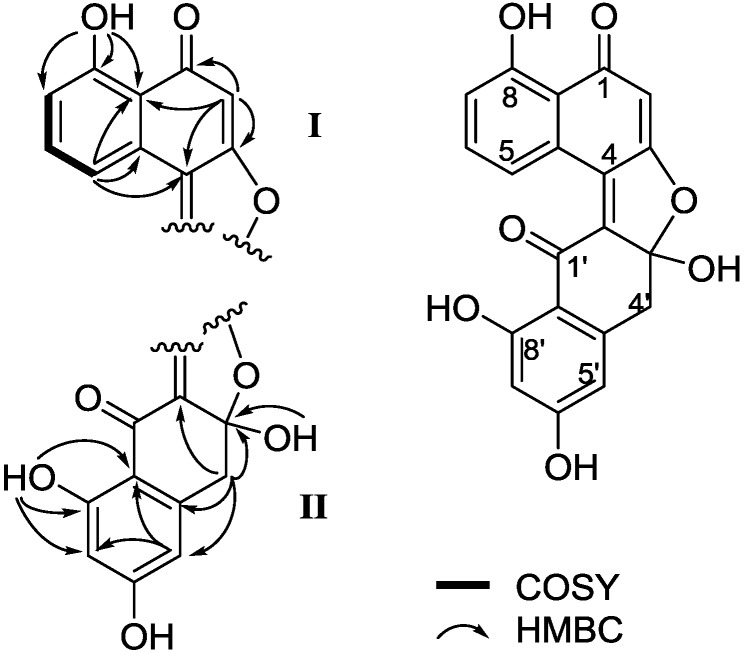
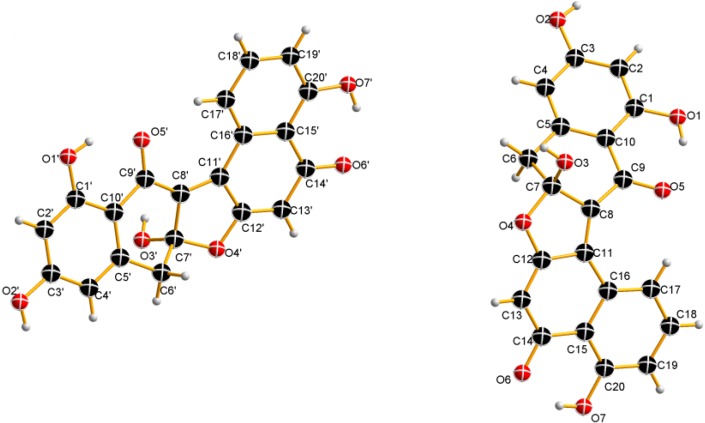
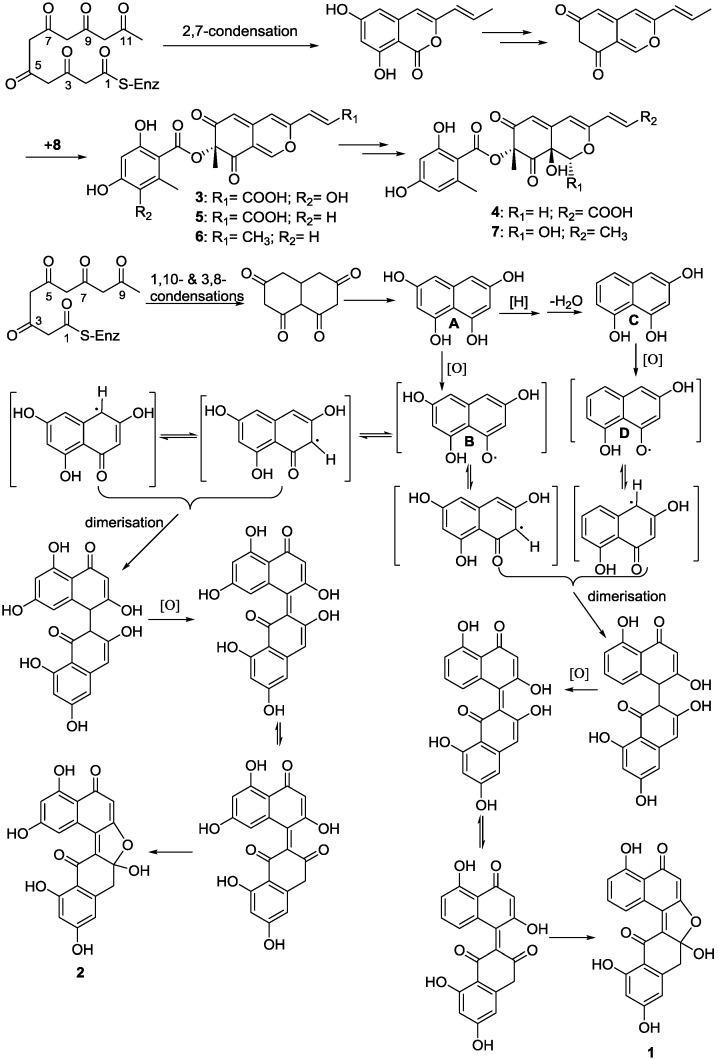
(±)-Asperlone A (1) was obtained as a red crystal (MeOH). The molecular formula C20H12O7 results from the HR-ESI mass spectrum (m/z = 363.0512, [M − H]−) and indicates 15 degrees of unsaturation. The presence of hydroxyl and carbonyl groups is shown by IR absorbtion bands at νmax 3394 and 1647 cm−1. The 1H NMR spectrum exhibits 12 proton signals in DMSO-d6, which revealed the presence of six aromatic/quinoid protons (δH 6.17, 6.25, 6.40, 7.20, 7.68 and 8.80), a methylene group (δH 3.41/3.58) and four exchangeable protons (δH 8.23, 11.30, 12.84, 13.58). The 13C NMR spectrum exhibits 20 carbon signals (Table 1). Besides the proton attached carbon atoms, the signals of 13 quaternary carbon atoms are observable, including two carbonyl groups (δC 186.7 and 191.8). Proton and carbon signal assignments result from the HSQC. Interpretation of the 1H–1H COSY indicated the presence of one substructure (bolded lines in Figure 1). In the HMBC spectrum, correlations from the quinoid proton at δH 6.17 (H-2) to C-1 (δC 191.8), C-3 (δC 171.6), C-4 (δC 129.8), C-8a (δC 113.8) and from aromatic proton at δH 8.80 (H-5) to C-4, C-4a (δC 128.4) and C-8a established the structure of fragment I. HMBC correlation from the signal at δH 8.23 (OH-3′) to the quaternary carbon signal (δC 113.5) suggested that C-3′ is a hemiacetal carbon. Furthermore, HMBC correlations from the methylene signals at δH 3.41/3.58 (H2-4′) to C-2′ (δC 142.7), C-3′, C-4a′ (δC 144.0), C-5′ (δC 110.4), from the exchangeable proton at δH 12.84 (OH-8′) to C-7′ (δC 101.6), C-8′ (δC 167.3), C-8a′ (δC 112.0), and from the aromatic proton at δH 6.40 (H-5′) to C-7′ and C-8a′ established the structure of fragment II. Taken together, the planar structure of 1 was defined (Figure 1). No CD (circular dichroism) spectrum could be obtained from 1, indicating a racemic mixture of the possible enantiomers, which results from the center of chirality (C-3′) of the hemiketal. Ultimately, the structure of 1 was subsequently confirmed by single-crystal X-ray diffraction experiments using Cu Kα radiation (Figure 2), named (±)-asperlone A. (±)-Asperlone A were attempted to separate by chiral HPLC and using four types of chiral columns, but all were unsuccessful.
Table 1.
13C NMR (100 MHz) and 1H NMR (400 MHz) data of 1 and 2 (DMSO-d6).
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) | |
| 1 | 191.8, qC | 190.5, qC | ||
| 2 | 102.1, CH | 6.17 s | 101.6, CH | 6.03 s |
| 3 | 171.6, qC | 170.6, qC | ||
| 4 | 129.8, qC | 130.2, qC | ||
| 4a | 128.4, qC | 129.8, qC | ||
| 5 | 121.8, CH | 8.80 d (7.8) | 111.2, CH | 8.34 d (2.2) |
| 6 | 135.3, CH | 7.68 m | 163.4, qC | |
| 7 | 122.5, CH | 7.20 d (8.3) | 106.7, CH | 6.47 d (2.2) |
| 8 | 162.1, qC | 164.4, qC | ||
| 8a | 113.8, qC | 107.2, qC | ||
| 1′ | 186.7, qC | 186.5, qC | ||
| 2′ | 142.7, qC | 142.2, qC | ||
| 3′ | 113.5, qC | 113.2, qC | ||
| 4′ | 41.0, CH2 | 3.41 d (15.7); 3.58 d (15.7) | 41.1, CH2 | 3.39 d (15.8); 3.55 d (15.8) |
| 4a′ | 144.0, qC | 144.0, qC | ||
| 5′ | 110.4, CH | 6.40 d (1.6) | 110.4, CH | 6.40 d (1.6) |
| 6′ | 167.1, qC | 167.1, qC | ||
| 7′ | 101.6, CH | 6.25 d (1.6) | 101.6, CH | 6.25 d (1.6) |
| 8′ | 167.3, qC | 167.0, qC | ||
| 8a′ | 112.0, qC | 112.0, qC | ||
| 6-OH | 10.83 brs | |||
| 8-OH | 13.58 s | 13.76 s | ||
| 3′-OH | 8.23 brs | 8.14 brs | ||
| 6′-OH | 11.30 brs | 11.17 brs | ||
| 8′-OH | 12.84 s | 12.93 s | ||
Figure 1.
COSY and key HMBC correlations of (±)-asperlone A.
Figure 2.
Perspective ORTEP illustrations of (±)-asperlone A.
(±)-Asperlone B (2), isolated as a red amorphous powder, exhibits one oxygen more than 1, resulting in the molecular formula C20H12O8 (HR-ESIMS m/z = 379.0461, [M − H]−). The close resemblance between the NMR spectra of 1 and 2 indicated that 2 was another dinaphthalenone derivative and the major difference was the 13C NMR chemical shift of C-6 increased from 135.3 to 163.4, while the 1H NMR chemical shifts of H-5/7 (δH 8.80 (d, 7.8)/ 7.20 (d, 8.3)) reduced to 8.34 (d, 2.2) and 6.47 (d, 2.2), respectively. The aromatic proton in 1 (δH 7.68, H-6) was replaced by a hydroxyl in 2 (δH 10.83), which suggested that 2 is a 6-oxygenated derivative of 1. The HMBC correlations from H-5 (δH 8.34) and H-7 (δH 6.47) to this aromatic carbon (δC 163.4) further confirmed that 2 was 6-hydroxyasperlone A. The absence of any CD spectrum indicating that 2 is also a racemic mixture. Unfortunately, the single crystal of compound 2 was unable to be obtained and the structure was named (±)-asperlone B. Resolution of the separation of 2 was also unsuccessful.
In most cases, natural products are produced in optically pure form, with only one enantiomer biosynthesized. Enantiomerically opposite products are also metabolized, but at a rare occurrence of less than 1% relative to the overall abundance of natural products, which often result from the action of stereochemically distinct enzymes that can give single and opposite enantiomeric products from achiral substrates [21,22].
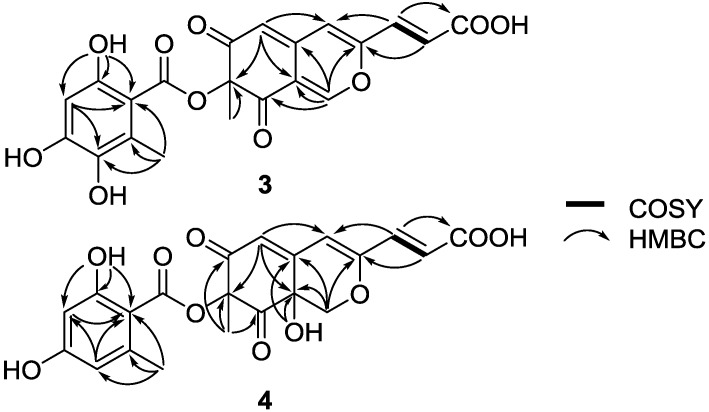
6″-Hydroxy-(R)-mitorubrinic acid (3) isolated as a yellow power was assigned the molecular formula C21H16O10 on the basis of HR-ESIMS (m/z = 427.0669, [M − H]−), consistent with 14 degrees of unsaturation. The IR spectrum showed the presence of a hydroxy (3425 cm−1) and a conjugated carbonyl (1722 and 1624 cm−1). The 1H NMR spectrum (Table 2) showed six olefinic protons (δH 5.73, 6.24, 6.43, 7.14, 7.28 and 8.32), four hydroxy signals (δH 8.00, 9.68, 10.28, and 12.86) and two methyl signals (δH 1.56 and 2.36). In addition, the 13C NMR spectrum exhibits two carbonyl groups (δC 192.2 and 192.6), suggesting the presence of an azaphilone core and an orsellinic acid [23] moiety for 3, analogous to (−)-mitorubrinic acid (5) [24]. The differences in NMR data between 3 and 5 could be explained by the replacement of an aromatic proton in 5 with a phenolic hydroxyl group (δH 8.00) in 3, indicating that 3 is the hydroxy derivative of 5. The key HMBC correlations from H-4″ (δH 6.24) and CH3-7″ (δH 2.36) to C-6″ (δC 137.3) suggested that the hydroxylation occurs at C-6″ (Figure 3). The optical rotation of = −267.8 clearly revealed the 7R-configuration, as same as 5. Thus, the structure of 3 was elucidated as 6″-hydroxy-(R)-mitorubrinic acid.
Table 2.
13C NMR (100 MHz) and 1H NMR (400 MHz) data of 3 and 4 (DMSO-d6).
| Position | 3 | 4 | ||
|---|---|---|---|---|
| δC, mult. | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 155.5, CH | 8.32 s | 69.8, CH2 | 3.96 d (12.4); 4.68 d (12.4) |
| 3 | 153.3, qC | 156.7, qC | ||
| 4 | 116.2, CH | 7.14 s | 109.1, CH | 6.46 s |
| 4a | 142.4, qC | 148.1, qC | ||
| 5 | 109.2, CH | 5.73 s | 120.2, CH | 6.21 s |
| 6 | 192.2, qC | 192.2, qC | ||
| 7 | 85.5, qC | 84.1, qC | ||
| 7-CH3 | 22.3, CH3 | 1.56 s | 23.9, CH2 | 1.82 s |
| 8 | 192.6, qC | 200.0, qC | ||
| 8a | 114.7, qC | 66.2, qC | ||
| 1′ | 134.0, CH | 7.28 d (15.7) | 136.7, CH | 7.13 d (15.6) |
| 2′ | 125.0, CH | 6.43 d (15.7) | 124.2, CH | 6.34 d (15.6) |
| 3′ | 166.6, qC | 167.0, qC | ||
| 1″ | 168.7, qC | 168.2, qC | ||
| 2″ | 105.0, qC | 105.1, qC | ||
| 3″ | 155.2, qC | 163.1, qC | ||
| 4″ | 101.0, CH | 6.24 s | 101.0, CH | 6.16 d (2.0) |
| 5″ | 152.2, qC | 162.8, qC | ||
| 6″ | 137.3, qC | 111.6, CH | 6.24 d (2.0) | |
| 7″ | 126.2, qC | 142.8, qC | ||
| 7″-CH3 | 14.6, CH2 | 2.36 s | 23.1, CH2 | 2.44 s |
| 8a-OH | 7.26 s | |||
| 3′-OH | 12.86 brs | 12.82 brs | ||
| 3″-OH | 9.68 s | 10.26 s | ||
| 5″-OH | 10.28 brs | 10.32 brs | ||
| 6″-OH | 8.00 brs | |||
Figure 3.
COSY and key HMBC correlations of 3 and 4.
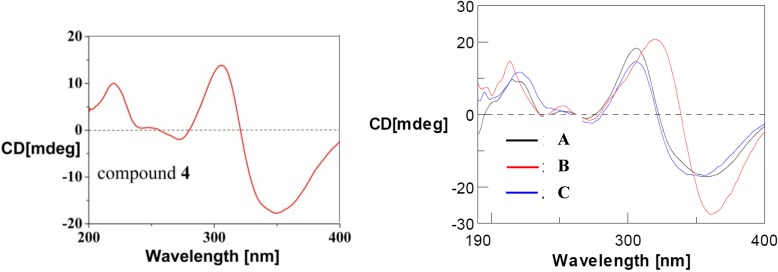
Purpurquinone D (4) was obtained as a yellowish powder and was assigned the molecular formula C21H18O10 from HR-ESIMS (m/z = 429.0824, [M − H]−), with one oxygen atom and two hydrogen atoms more than that of 5 [24]. Analysis of the NMR data for 4 (Table 2) revealed the presence of similar structural features to those found in 5, except that an olefinic double bond was replaced by a methylene (δH/δC 3.96, 4.68/69.8) and an oxygenated quaternary carbon (δC 66.2), indicating that 4 is the hydroxylated derivative of 5. In addition, the key HMBC correlations from OH-8a (δH 7.26) to C-4a/8a/1 (δC 148.1/66.2/69.8), and from H2-1 (δH 3.96, 4.68) to C-4a/8a/3 (δC 148.1/66.2/156.7) revealed that the hydration occurs at C1–C8a double bond (Figure 3). The NOESY correlations among OH-8a and H3-7 (δH 1.82) suggested a cis-configuration between OH-8a and H3-7. Further more, the similar CD Cotton effects (Figure 4) at 350 nm (Δε −23.1), 305 nm (Δε +18.1), and 220 nm (Δε +13.0) indicated that 4 has the same (7R, 8aS)-configuration as purpurquinone C [25]. Thus, the structure of 4 was identified and named purpurquinone D.
Figure 4.
CD curves of compound 4 and purpurquinones A–C.
The remaining four known compounds were identified as (−)-mitorubrinic acid (5) [24], (−)-mitorubrin (6) [24], purpurquinone A (7) [25] and orsellinic acid (8) [23], respectively, by comparison of their NMR and MS data with those reported.
In light of our hypothesis that all these compounds are the product of a fungal polyketide synthase [26]. The unusual dinaphthalenone derivatives, named (±)-asperlones A (1) and B (2), are presumed to be started from five acetate units through a series of polyketide biosynthesis, reduction, dehydration, oxidation and dimerization [27,28,29], as shown in Scheme 2. The initial condensations of tetraoxodecanoic acid and further reduction leading to compound A, followed by oxidation to generate compound B and subsequent dimerization, oxidation and rearrangement to generate (±)-asperlone B (2). On the other way, the reduction and dehydration of A leading to C and further oxidation to generate D. Subsequent dimerization of the rearrangement structure of B and D afford the dinaphthalenone skeleton and then further oxidize and rearrange to generate (±)-asperlone A (1).
Scheme 2.
Proposed biosynthetic pathways for compounds (1)–(7).
The activity of compounds 1–3 and 5–6 against MptpB was evaluated with sodium orthovanadate as the positive control (Table 3). The results showed that compounds (±)-1, (±)-2 and 6 were strong inhibitors of of MptpB with IC50 value of 4.24 ± 0.41, 4.32 ± 0.60 and 3.99 ± 0.34 μM, respectively, which revealed that isolated compounds 1, 2 and 6 could be potential antituberculosis drugs.
Table 3.
Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB) assay for 1–3 and 5–6.
| Compound | (±)-1 | (±)-2 | 3 | 5 | 6 | Positive Control |
|---|---|---|---|---|---|---|
| IC50 (μM) | 4.24 ± 0.41 | 4.32 ± 0.60 | >50 | >50 | 3.99 ± 0.34 | 0.05 |
3. Experimental Section
3.1. General
Melting points were measured on an X-4 micromeltin-point apparatus (Cany Precision Instruments Co., Ltd., Shanghai, China) and are uncorrected. Optical rotations were determined with a RUDOLPH Autopol III polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA) at 20 °C. UV data were measured on a PERSEE TU-1900 spectrophotometer (Purkinje General Instrument Co., Ltd., Beijing, China). IR spectra were measured on a Nicolet Nexus 670 spectrophotometer (Thermo Fisher Scientific, Inc., Hudson, NH, USA), in KBr discs. CD data were measured on a Chirascan™ CD spectrometer (Applied Photophysics, London, UK). 1H and 13C NMR data were recorded on a Bruker AVANCE 400 spectrometer (Bruker BioSpin Corporation, Billerica, MA, USA) in DMSO-d6. ESIMS spectra were obtained from a Micro mass Q-TOF spectrometer (Waters Corporation, Milford, MA, USA). HR-ESIMS spectra were measured on a Thermo Scientific LTQ Orbitrap Elite high-resolution mass spectrometer (Thermo Fisher Scientific, Inc., Hudson, NH, USA). Silica gel (Qing Dao Hai Yang Chemical Group Co., Qingdao, China; 200–300 mesh), octadecylsilyl silica gel (Unicorn, Palarivattom, Kerala, India; 45 μm) and Sephadex LH-20 (GE Healthcare, Buckinghamshire, UK) were used for column chromatography. Precoated silica gel plates (Qing Dao Huang Hai Chemical Group Co., Qingdao, China; G60, F-254) were used for thin layer chromatography.
3.2. Fungal Material
The fungus used in this study was isolated from a mangrove endophytic fungus from the leaves of S. apetala, which were collected in Hainan Island, China. The fungus was identified as Aspergillus sp. according to a molecular biological protocol by DNA amplification and sequencing of the ITS region (deposited in GenBank, accession No. JX993829). A voucher strain was deposited in School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou, China, with the access code, 16-5C.
3.3. Extraction and Isolation
The fungal strain Aspergillus sp. 16-5C was cultivated in potato glucose liquid medium (15 g glucose and 3 g sea salt in 1 L potato infusion) in 1 L Erlenmeyer flasks, each containing 300 mL of culture broth, at 28 °C without shaking for 30 days. The culture (50 L) was filtered to separate the culture broth from the mycelia, and then the mycelia were extracted three times with EtOAc. The organic solvent was filtered and concentrated under reduced pressure to yield 4.7 g of organic extract, which was subjected to silica gel column chromatography (CC) (petroleum ether-EtOAc from 90:10 to 0:100 (v/v), gradient) to generate six fractions (Fr. 1–6). Fr.2 was further purified by silica gel CC using 30% EtOAc-light petroleum, then subjected to Sephadex LH-20 CC eluting with CHCl3/MeOH (1:1) to obtain compounds 1 (1.0 mg) and 2 (1.5 mg). Meanwhile, Fr. 4 was further purified silica gel CC using 40% EtOAc-light petroleum to afford seven subfractions (Frs. 4.1–4.7). Fr.4.3 was applied to Sephadex LH-20 CC, eluted with CHCl3/MeOH (1:1), to obtain two subfractions (Frs. 4.3.1–4.3.2). Fr. 4.3.2 was further purified by RP-HPLC (55% MeOH in H2O, 2.0 mL/min) to afford 3 (6.3 mg, tR 14.5 min) and 4 (0.6 mg, tR 21.0 min).
Compound (±)-1: Red crystal, m.p. 172–173 °C (MeOH); UV (MeOH) (λmax) 224, 325 and 458 nm; 1H and 13C NMR spectroscopic data, see Table 1; HR-ESIMS m/z 363.0512 ([M − H]−, C20H11O7, calcd 363.0510). Crystal data: C20H12O7, Mr = 364.30, Triclinic, a = 7.4538 (5) Å, b = 12.9674 (7) Å, c = 15.7108 (10) Å, α =89.036 (5)°, β = 89.417 (5)°, γ = 80.470 (5)°, V = 1497.34 (16) Å3, space group P-1, Z = 4, Dx = 1.616 mg/m3, μ (Cu Kα) = 1.051 mm−1, and F (000) = 752. Crystal dimensions: 0.32 mm × 0.24 mm × 0.15 mm. Independent reflections: 5396 (Rint = 0.0287). The final R1 values were 0.0356, wR2 = 0.0875 [I > 2σ(I)]. The X-ray diffraction data were collected at 150 K on an Gemini S Ultra (Oxford Diffraction Ltd., Oxfordshire, UK) with Cu Kα radiation (λ = 1.54178 Å). Structures were identified using direct methods (SHELXS-97) and refined using full-matrix least-squares difference Fourier techniques. H atoms bonded to C were placed on geometrically ideal positions by the “ride on” method, while H atoms bonded to O were located by the difference Fourier method and included in the calculation of structure factors with isotropic temperature factors. The crystallographic data obtained were deposited in the Cambridge Crystallographic Data Centre (Cambridge, UK). Copies of the data may be obtained free of charge through an application addressed to The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-(0)1223-336033, or E-mail: deposit@ccdc.cam.ac.uk). CCDC number: 979498.
Compound (±)-2: Red amorphous powder; m.p. >300 °C; UV (MeOH) (λmax) 210, 247, 339 and 441 nm; 1H and 13C NMR spectroscopic data, see Table 1; HR-ESIMS m/z 379.0461 ([M − H]−, C20H11O8, calcd. 379.0459).
Compound 3: Yellow power; m.p. >300 °C; −267.8 (c 0.28, MeOH); UV (MeOH) (λmax) 225, 260 and 342 nm; 1H and 13C NMR spectroscopic data, see Table 2; HR-ESIMS m/z 427.0669 ([M − H]−, C21H15O10, calcd. 427.0671).
Compound 4: Yellow power; m.p. >300 °C; −681.8 (c 0.11, MeOH); UV (MeOH) (λmax) 215, 268 and 354 nm; 1H and 13C NMR spectroscopic data, see Table 2; HR-ESIMS m/z 429.0824 ([M − H]−, C21H17O10, calcd. 429.0827).
3.4. Materials and Methods for mPTPB Assay
3.4.1. Cloning, Expression and Purification of MptpB
The full-length PTPB gene was amplified from genomic DNA of the Mtb H37Rv strain (School of Life Sciences, Sun Yat-sen University: Guangzhou, China). PCR products were cloned in frame with an N-terminal His6 tag into the pET28a (+) vector (Novagen, Merck KGaA, Darmstadt, Germany). For protein expression, plasmids were transformed into Escherichia coli BL21(DH3) cells (Invitrogen, Thermo Fisher Scientific, Inc., Hudson, NH, USA) and grown in LB medium containing 50 μg/mL kanamycin at 37 °C till the OD600 of the solution was about 0.6. After the addition of 0.1 mM IPTG, the culture was grown for another 16 h at 20 °C. The cells were harvested by centrifugation at 5000 rpm for 5 min at 4 °C. The bacterial cell pellets were resuspended in the buffer containing 20 mM Tris, pH 7.9, 500 mM NaCl, 5 mM imidazole and were lysed by sonication on ice. Cellular debris was removed by centrifugation at 10,000 rpm for 30 min at 4 °C. The protein was purified from the supernatant using glutathione-Sepharose 4B (GE Healthcare, Buckinghamshire, UK), according to the manufacturer’s instructions. Protein concentration was determined using the Bradford dye binding assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturer’s recommendations, with bovine serum albumin as the standard. The purified MptpB was stored in 20% glycerol at −20 °C.
3.4.2. MptpB Inhibition Assay
The inhibition assays were performed using the RediPlate 96 EnzChek Tyrosine Phosphatase Assay kit (Invitrogen, Thermo Fisher Scientific, Inc., Hudson, NH, USA) by monitoring the hydrolysis of the fluorogenic phosphatase substrate, 6,8-difluoro-methylumbelliferyl phosphate (DiFMUP), according to the manufacturer’s instruction. The IC50 value was determined at five different substrate concentrations by non-linear regression fitting of the inhibitor concentration versus inhibition rate. All measurements were done in triplicate from three independent experiments. The reported IC50 were the average value of three independent experiments.
4. Conclusions
Aspergillus sp. 16-5C, an endophytic fungus from the South China Sea, was proven as a prolific producer of bioactive metabolites. Two unusual pairs of enantiomers of dinaphthalenone derivatives and two new azaphilones, together with four known compounds, were isolated from this fungal strain, and the structures of 1–8 were elucidated primarily by NMR experiments. The structure of 1 confirmed by single-crystal X-ray diffraction analysis, while 3 was determined by the optical rotation and 4 was assigned on the basis of the CD and NOESY spectra. The biosynthetic pathways for all these compounds were proposed and suggested that the secondary metabolites are the product of a fungal polyketide synthase. In the bioassay, the isolated compounds were evaluated for their inhibitory activity against MptpB; compounds 1, 2 and 6 exhibited strong inhibitory activity that suggested they could represent a new type of lead compounds for the development of new anti-tuberculosis drugs.
Acknowledgments
We thank the National Natural Science Foundation of China (41406134, 41276146, 21472251), the Science and Technology Plan Project of Guangdong Province of China (2013B021100011), Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (GD2012-D01-001), the China Postdoctoral Science Foundation (2014M552281) and China’s Marine Commonweal Research Project (201305017) for generous support.
Author Contributions
Conceived and designed the experiments: ZS, XH, ZX, SL, YL, LH, CT. Performed the experiments: ZX, XH, SL, LH, CT. Analyzed the data: ZX, SL, XH. Wrote the paper: ZX, XH, SL. Read and approved the final manuscript: ZS, XH, ZX.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Global Tuberculosis Report 2014. [(assessed on 17 July 2014)]. Available online: http://www.who.int/tb/publications/global_report/en/
- 2.Butler D. New fronts in an old war. Nature. 2000;406:670–672. doi: 10.1038/35021291. [DOI] [PubMed] [Google Scholar]
- 3.Singh R., Rao V., Shakila H., Gupta R., Khera A., Dhar N., Singh A., Koul A., Singh Y., Naseema M., et al. Disruption of mptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol. Microbiol. 2003;50:751–762. doi: 10.1046/j.1365-2958.2003.03712.x. [DOI] [PubMed] [Google Scholar]
- 4.Bialy L., Waldmann H. Inhibitors of Protein Tyrosine Phosphatases: Next-Generation Drugs? Angew. Chem. Int. Ed. 2005;44:3814–3839. doi: 10.1002/anie.200461517. [DOI] [PubMed] [Google Scholar]
- 5.Singh R., Singh A., Tyagi A.K. Deciphering the genes involved in pathogenesis of Mycobacterium tuberculosis. Tuberculosis. 2005;85:325–335. doi: 10.1016/j.tube.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Castandet J., Prost J.F., Peyron P., Astarie-Dequeker C., Anes E., Cozzone A.J., Griffiths G., Maridonneau-Parini I. Tyrosine phosphatase MptpA of Mycobacterium tuberculosis inhibits phagocytosis and increases actin polymerization in macrophages. Res. Microbiol. 2005;156:1005–1013. doi: 10.1016/j.resmic.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Beresford N., Patel S., Armstrong J., Szöor B., Fordham-Skelton A.P., Tabernero L. MptpB, a virulence factor from Mycobacterium tuberculosis, exhibits triple-specificity phosphatase activity. Biochem. J. 2007;406:13–18. doi: 10.1042/BJ20070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nören-Müller A., Wilk W., Saxena K., Schwalbe H., Kaiser M., Waldmann H. Discovery of a New Class of Inhibitors of Mycobacterium tuberculosis Protein Tyrosine Phosphatase B by Biology-Oriented Synthesis. Angew. Chem. Int. Ed. 2008;47:5973–5977. doi: 10.1002/anie.200801566. [DOI] [PubMed] [Google Scholar]
- 9.Beresford N.J., Mulhearn D., Szczepankiewicz B., Liu G., Johnson M.E., Fordham-Skelton A., Adab-Zapatero C., Cavet J.S., Tabernero L.J. Inhibition of MptpB phosphatase from Mycobacterium tuberculosis impairs mycobacterial survival in macrophages. Antimicrob. Chemother. 2009;63:928–936. doi: 10.1093/jac/dkp031. [DOI] [PubMed] [Google Scholar]
- 10.Vintonyak V.V., Warburg K., Kruse H., Grimme S., Hubel K., Rauh D., Waldmann H. Identification of Thiazolidinones Spiro-Fused to Indolin-2-ones as Potent and Selective Inhibitors of the Mycobacterium tuberculosis Protein Tyrosine Phosphatase B. Angew. Chem. Int. Ed. 2010;49:5902–5905. doi: 10.1002/anie.201002138. [DOI] [PubMed] [Google Scholar]
- 11.Ecco G., Vernal J., Razzera G., Martins P.A., Matiollo C., Terenzi H. Mycobacterium tuberculosis tyrosine phosphatase A (PtpA) activity is modulated by S-nitrosylation. Chem. Commun. 2010;46:7501–7503. doi: 10.1039/c0cc01704c. [DOI] [PubMed] [Google Scholar]
- 12.Silva A.P.G., Tabernero L. New strategies in fighting TB: Targeting Mycobacterium tuberculosis-secreted phosphatases MptpA & MptpB. Future Med. Chem. 2010;2:1325–1337. doi: 10.4155/fmc.10.214. [DOI] [PubMed] [Google Scholar]
- 13.Zhou B., He Y., Zhang X., Xu J., Luo Y., Wang Y., Franzblau S.G., Yang Z., Chan R.J., Liu Y., et al. Targeting Mycobacterium protein tyrosine phosphatase B for antituberculosis agents. Proc. Natl. Acad. Sci. USA. 2010;107:4573–4578. doi: 10.1073/pnas.0909133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen L., Cai X., Xu F., She Z., Chan W., Vrijmoed L.L.P., Jones E.B.G., Lin Y. Three Metabolites from the Mangrove Endophytic Fungus Sporothrix sp. (#4335) from the South China Sea. J. Org. Chem. 2009;74:1093–1098. doi: 10.1021/jo802096q. [DOI] [PubMed] [Google Scholar]
- 15.Huang H., Feng X., Xiao Z., Liu L., Li H., Ma L., Lu Y., Ju J., She Z., Lin Y. Azaphilones and p-Terphenyls from the Mangrove Endophytic Fungus Penicillium chermesinum (ZH4-E2) Isolated from the South China Sea. J. Nat. Prod. 2011;74:997–1002. doi: 10.1021/np100889v. [DOI] [PubMed] [Google Scholar]
- 16.Li H., Huang H., Shao C., Huang H., Jiang J., Zhu X., Liu Y., Liu L., Lu Y., Li M., et al. Cytotoxic Norsesquiterpene Peroxides from the Endophytic Fungus Talaromyces flavus Isolated from the Mangrove Plant Sonneratia apetala. J. Nat. Prod. 2011;74:1230–1235. doi: 10.1021/np200164k. [DOI] [PubMed] [Google Scholar]
- 17.Wang C., Wang J., Huang Y.H., Chen H., Li Y., Zhong L.L., Chen Y., Chen S.P., Wang J., Kang J.L., et al. Anti-mycobacterial activity of marine fungus-derived 4-deoxybostrycin and nigrosporin. Molecules. 2013;18:1728–1740. doi: 10.3390/molecules18021728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X.S., Huang H.B., Li H.X., Sun X.F., Huang H.R., Lu Y.J., Lin Y.C., Long Y.H., She Z.G. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5C. Org. Lett. 2013;15:721–723. doi: 10.1021/ol303549c. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z., Huang H., Shao C., Xia X., Ma L., Huang X., Lu Y., Lin Y., Long Y., She Z. Asperterpenols A and B, New Sesterterpenoids Isolated from a Mangrove Endophytic Fungus Aspergillus sp. 085242. Org. Lett. 2013;15:2522–2525. doi: 10.1021/ol401005j. [DOI] [PubMed] [Google Scholar]
- 20.Li H.X., Jiang J.Y., Liu Z.M., Lin S.E., Xia G.P., Xia X.K., Ding B., He L., Lu Y.J., She Z.G. Peniphenones A–D from the mangrove fungus Penicillium dipodomyicola HN4–3A as inhibitors of Mycobacterium tuberculosis Phosphatase MptpB. J. Nat. Prod. 2014;77:800–806. doi: 10.1021/np400880w. [DOI] [PubMed] [Google Scholar]
- 21.Miller K.A., Tsukamoto S., Williams R.M. Asymmetric total syntheses of (+)- and (−)-versicolamide B and biosynthetic implications. Nat. Chem. 2009;1:63–68. doi: 10.1038/nchem.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finefield J.M., Sherman D.H., Kreitman M., Williams R.M. Enantiomeric Natural Products: Occurrence and Biogenesis. Angew. Chem. Int. Ed. 2012;51:4802–4836. doi: 10.1002/anie.201107204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes T.I.B., Coelho R.G., Yoshida N.C., Honda N.K. Radical-Scavenging Activity of Orsellinates. Chem. Pharm. Bull. 2008;56:1551–1554. doi: 10.1248/cpb.56.1551. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J.L., Porco J.A. Asymmetric Syntheses of (−)-Mitorubrin and Related Azaphilone Natural Products. Org. Lett. 2006;8:5169–5171. doi: 10.1021/ol062233m. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Wang Y., Wang W., Fu P., Liu P.P., Zhu W.M. Anti-influenza Virus Polyketides from the Acid-Tolerant Fungus Penicillium purpurogenum JS03-21. J. Nat. Prod. 2011;74:2014–2018. doi: 10.1021/np2004769. [DOI] [PubMed] [Google Scholar]
- 26.Bell A.A., Wheeler M.H. Biosynthesis and function of fungal melanins. Ann. Rev. Phytopathol. 1986;24:411–415. doi: 10.1146/annurev.py.24.090186.002211. [DOI] [Google Scholar]
- 27.Bode H.B., Zeeck A. Sphaerolone and dihydrosphaerolone, two bisnaphthyl-pigments from the fungus Sphaeropsidales sp. F-24′707. Phytochemistry. 2000;54:597–601. doi: 10.1016/S0031-9422(00)00145-X. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y.L., Zhang J., Jiang N., Lu Y.H., Wang L., Xu S.H., Wang W., Zhang G.F., Xu Q., Ge H.M., et al. Immunosuppressive Polyketides from Mantis-Associated Daldinia eschscholzii. J. Am. Chem. Soc. 2011;133:5931–5940. doi: 10.1021/ja110932p. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y.L., Ge H.M., Zhao W., Dong H., Xu Q., Li S.H., Li J., Zhang J., Song Y.C., Tan R.X. Unprecedented Immunosuppressive Polyketides from Daldinia eschscholzii, a Mantis-Associated Fungus. Angew. Chem. Int. Ed. 2008;47:5823–5826. doi: 10.1002/anie.200801284. [DOI] [PubMed] [Google Scholar]