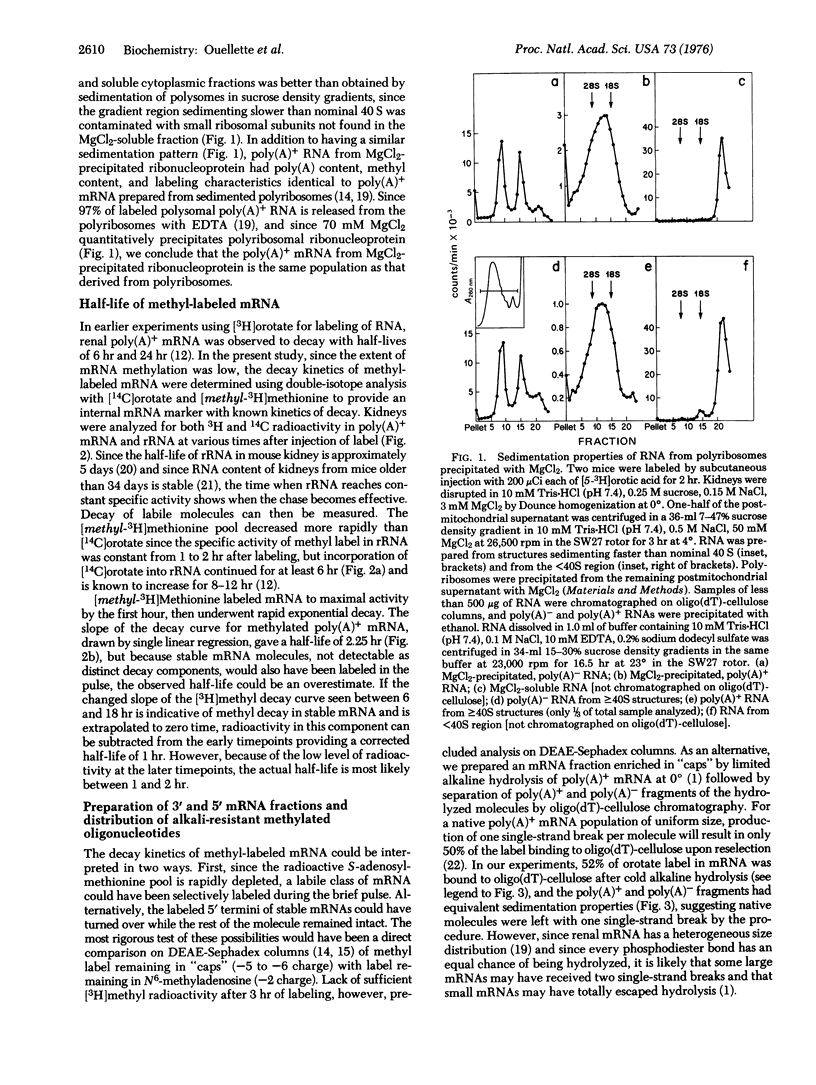
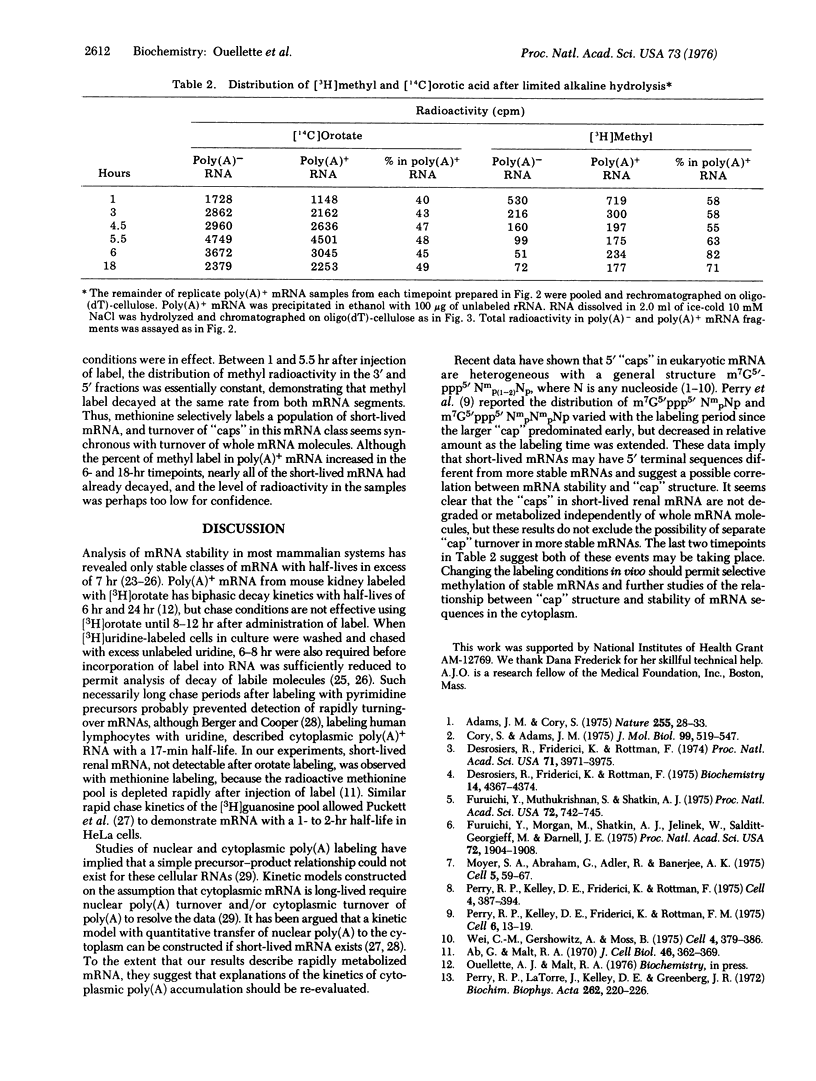
Abstract
In experiments originally designed to examine selective turnover of methylated "caps" in renal mRNA, we observed that [3H]methyl label decayed from mRNA containing poly(A) with a half-life of 1-2 hr. (Caps are blocked, methylated mRNA sequences of the general structure m7GpppNm p(1 or 2)Np.). To distinguish between metabolism of short-lived mRNA and discriminate turnover of "caps", we compared residual [3H]methyl label in 5' and 3'mRNA fragments prepared from mRNA isolated during the decay period. Hydrolysis of mRNA at 0 degrees with dilute KOH before oligo(dT)-cellulose selection produced 5' mRNA fragments enriched with an alkali-resistant oligonucleotide with a -5 charge; the 3' mRNA fraction was correspondingly reduced in oligonucleotide content. Since methyl label disappeared at the same rate from both fractions, we conclude that mouse kidney contains short-lived mRNA and that the "caps" of these labile mRNAs turn over with the rest of the mRNA molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ab G., Malt R. A. Metabolism of ribosomal precursor ribonucleic acid in kidney. J Cell Biol. 1970 Aug;46(2):362–369. doi: 10.1083/jcb.46.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. M., Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975 May 1;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Cooper H. L. Very short-lived and stable mRNAs from resting human lymphocytes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3873–3877. doi: 10.1073/pnas.72.10.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. The modified 5'-terminal sequences in messenger RNA of mouse myeloma cells. J Mol Biol. 1975 Dec 25;99(4):519–547. doi: 10.1016/s0022-2836(75)80170-7. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Shatkin A. J. 5'-Terminal m-7G(5')ppp(5')G-m-p in vivo: identification in reovirus genome RNA. Proc Natl Acad Sci U S A. 1975 Feb;72(2):742–745. doi: 10.1073/pnas.72.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Brawerman G. Pulse-labeled ribonucleic acid complexes released by dissociation of rat liver polysomes. Biochemistry. 1971 Feb 2;10(3):510–516. doi: 10.1021/bi00779a025. [DOI] [PubMed] [Google Scholar]
- Melvin W. T., Kumar A., Malt R. A. Conservation of ribosomal RNA during compensatory renal hypertrophy. A major mechanism in RNA accretion. J Cell Biol. 1976 Jun;69(3):548–556. doi: 10.1083/jcb.69.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Abraham G., Adler R., Banerjee A. K. Methylated and blocked 5' termini in vesicular stomatitis virus in vivo mRNAs. Cell. 1975 May;5(1):59–67. doi: 10.1016/0092-8674(75)90092-6. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Frederick D., Malt R. A. Methylated messenger RNA in mouse kidney. Biochemistry. 1975 Oct 7;14(20):4361–4367. doi: 10.1021/bi00691a003. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Kumar A., Malt R. A. Physical aspects and cytoplasmic distribution of messenger RNA in mouse kidney. Biochim Biophys Acta. 1976 Apr 2;425(4):384–395. doi: 10.1016/0005-2787(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Friderici K., Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5' terminus. Cell. 1975 Apr;4(4):387–394. doi: 10.1016/0092-8674(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Methylated constituents of heterogeneous nuclear RNA: presence in blocked 5' terminal structures. Cell. 1975 Sep;6(1):13–19. doi: 10.1016/0092-8674(75)90068-9. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Priestley G. C., Malt R. A. Development of the metanephric kidney. Protein and nucleic acid synthesis. J Cell Biol. 1968 Jun;37(3):703–715. doi: 10.1083/jcb.37.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett L., Chambers S., Darnell J. E. Short-lived messenger RNA in HeLa cells and its impace on the kinetics of accumulation of cytoplasmic polyadenylate. Proc Natl Acad Sci U S A. 1975 Jan;72(1):389–393. doi: 10.1073/pnas.72.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Gershowitz A., Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975 Apr;4(4):379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]



