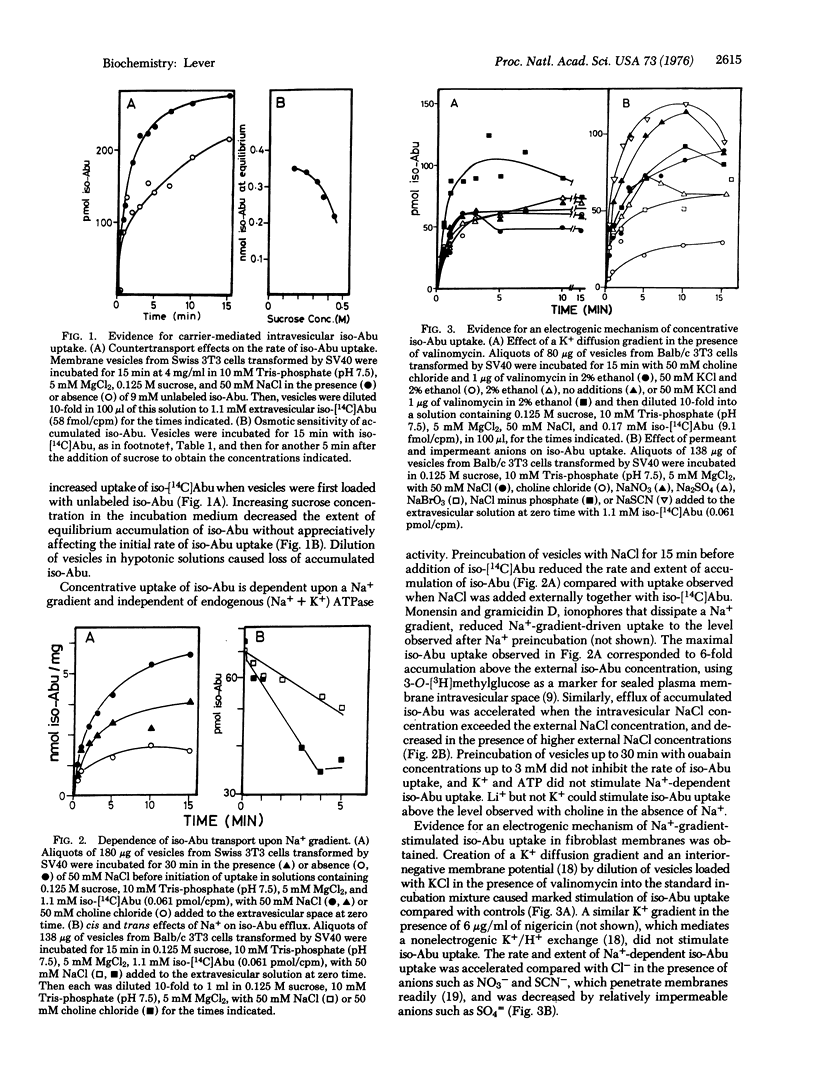
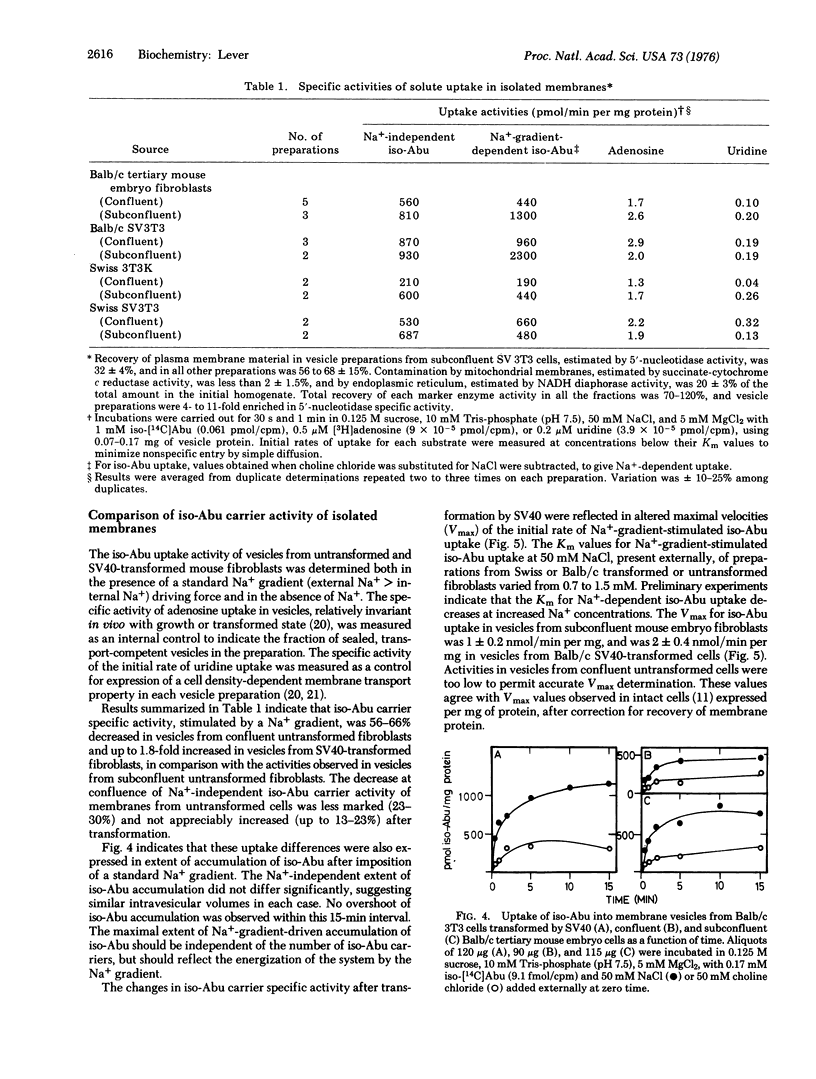
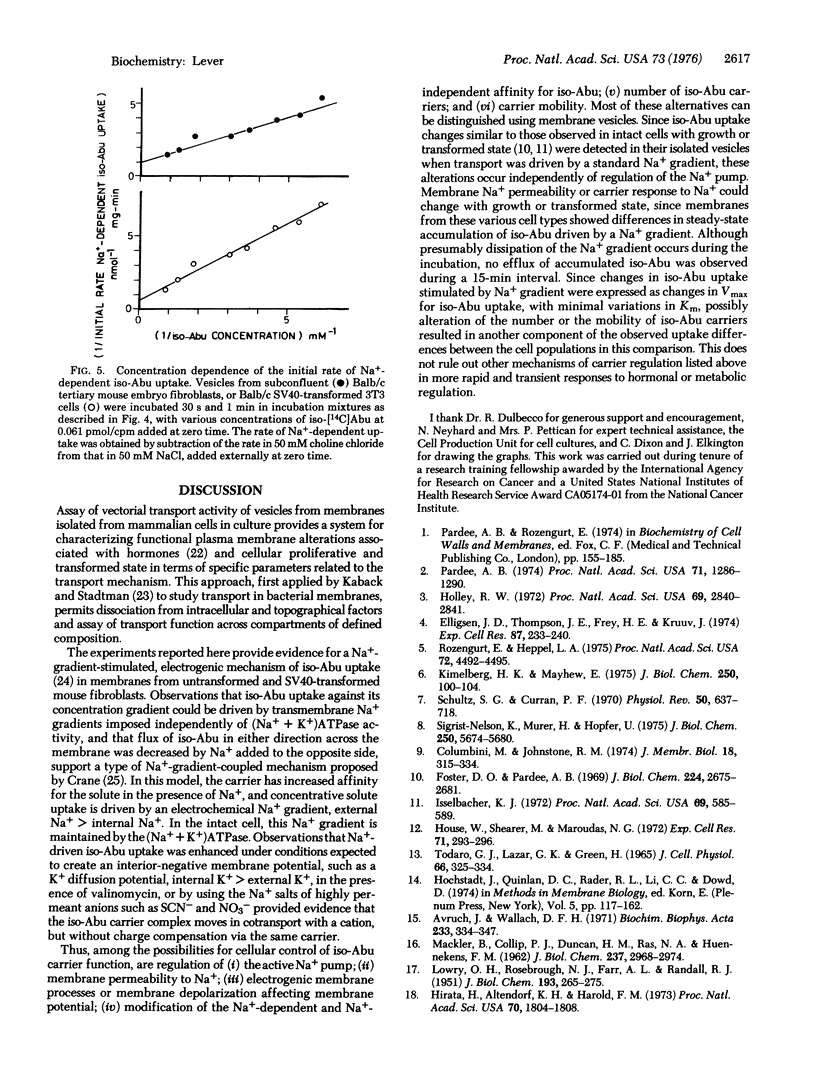
Abstract
Membrane vesicles isolated from untransformed Balb/c and Swiss mouse fibroblasts and from those transformed by simian virus 40 catalyzed carrier-mediated uptake of L-alpha-aminoisobutyric acid. Concentrative uptake required the presence of a Na+ gradient (external Na+ greater than internal Na+) and occurred independently of endogenous (Na+ + K+) ATPase activity. This process is electrogenic, since uptake was stimulated by a K+ diffusion gradient (internal greater external) in the presence of valinomycin or by the addition of the Na+ salt of a permeant ion, conditions expected to create an interior-negative membrane potential. Both the initial rate of concentrative uptake of L-alpha-aminoisobutyric acid and its maximal accumulation, driven by a standard Na+ gradient, were decreased in vesicles from density-inhibited, untransformed cells and increased in those from cells transformed by simian virus 40 compared with vesicles from proliferating untransformed cells. An increased maximal velocity (Vmax) of uptake stimulated by Na+ gradient was observed in vesicles from transformed cells compared with those from untransformed cells, suggesting an increase in the number of carriers or in their mobility. Since the relative extent of accumulation of this model amino acid driven by a standard Na+ gradient also differed with growth or transformed status, an additional possibility for cellular regulation of this process could be alteration of membrane Na+ permeability or carrier response to Na+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Colombini M., Johnstone R. M. Na+-gradient-stimulated AIB transport in membrane vesicles from Ehrlich ascites cells. J Membr Biol. 1974;18(3-4):315–334. doi: 10.1007/BF01870120. [DOI] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elligsen J. D., Thompson J. E., Frey H. E., Kruuv J. Correlation of (Na+--K+)-ATPase activity with growth of normal and transformed cells. Exp Cell Res. 1974 Aug;87(2):233–240. doi: 10.1016/0014-4827(74)90475-3. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- House W., Shearer M., Maroudas N. G. Method for bulk culture of animal cells on plastic film. Exp Cell Res. 1972;71(2):293–296. doi: 10.1016/0014-4827(72)90296-0. [DOI] [PubMed] [Google Scholar]
- Inui Y., Christensen H. N. Discrimination of single transport systems. The Na plus-sensitive transport of neutral amino acids in the Ehrlich cell. J Gen Physiol. 1966 Sep;50(1):203–224. doi: 10.1085/jgp.50.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K., Mayhew E. Increased ouabain-sensitive 86Rb+ uptake and sodium and potassium ion-activated adenosine triphosphatase activity in transformed cell lines. J Biol Chem. 1975 Jan 10;250(1):100–104. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan D. C., Hochstadt J. An altered rate of uridine transport in membrane vesicles isolated from growing and quiescent mouse 3T3 fibroblast cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5000–5003. doi: 10.1073/pnas.71.12.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Murer H., Hopfer U. Active alanine transport in isolated brush border membranes. J Biol Chem. 1975 Jul 25;250(14):5674–5680. [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]


