Abstract
Numerous explanations have been proposed for the increase in fungal infections including the use of broad-spectrum antibiotics, antineoplastic agents and prosthetic devices. Also increase in proportion of immunocompromised population predisposed to fungal infection might be a contributory factor.
Candida albicans is a part of normal flora of the oral cavity. However, it is rarely implicated in maxillary osteomyelitis. Diagnosis of Candida infection is challenging as most symptoms are non-specific and cultures may only become positive late in the course of the infection. Due to scarcity of literature, there are no robust guidelines regarding the most appropriate therapeutic regimens to be employed in such cases.
A case of candidal osteomyelitis of mid face is reported suggesting the need for more multicentric long-term studies to formulate and establish appropriate treatment regimens.
Keywords: Candida, Fungal, Osteomyelitis, Maxilla
A 55-year-male presented with the chief complaint of fever and severe pain in relation to extracted left maxillary first molar. Patient did not recall any mobility or decay with respect to the tooth. Extraction did not provide any relief from pain. Mouth opening was restricted (18 mm).
Local examination revealed a 1 × 1 cm large unhealed extraction socket with respect to left maxillary first molar. Exposed bone was dark yellowish brown in color (Fig. 1). Buccal vestibule was partially obliterated with diffuse rubbery soft tissue. Although communication between oral cavity and antrum was present valsalva maneuver was negative. CT scan revealed presence of soft tissue density blocking the osteomeatal complex and perforating the postero-inferior wall of left maxillary sinus (Fig. 2).
Fig. 1.
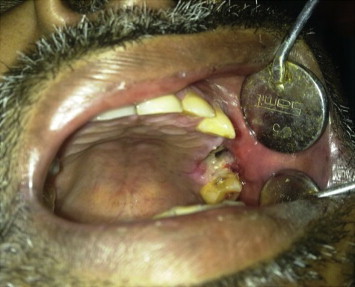
Preoperative intraoral view showing unhealed socket.
Fig. 2.
Preoperative CT scan showing dehiscence of postero-inferior wall of left maxillary sinus.
An incisional biopsy was carried out. Histomorphologic features comprised budding yeast cells and pseudo-hyphae suggesting Candida albicans (Fig. 3). Antibiotic susceptibility of tissue culture showed that it was sensitive to fluconazole, flucytosine, voriconazole and amphotericin B. A diagnosis of chronic candidal osteomyelitis of maxillary sinus was made and fluconazole 300 mg OD was started for 8 weeks. A Caldwell Luc operation was performed for thorough removal of necrotic bone, sinus lining and soft tissue. The sinus was loosely packed with iodoform gauze. Patient's mouth opening gradually increased to 36 mm after eight weeks of the therapy.
Fig. 3.
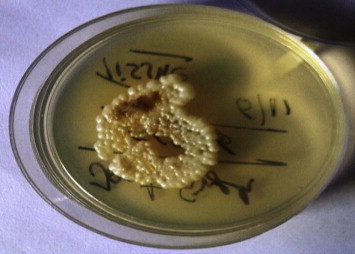
Culture plate showing growth of Candida albicans.
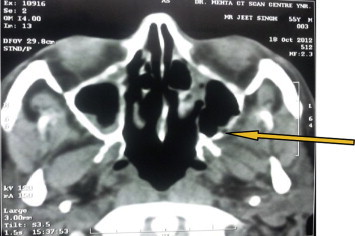
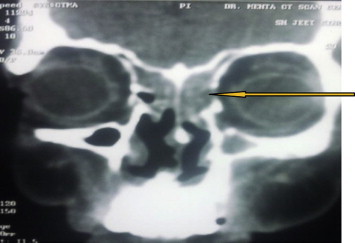
At three months follow up, CT scan was repeated which showed soft tissue density in ethmoidal and sphenoidal sinuses bilaterally (Fig. 4). The left medial orbital wall was invaded by the soft tissue density. An immediate biopsy was carried out in coordination with the ENT surgeon using functional endoscopic sinus surgery. The sinus mucosa was biopsied again at this time. Both histopathologies were suggestive of invasion by C. albicans. Patient was admitted and was administered injection amphotericin B 1.5 g OD intravenously. After forty-five injections of amphotericin B, CT scan and biopsy from both sites were repeated (Fig. 5). The CT scan showed complete resolution of soft tissues density in all the sinuses. The tissue samples were negative for growth of C. albicans. Amphotericin B 1.5 g OD was continued for 1 week as maintenance therapy, after which the injections were stopped. Subsequent follow up CT scans at 3 and 6 months have shown no evidence of re-infection and the patient remains clinically disease free.
Fig. 4.
CT scan at three months follow up showing soft tissue density in ethmoidal and sphenoidal sinuses bilaterally and invasion of left medial orbital wall.
Fig. 5.
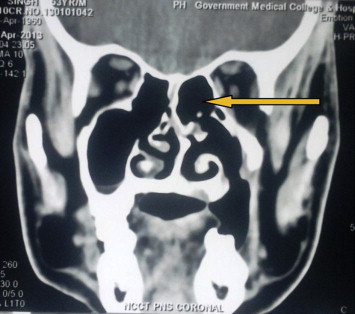
Postoperative CT scan showing complete resolution.
In the past fungal infections have predominantly occurred in immunocompromised patients, however, recently cases of these infections in otherwise healthy individuals, have also been reported.1 This changing trend could be because of the antibiotic abuse that may be leading to increased incidence of fungal infections.2 C. albicans is a part of normal flora of the oral cavity3,4 in healthy individuals. Candidal osteomyelitis rarely affects the maxilla and management remains a clinical challenge. No accurate treatment protocols are available for such fungal infections. Presently the treatment is based on antifungal chemotherapy with or without surgical debridement. Patients reported are apparently cured following disparate therapeutic regimens with insufficient follow up. There are no clear guidelines on drug, dosages, duration and surrogate end point for such treatment. Different drugs are available out of them amphotericin B is considered the drug of choice however the cost and side effects associated with it are very high. In patients who are unable to start or complete an adequate course of amphotericin B, fluconazole seems to be a reasonable alternative. However some authors caution against the use of flucytosine5,6 and fluconazole7,8 alone since resistant strains emerge rapidly during treatment.
Antifungal susceptibility, histopathological examination and multiple factors like cost, risk verses benefit ratio, patient's compliance and clinico-radiological response should be taken into account while determining the exact regimen. According to some authors, patients should be treated for two to four weeks after resolution of clinical signs of infection coupled with microbiological evidence of eradication of the infection. Others recommend that therapy should continue for atleast six to twelve months beyond the resolution of infection.9
We initially instituted eight weeks of oral fluconazole therapy with complete resolution of clinical signs of the infection and a negative histopathology report. However repeat CT scan at three months follow up and subsequent positive biopsies pointed towards a relapse of the infection with imminent threat of optic and intracranial spread. We treated this with one and half months of IV amphotericin B and the patient could finally be relieved of the infection.
The present case suggests that there is a lack of clear guidelines regarding the accurate therapeutic regimen for such fungal infections. This poses difficulty in deciding the exact choice of drug and its duration. In our view serial biopsies and CT scans can help in determining the adequacy of response of a particular regimen and surrogate end point. The patients should be followed clinically, radiologically and histopathologically for atleast six months after stopping the drug treatment. This would not only increase the chances of definitive eradication of the disease process in most cases but also ensure early treatment should there be a relapse.
The optimum therapy and duration for such fungal infections needs to be established on the basis of multicentric long term randomized control trials. This would facilitate the clinician in instituting the right therapeutic regimen and thus bringing down the morbidity associated with this disease.
Conflicts of interest
All authors have none to declare.
References
- 1.Singh V., Sharma B., Sen R., Agrawal S., Bhagol A., Bali R. Rhinocerebral mucormycosis: a diagnostic challenge and therapeutic dilemma in immunocompetent host. J Oral Maxillofac Surg. 2012;70(6):1369–1375. doi: 10.1016/j.joms.2011.06.209. [DOI] [PubMed] [Google Scholar]
- 2.Richardson M.D. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother. 2005;56(SI) doi: 10.1093/jac/dki218. i5–i11. [DOI] [PubMed] [Google Scholar]
- 3.Epstein J.B., Fleischmann J., Silverman S. Oral fungal infections. In: Silverman S., Eversole L.R., Truelove E.L., editors. Essentials of Oral Medicine. BC Decker Inc.; Hamilton, London: 2001. p. 170. [Google Scholar]
- 4.Rautemaa R., Rusanen P., Richardson M., Meurman J.H. Optimal sampling site for mucosal candidosis in oral cancer patients is the labial sulcus. J Med Microbiol. 2006;55:1447. doi: 10.1099/jmm.0.46615-0. [DOI] [PubMed] [Google Scholar]
- 5.Neale T.J., Muir J.C., Mills H., Horne J.G., Jones M.R. Candida albicans vertebral osteomyelitis in chronic renal failure. Postgrad Med J. 1987;63:695–698. doi: 10.1136/pgmj.63.742.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirschmann J.V., Everetti E.D. J Bone Joint Surg. 1976;58(4):573–575. [PubMed] [Google Scholar]
- 7.Ben-Ami R., Keren Pops O., Krieger M. Antibiotic exposure as a risk factor for fluconazole-resistant Candida spp. bloodstream infection. Antimicrob Agents Chemother. 2012;56(5):2518–2523. doi: 10.1128/AAC.05947-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siavoshi F., Tavakolian A., Foroumadi A. Comparison of the effect of non antifungal and antifungal agents on Candida isolates from the gastrointestinal tract. Arch Iran Med. 2012;15(1):27–31. [PubMed] [Google Scholar]
- 9.Arias F., Essayag S.M., Landaeta M.E. Candida albicans osteomyelitis: case report and literature review. Int J Infect Dis. 2004;8:307–314. doi: 10.1016/j.ijid.2003.12.006. [DOI] [PubMed] [Google Scholar]


