Abstract
Surveillance for Rickettsia spp. is urgently needed due to the recent emergence of many novel rickettsioses around the globe, but previous studies in Taiwan have been limited to small areas and no investigation of infections in vertebrate hosts has ever been attempted. We surveyed rickettsial infections systematically in small-mammal hosts trapped between 2006 and 2010 throughout Taiwan. Fragments of ompB and gltA genes in the liver, spleen, and kidney of mammals were targeted by nested polymerase chain reaction. We trapped 1375 individuals of 10 species, among which Rattus losea was the most common (54.6%), followed by Suncus murinus (20.6%) and Mus caroli (10.6%). The overall rate of Rickettsia infections in the liver, spleen, or kidney of 309 assayed small mammals was 60.5%, with a rate of infection ≥50% for each mammal species. DNA nucleotide sequences of 184 successfully sequenced genes were most similar to nine Rickettsia species: Rickettsia conorii, R. felis, R. japonica, R. raoultii, R. rickettsii, Rickettsia sp. IG-1, Rickettsia sp. TwKM01, Rickettsia sp. TwKM02, and R. typhi. Our results suggest that several novel Rickettsia spp. are common and widespread across various habitats throughout Taiwan and suggest the need for further study of emerging rickettsioses in Taiwan.
Key Words: : Rickettsia, Mammalian hosts, Molecular detection, Taiwan
Introduction
Rickettsia spp. are obligate intracellular bacteria belonging to the family Rickettsiaceae, order Rickettsiales. Some rickettsial diseases, such as epidemic typhus, which is caused by Rickettsia prowazekii, are among the oldest infectious diseases. Others, such as Japanese spotted fever, Flinders Island spotted fever, and African tick bite fever, caused by R. japonica, R. honei, and R. africae, respectively, were identified only in the past few decades (Raoult and Roux 1997). Rickettsia are transmitted to humans mainly by arthropods, including ticks, fleas, mites, and lice, with many of these arthropod vectors also serving as reservoirs. In comparison, the role of vertebrates in sustaining these pathogens remains little studied, although limited studies have suggested their involvement in Rickettsia persistence (Parola et al. 2013). For instance, dogs may act as reservoir hosts of R. felis and R. conorii (Hii et al. 2011, Levin et al. 2012), and R. rickettsii has been isolated from several small mammal species, including the cottontail rabbit, white-footed mouse, and meadow vole (Bozeman et al. 1967).
Nine Rickettsia species or closely related pathogens have been identified in Taiwan, including R. conorii, R. felis, R. japonica, Rickettsia sp. IG-1, R. rhipicephali, Rickettsia sp. TwKM01, Rickettsia sp. TwKM02, Rickettsia sp. TwKM03, and R. typhi. Murine typhus, transmitted by R. typhi–infected fleas, is a notifiable disease in Taiwan, with 13–61 human cases each year between 2004 and 2013 (online data, Taiwan Centers for Disease Control). R. felis is another flea-borne rickettsia that was recently isolated from humans and several flea species in Taiwan (Tsai et al. 2008a, Hsu et al. 2011, Tsai et al. 2011, Kuo et al. 2012). Rickettsia sp. IG-1, R. rhipicephali, Rickettsia sp. TwKM01, and Rickettsia sp. TwKM03 were detected in ticks (Tsui et al. 2007, Tsai et al. 2008b, Hsu et al. 2011), whereas Rickettsia sp. TwKM02 and Rickettsia sp. TwKM03 were identified in Leptotrombidium chigger mites (Tsui et al. 2007), R. conorii, R. japonica, and Rickettsia sp. TwKM01 in fleas (Kuo et al. 2012), and Rickettsia sp. TwKM03 in Mesostigmata mites (Tsui et al. 2007). A seroprevalence study of humans in southern Taiwan revealed a moderate rate of antibodies against R. typhi (23.9%), but low rates of reactivity against spotted fever group (SFG) rickettsiae, including R. japonica (3.5%), R. sibirica (4.4%), and Rickettsia sp. Thai TT-118 (4.4%; Takada et al. 1993).
Rickettsial diseases are emerging around the world and are expanding rapidly in geographic distribution (Parola et al. 2013). Furthermore, except for R. typhi, the endemic Rickettsia species in Taiwan were identified only in the past few years, suggesting that many rickettsiae remain to be discovered in Taiwan. Moreover, these studies have mostly been confined to limited areas, leaving many regions of Taiwan uninvestigated. More widespread surveillance for Rickettsia is therefore needed in Taiwan. The aim of this study was thus systematically to investigate and identify Rickettsia infections throughout Taiwan. We focus on small mammals to investigate their potential to facilitate rickettsiae circulation. To our knowledge, this is the first study of Rickettsia infections in vertebrates in Taiwan.
Materials and Methods
Small-mammal trapping
From 2006 to 2010, small mammals were trapped at nine study sites in Taiwan, with three sites each in eastern Taiwan (Yilan, Hualien, Taitung), western Taiwan (Taoyuan, Taichung, Kaoping), and surrounding islets (Matsu, Kinmen, Penghu; Fig. 1), encompassing both fields and domestic areas. The fields were mainly located in agricultural lands, and the domestic areas were mainly located in rural villages. Trapping was undertaken on 22 separate occasions, two to three times per site. Each round of trapping lasted for one night.
FIG. 1.
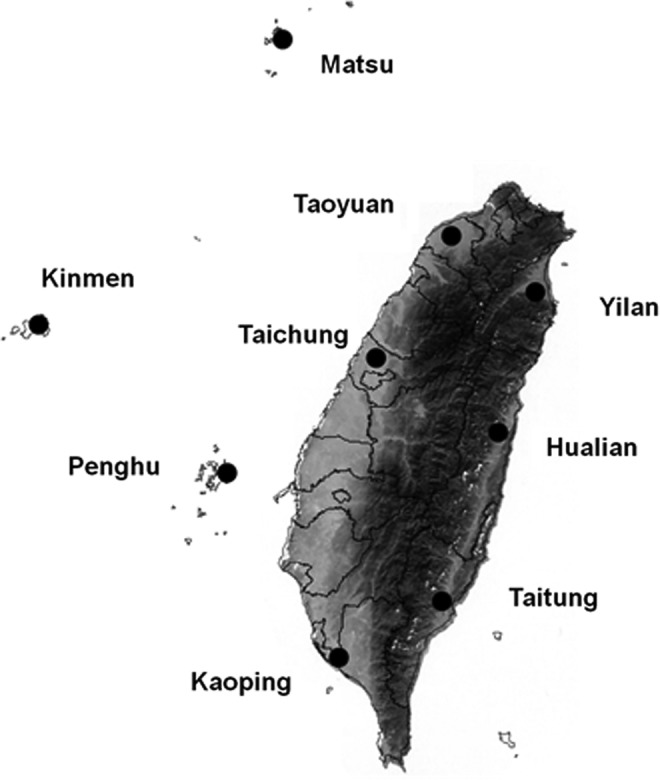
Study sites for the surveillance of Rickettsia spp. infections in small mammals in Taiwan.
Trapped small mammals (rodents and shrews) were first transferred to clean nylon mesh bags. Small mammals were anesthetized with an overdose of Zoletil 50 (Virbac SA, Carros, France), and their ectoparasites, including ticks, fleas, and mites, were recovered for parallel studies on molecular detection of rickettsial infections (H.C. Wang, unpublished data). Rodents and shrews were dissected, and small portions (∼0.5 cm) of their spleens, livers, and kidneys were retrieved and preserved separately in 70% ethanol and stored at −70°C for subsequent detection of Rickettsia infections. All trapping and handling procedures met Taiwanese legal requirements.
Immunofluorescent antibody assay
Only rodents were assayed serologically for exposure to Rickettsia infections. We followed the method described by Kuo et al. (2011, 2012) for the assessment of seroprevalence of Rickettsia spp. Briefly, each rodent serum sample was diluted 1:40 in phosphate-buffered saline (PBS), applied to slides coated with R. conorii, R. rickettsii, or R. typhi antigens (Focus Technologies, Inc., Cypress, CA), and mixed by pipetting with fluorescein isothiocyanate–goat anti-mouse immunoglobulin G (IgG)+A+M (H+L; Zymed Laboratories, Inc., San Francisco, CA) diluted 1:40 in PBS. PBS was used as a negative control; positive controls were not included in this study because our parallel serological studies (Kuo et al. 2011, 2012) confirmed the validity of the test, and excluding positive controls would allow an assay of more individuals for each antigen slide. We chose 1:40 as the cutoff titer because this is the same criterion used for confirmation of human cases of rickettsial diseases (mainly scrub typhus) in Taiwan by the Taiwan Centers for Disease Control. Because R. conorii and R. rickettsii had high antigenic cross-reactivity with each other and with other SFG rickettsiae, whereas R. typhi cross-reacted with other typhus group (TG) rickettsiae, such as R. prowazekii (Fang and Raoult 2003). Seropositivity for R. conorii or R. rickettsii was combined to represent exposure to SFG rickettsiae, and R. typhi seropositivity was taken to represent exposure to TG rickettsiae. Nevertheless, the lack of specificity of this assay, along with the lower-titer cutoff (1:40) applied in this study, yields relative exposure to rickettsial infections across mammalian species and localities, rather than a definitive identification of specific Rickettsia species.
Detection of Rickettsia spp. in small mammals by nested PCR
Rodent and shrew DNA was extracted separately from livers, kidneys (each with ∼25 mg), and spleens (with ∼10 mg) for each individual and purified with a QIAamp DNA Mini Kit (QIAGEN, Gmbh, Hilden, Germany). Each study site included at least 30 individual small mammals (range, 30–55). An individual was considered Rickettsia-positive when Rickettsia was detected in the liver, kidney, or spleen after the method of Kuo et al. (2012). This method targets the 120- to 135-kDa surface antigen (ompB: outer primer pair, ompB OF, 5′-GTA ACC GGA AGT AAT CGT TTC GTA A-3′; ompB OR, 5′-GCT TTA TAA CCA GCT AAA CCA CC-3′; inner primer pair, ompB SFG IF, 5′-GTT TAA TAC GTG CTG CTA ACC AA-3′; ompB SFG/TG IR, 5′-GGT TTG GCC CAT ATA CCA TAA G-3′; ompB TG IF, 5′-AAG ATC CTT CTG ATG TTG CAA CA-3′) and citrate synthase (gltA genes: outer primer pair, RpCS.877p, 5′-GGG GGC CTG CTC ACG GCG G-3′; RpCS.1258n, 5′-AAT GCA AAA AGT ACA GTG AAC A-3′; inner primer pair, RpCS.896, 5′-GGC TAA TGA AGC AGT GAT AA-3′; RpCS.1233n, 5′-GCG ACG GTA TAC CCA TAG C-3′) with nested PCR. The length of the ompB gene amplified was 426 bp for SFG and 250 bp for TG and 338 bp for both SFG and TG in gltA gene. The PCR products were separated by electrophoresis in 1.5% agarose gels, stained with ethidium bromide, and identified under UV fluorescence. The PCR products were purified with a QIAquick Gel Extraction Kit (QIAGEN) and then sequenced twice in each direction. DNA nucleotide sequences were assessed with the Basic Local Alignment Search Tool (www.ncbi.nlm.nih.gov) for any resemblance to known Rickettsia spp.
Results
Small-mammal trapping
Across the 22 rounds of trapping, we captured 1375 individuals of 10 species, including one species of shrew and nine species of rodents (including one squirrel species, Callosciurus erythraeus Pallas). The most common species were Rattus losea Swinhoe (54.6% of total captures), followed by Suncus murinus Linneaus (20.6%), Mus caroli Bonhote (10.6%), and Bandicota indica Bechstein (6.0%; Table 1). Each of the other six species had capture rates <5%.
Table 1.
Species Composition, Abundance, and Seropositivity Rates for Rickettsia, and Rate of Infections with Rickettsia in Small Mammals Trapped Between 2006 and 2010 in Taiwan
| Rickettsia seropositivity rate (%) | Rickettsia DNA detection rate (%)a | ||||||
|---|---|---|---|---|---|---|---|
| Host species | Captures, n (% of total) | R. conorii or R. rickettsii | R. typhi | SFG | TG | Overallb | Rickettsia spp. co-infection rate (%) |
| Shrews | |||||||
| Suncus murinus | 283 (20.6%) | NA | NA | 22.2 (8/36) | 11.1 (4/36) | 50.0 (18/36) | 8.3 (3/36) |
| Rodents | |||||||
| Rattus losea | 751 (54.6%) | 94.6 (694/734) | 2.3 (17/734) | 40.3 (73/181) | 9.9 (18/181) | 55.3 (100/181) | 9.9 (18/181) |
| Mus caroli | 145 (10.6%) | 48.9 (66/135) | 5.9 (8/135) | 43.2 (19/44) | 6.8 (3/44) | 70.5 (31/44) | 9.1 (4/44) |
| Bandicota indica | 83 (6.0%) | 88.0 (73/83) | 0.0 (0/83) | 41.2 (7/17) | 5.9 (1/17) | 76.5 (13/17) | 11.8 (2/17) |
| Mus musculus | 48 (3.5%) | 68.8 (33/48) | 6.3 (3/48) | 100 (6/6) | 0 (0/6) | 100.0 (6/6) | 33.3 (2/6) |
| Rattus exulans | 26 (1.9%) | 92.3 (24/26) | 0.0 (0/26) | 50.0 (3/6) | 16.7 (1/6) | 100.0 (6/6) | 16.7 (1/6) |
| Apodemus agrarius | 24 (1.8%) | 47.8 (11/23) | 8.7 (2/23) | 63.6 (7/11) | 9.1 (1/11) | 72.7 (8/11) | 18.2 (2/11) |
| Rattus norvegicus | 11 (0.8%) | 90.9 (10/11) | 0.0 (0/11) | 50.0 (3/6) | 16.7 (1/6) | 50.0 (3/6) | 33.3 (2/6) |
| Niviventer coxingi | 2 (0.2%) | 50.0 (1/2) | 0.0 (0/2) | 50.0 (1/2) | 0 (0/6) | 100.0 (2/2) | 0 (0/2) |
| Callosciurus erythraeus | 2 (0.2%) | 0.0 (0/2) | 0.0 (0/2) | NA | NA | NA | NA |
| Total | 1375 | 85.7 (912/1064) | 2.8 (30/1064) | 41.1 (127/309) | 9.4 (29/309) | 60.5 (187/309) | 11.0 (34/309) |
An individual was considered positive for Rickettsia infection when Rickettsia was detected in the liver, kidney, or spleen.
Overall detection rate is not the sum of SFG and TG because some positive samples can not be sequenced successfully to reveal its identity (so can not be assigned to SFG or TG). This is also due to co-infections with both SFG and TG in some rodent individual and the exclusion of Rickettsia felis in the categorization of SFG and TG.
SFG, spotted fever group rickettsiae, including Rickettsia conorii, Rickettsia japonica, Rickettsia raoultii, Rickettsia rickettsia, Rickettsia sp. IG-1, Rickettsia sp. TwKM01, and Rickettsia sp. TwKM02; TG, typhus-group rickettsiae, including Rickettsia typhi. Rickettsia felis is considered a transitional group distinct from the SFG and TG rickettsiae (Reif and Macaluso, 2009) and was thus excluded.
Seroprevalence for Rickettsia in rodents
A total of 1064 rodents were assayed for exposure to R. conorii, R. rickettsii, and R. typhi infections. Seropositivity rates across all rodents for R. conorii or R. rickettsii were 85.7%, with very high positivity rates (>90%) in Rattus species (R. losea, R. exulans Peale, R. norvegicus Berkenhout) and B. indica (88.0%; Table 1). Among the regions, Yilan had the highest seropositivity rate (96.3%), followed by Kinmen (95.3%) and Taoyuan (90.3%; Table 2). Seropositivity rates across all rodents against R. typhi were 2.8%, with higher positivity rates occurring in A. agrarius Pallas (8.7%), Mus musculus Linneaus (6.3%), and M. caroli (5.9%). Rodents in Kaoping and Penghu had higher positivity rates (8.4% and 8.0%, respectively) than those in other study sites (Table 2).
Table 2.
Seropositivity Rates of Exposure to Rickettsia and Rates of Rickettsia Infection in Small Mammals Trapped Between 2006 and 2010 at Different Study Sites in Taiwan
| Rickettsia seropositivity rate (%) | Rickettsia DNA detection rate (%)a | |||||
|---|---|---|---|---|---|---|
| Study site | R. conorii or R. rickettsii | R. typhi | SFG | TG | Overallb | Rickettsia spp. co-infection rate (%) |
| Yilan | 96.3 (101/105) | 1.9 (2/105) | 60.0 (18/30) | 46.7 (14/30) | 86.7 (26/30) | 36.7 (11/30) |
| Hualien | 84.0 (105/125) | 1.6 (2/125) | 73.3 (22/30) | 16.7 (5/30) | 100.0 (30/30) | 40.0 (12/30) |
| Taitung | 73.9 (68/92) | 2.2 (2/92) | 10.9 (6/55) | 5.5 (3/55) | 45.5 (25/55) | 1.8 (1/55) |
| Taoyuan | 90.3 (168/186) | 0.5 (1/186) | 36.7 (11/30) | 0 (0/30) | 43.3 (13/30) | 3.3 (1/30) |
| Taichung | 64.3 (45/70) | 0 (0/70) | 38.6 (17/44) | 0 (0/44) | 38.6 (17/44) | 2.3 (1/44) |
| Kaoping | 81.9 (68/83) | 8.4 (7/83) | 40.0 (12/30) | 0 (0/30) | 70.0 (21/30) | 0 (0/30) |
| Matsu | 83.9 (47/56) | 1.8 (1/56) | 70.0 (21/30) | 0 (0/30) | 70.0 (21/30) | 10 (3/30) |
| Kinmen | 95.3 (224/235) | 2.5 (6/235) | 20.0 (6/30) | 6.7 (2/30) | 70.0 (21/30) | 0 (0/30) |
| Penghu | 76.8 (86/112) | 8.0 (9/112) | 33.3 (10/30) | 16.7 (5/30) | 43.3 (13/30) | 16.7 (5/30) |
| Total | 85.7 (912/1064) | 2.8 (30/1064) | 41.1 (127/309) | 9.4 (29/309) | 60.5 (187/309) | 11.0 (34/309) |
An individual was considered positive for Rickettsia infection when Rickettsia was detected in the liver, kidney, or spleen.
Overall detection rate is not the sum of SFG and TG because some positive samples cannot be successfully sequenced to reveal its identity (so cannot be assigned to SFG or TG). This is also due to co-infections with both SFG and TG in some rodent individual and the exclusion of Rickettsia felis in the categorization of SFG and TG.
SFG, spotted fever group rickettsiae, including R. conorii, R. japonica, R. raoultii, R. rickettsia, Rickettsia sp. IG-1, Rickettsia sp. TwKM01, and Rickettsia sp. TwKM02; TG, typhus-group rickettsiae, including R. typhi. R. felis is considered a transitional group distinct from the SFG and TG rickettsiae (Reif and Macaluso, 2009) and was thus excluded.
Detection of Rickettsia spp. in small mammals
A total of 927 samples from 309 small-mammal individuals (each individual included one sample each from liver, kidney, and spleen) were subjected to nested PCRs. The overall rate of infection was 60.5%. All assayed mammal species had ≥50% rate of infection (Table 1). Among the three main study areas, the rate of infection was highest in eastern Taiwan (81/115=70.4%), followed by the surrounding islets (55/90=61.1%) and western Taiwan (51/104=49.0%). Specifically, Hualien and Yilan had very high rates of infection (100% and 86.7%, respectively), whereas Taichung had a relatively lower rate of infection (38.6%; Table 2).
Among the 187 Rickettsia-positive individuals, we successfully sequenced DNA from 140 small mammals, yielding 184 sequences (some individuals had multiple infections). These DNA sequences had the highest similarity (ranges=96.3–100%) to nine Rickettsia species: R. conorii (99.0–100% similarity), R. felis (98.3–100%), R. japonica (98.0–100%), R. raoultii (99.7–100%), R. rickettsii (96.3–100%), Rickettsia sp. IG-1 (98.7–100%), Rickettsia sp. TwKM01 (96.9–100%), Rickettsia sp. TwKM02 (98.7–99.7%), and R. typhi (97.3–100%). Most small-mammal species were infected with four or more Rickettsia species (Table 3). Relatively fewer Rickettsia species were found in western Taiwan (Taoyuan, Taichung, Kaoping; Table 4). R. japonica, Rickettsia sp IG-1, R. typhi, R. rickettsii, and Rickettsia sp. TwKM01 were detected in more mammal species (five to eight species). Among these species, R. japonica, R. typhi, and R. rickettsii were more widely distributed (four to six sites), whereas Rickettsia sp IG-1and Rickettsia sp. TwKM01 were more geographically confined (two to three sites; Table 5). On the contrary, R. conorii and R. felis were limited to a few mammal species (two to three species) but occurred in more localities (both five sites; Table 5). In comparison, R. raoultii and Rickettsia sp. TwKM02 infected only two species and were detected in only one and three regions, respectively (Table 5).
Table 3.
Rickettsia spp. or Closely Related Species Detected in Small Mammals Trapped Between 2006 and 2010 in Taiwan
| Host species | Number of Rickettsia spp. detected | Rickettsia spp. |
|---|---|---|
| Shrews | ||
| Suncus murinus | 4 | R. japonica (4); R. rickettsii (5); Rickettsia sp. TwKM01 (1); R. typhi (4) |
| Rodents | ||
| Rattus losea | 7 | R. conorii (33); R. felis (8); R. japonica (14); R. rickettsii (21); Rickettsia sp. IG-1 (5); Rickettsia sp. TwKM02 (3); R. typhi (18) |
| Mus caroli | 6 | R. conorii (1); R. felis (1); R. japonica (7); R. rickettsii (10); Rickettsia sp. IG-1 (2); R. typhi (3) |
| Bandicota indica | 5 | R. japonica (4); R. raoultii (2); R. rickettsii (3); Rickettsia sp. IG-1 (1); R. typhi (1) |
| Mus musculus | 5 | R. japonica (4); R. raoultii (1); R. rickettsii (2); Rickettsia sp. IG-1 (1); Rickettsia sp. TwKM01 (1) |
| Rattus exulans | 4 | R. japonica (2); Rickettsia sp. IG-1 (1); Rickettsia sp. TwKM01 (1); R. typhi (1) |
| Apodemus agrarius | 5 | R. japonica (1); R. rickettsii (2); Rickettsia sp. IG-1 (5); Rickettsia sp. TwKM01 (1); R. typhi (1) |
| Rattus norvegicus | 5 | R. japonica (2); Rickettsia sp. IG-1 (1); Rickettsia sp. TwKM01 (1); Rickettsia sp. TwKM02 (1); R. typhi (1) |
| Niviventer coxingi | 1 | R. conorii (1) |
| Total | 9 | R. conorii (35); R. felis (9); R. japonica (38); R. raoultii (3); R. rickettsii (43); Rickettsia sp. IG-1 (16); Rickettsia sp. TwKM01 (7); Rickettsia sp. TwKM02 (4); R. typhi (29) |
Table 4.
Rickettsia spp. or Closely Related Species Detected in Small Mammals Trapped Between 2006 and 2010 in Different Study Sites in Taiwan
| Study site | Number of Rickettsia spp. detected | Rickettsia spp. or species with similar nucleotide sequences detected |
|---|---|---|
| Yilan | 3 | R. conorii (17); Rickettsia sp. TwKM02 (4); R. typhi (14) |
| Hualien | 6 | R. japonica (13); R. raoultii (1); R. rickettsii (4); Rickettsia sp IG-1 (13); Rickettsia sp. TwKM01 (6); R. typhi (5) |
| Taitung | 6 | R. conorii (2); R. felis (3); R. raoultii (1); R. rickettsii (1); Rickettsia sp. IG-1 (2); R. typhi (3) |
| Taoyuan | 2 | R. japonica (11); R. felis (1) |
| Taichung | 4 | R. japonica (2); R. raoultii (1); R. rickettsii (15); Rickettsia sp. TwKM01 (1) |
| Kaoping | 1 | R. japonica (12) |
| Matsu | 4 | R. conorii (10); R. felis (2); R. rickettsii (12); Rickettsia sp. IG-1 (1) |
| Kinmen | 4 | R. conorii (3); R. felis (2); R. rickettsii (3); R. typhi (2) |
| Penghu | 4 | R. conorii (3); R. felis (1); R. rickettsii (8); R. typhi (5) |
| Total | 9 | R. conorii (35); R. felis (9); R. japonica (38); R. raoultii (3); R. rickettsii (43); Rickettsia sp. IG-1 (16); Rickettsia sp. TwKM01 (7); Rickettsia sp. TwKM02 (4); R. typhi (29) |
Table 5.
Number of Small-Mammal Species and Study Sites for the Detection of Rickettsia spp. or Closely Related Species in Taiwan
| Rickettsia spp. | Number of small mammal species | Number of study sites |
|---|---|---|
| R. conorii | 3 | 5 |
| R. felis | 2 | 5 |
| R. japonica | 8 | 4 |
| R. raoultii | 2 | 3 |
| R. rickettsii | 6 | 6 |
| Rickettsia sp. IG-1 | 7 | 3 |
| Rickettsia sp. TwKM01 | 5 | 2 |
| Rickettsia sp. TwKM02 | 2 | 1 |
| R. typhi | 7 | 5 |
The rates of infections were further divided into SFG rickettsiae and TG rickettsiae on the basis of the Rickettsia spp. identified. SFG rickettsiae include R. conorii, R. japonica, R. raoultii, R. rickettsii, Rickettsia sp. IG-1, Rickettsia sp. TwKM01, and Rcikettsia sp. TwKM02, and TG rickettsiae include R. typhi. R. felis is considered a transitional group distinct from SFG and TG rickettsiae (Reif and Macaluso 2009) and was thus excluded from the analysis. The rate of infection with SFG rickettsiae was highest in M. musculus (100%) and lowest in S. murinus (22.2%; Table 1). The rate of infection with TG rickettsiae was higher in R. exulans and R. norvegicus (both 16.7%) and lower in M. musculus and Niviventer coxingi Swinhoe (both 0%; Table 1). Hualien had the highest rate of infection with SFG rickettsiae (73.3%), and Yilan had the highest rate of infection with TG rickettsiae (46.7%; Table 2).
The overall rate of co-infection was 11.0% (34 out of 309). M. musculus and R. norvegicus Berkenhout had the highest rates of co-infection (both 33.3%), potentially as an artifact of the small sample size (six individuals each; Table 1). The rates of co-infection were much higher in Hualien (40.0%) and Yilan (36.7%) than at the other study sites (Table 2). The most common co-infection was R. conorii and R. typhi (4.89%), followed by R. japonica and Rickettsia sp. IG-1 (2.72%) and R. rickettsii and R. typhi (2.72%).
Discussion
This is the first report of the detection of Rickettsia in small mammals and the presence of R. raoultii and R. rickettsii or related species in Taiwan. We also found high rates of infection in these mammals (≥50% in all host species), a high diversity of Rickettsia species (nine species), and high geographical variability in the rate of infection (38.6–100%). These results are highly significant to the prevention and control of rickettsial diseases in Taiwan.
The rate of seropositivity for R. conorii or R. rickettsii antigens across all mammals was high (85.7%). These findings are comparable to the results of our previous study on seroprevalence for R. conorii in rodents in eastern Taiwan (Hualien, 91.9%), which was conducted using the same methods (Kuo et al. 2011). Such a high seroprevalence may not be surprising in light of the high rate of infection (60.5%) among small mammals, which indicates that rickettsiae infections circulate at high numbers in fields and suggests a high human risk for Rickettsia infections, although some species may not be human pathogens.
The overall rate of Rickettsia spp. infection in the liver, kidney, or spleen was high (60.5%). Moreover, except for squirrel C. erythraeus, which was not tested, all nine of the other mammal species, whether peridomestic (such as S. murinus, M. musculus, or R. norvegicus) or field-dwelling (such as R. losea, M. caroli, or A. agrarius), had rates of infection >50%. Capitalizing on mammalian hosts as sentinels of disease surveillance, the current results indicate widespread circulation of Rickettsia. spp. in high numbers across various habitats in Taiwan.
We detected nine Rickettsia species in small mammals, including two species or closely related species (R. raoultii and R. rickettsii) newly identified in Taiwan; however, it should be emphasized that these Rickettsia species are identified on the basis of partial nucleotide fragments and further study is required for a definitive identification. The recently named tick-borne pathogen R. raoultii was first isolated from ticks in Russia (Mediannikov et al. 2008). This species was later isolated from patients in Europe and from ticks in Africa, Europe, and Asian countries such as China, Japan, Mongolia, and Thailand (Parola et al. 2013). R. raoultii was primarily identified in ticks of the genera Dermacentor and Haemaphysalis (Parola et al. 2013), including Haemaphysalis hystricis that also exists in Taiwan. In this study, we demonstrated the circulation of R. raoultii–like rickettsia in Taiwan, although this pathogen was confined to fewer places and mammal species compared to other rickettsiae. R. rickettsii, the etiological agent of Rocky Mountain spotted fever, is the most lethal tick-borne rickettsiosis in the world (Raoult and Roux 1997). It is distributed predominately in the Americas (Parola et al. 2013), but has also been detected in ticks (Haemaphysalis longicornis) in Korea (Lee et al. 2003, Kim et al. 2006). Rhipicephalus sanguineus, the brown dog tick that distributes worldwide including Taiwan, is among the most important vectors of R. rickettsii (Parola et al. 2013). We detected this pathogen in most mammal species and localities, suggesting a widespread distribution of an R. rickettsia–like bacterium in Taiwan.
Previously, we identified species similar to R. conorii and R. japonica in fleas recovered from small mammals for the first time in Taiwan (Kuo et al. 2012); however, both rickettsiae are primarily transmitted by ticks (Parola et al. 2013) instead of the fleas in which they were detected, suggesting that R. conorii and R. japonica might also be detected in ticks or in both ticks and mammalian hosts (Kuo et al. 2012). In this study, we corroborated that R. conorii and R. japonica or rickettsiae with similar nucleotide sequences do exist in mammalian hosts, thus necessitating a further search for potential tick vectors in Taiwan. R. conorii causes Mediterranean spotted fever and occurs most commonly in Europe (Parola et al. 2013), although human cases have been reported in Korea (Choi et al. 2005). In the current study, a R. conorii–like bacterium was predominately isolated from R. losea, and likely as a result of the commonness of this rodent species, this rickettsia was found in several localities in Taiwan. In comparison, R. japonica occurred mainly in western Taiwan, although it was also found in Hualien, eastern Taiwan, but was detected in nearly all mammal species except the rarely trapped N. coxingi. R. japonica is confined to Asia, including Japan, Korea, and Thailand (Parola et al. 2013).
Rickettsia sp IG-1, Risckettsia sp. TwKM01, and Risckettsia sp. TwKM02 were first identified in Taiwan. Their pathogenicity remains undetermined (Tsui et al. 2007, Tsai et al. 2008b). Rickettsia sp. IG-1 was first isolated from Ixodes granulatus ticks in Taitung (Tsai et al. 2008b). Later, a similar rickettsia was also isolated from I. granulatus in Okinawa, Japan (Fujita et al. 2008). In this study, Rickettsia sp. IG-1 or a similar agent was identified in the majority of mammal species but its distribution was limited to eastern Taiwan. Rickettsia sp. TwKM01 was first isolated from Rhipicephalus haemaphysaloides ticks (Tsui et al. 2007). Importantly, our study revealed that this rickettsia was more commonly associated with peridomestic rodents, including S. murinus, M. musculus, and R. norvegicus. Therefore, the human health risk posed by Rickettsia sp. TwKM01 warrants further scrutiny despite the very confined distribution of this pathogen in Taiwan. Last, Rickettsia sp. TwKM02 seemed to be less common in Taiwan, having been detected only in two host species in one locality. This species was phylogenetically closely related to tick-borne R. australis but was initially recovered from Leptotrombidium chigger mites (Tsui et al. 2007). It was suspected that this rickettsia might come from rodents (Tsui et al. 2007), and our study did detect Rickettsia sp. TwKM02 in some rodents.
Unlike the above-mentioned rickettsiae, which are mainly transmitted by ticks, fleas are the vectors of R. typhi and R. felis. Murine typhus, caused by R. typhi, has long been endemic in Taiwan, but no investigation of the infection status of mammalian hosts in Taiwan has ever been attempted. This study showed that in Taiwan, R. typhi occurs not only in the typical commensal rats (such as R. norvegicus; Traub and Wisseman 1978) but also in rodents living away from housing, such as A. agrarius and M. caroli, indicating a widespread circulation of R. typhi both around human dwellings and in fields. Similarly, R. felis was detected in several localities, especially in the islets. This rickettsia was mostly identified in the rodent species R. losea (Table 3), which was also heavily infested with fleas (79.4% of total fleas collected from this species; H.C. Wang, unpublished data). In Taiwan, R. losea is found in fields; however, the moderate prevalence of R. felis infections in cat fleas (Ctenocephalides felis) recovered from stray cats and dogs in Taiwan (44.3% in Hsu et al. 2011; 21.4% in Tsai et al. 2011) suggests that this globally emergent pathogen (Pérez-Osorio et al. 2008) may also be prevalent in fields and in domestic areas.
In Taiwan, the definitive etiologic agent of many suspected rickettsial diseases cannot be confirmed. For instance, the causative agents of 98% of suspected human cases of murine typhus could not be verified in 2006 (Tsai et al. 2008b). Scrub typhus, the most prevalent rickettsiosis in Taiwan, is caused by infection with Orientia tsutsugamushi but could not be confirmed in 71% of suspected human cases between 2000 and 2004 in eastern Taiwan (Lee et al. 2006). This difficulty might arise from the similar clinical symptoms of most rickettsial diseases as well as serological cross-reactivity between rickettsial species, which render the identification of causative agents challenging (Raoult and Roux 1997). The many unrecognized rickettsiae circulating in Taiwan also complicate their identification, as revealed in this study. Our next step is to identify other Rickettsia infections in arthropod vectors recovered from mammalian hosts to help evaluate the potential risks to human health posed by these disease vectors. More studies are certainly needed to develop a more thorough understanding of these emerging rickettsioses in Taiwan, including such as the status of Rickettsia infections in humans and the role of birds as the hosts of ticks.
Acknowledgments
This study was financially supported by Taiwan Centers for Disease Control (DOH97-DC-2004, DOH98-DC-2012, DOH99-DC-2028).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Bozeman FM, Shirai A, Humphries JW, Fuller HS. Ecology of Rocky Mountain spotted fever II. Natural infection of wild mammals and birds in Virginia and Maryland. Am J Trop Med Hyg 1967; 16:48–59 [PubMed] [Google Scholar]
- Choi YJ, Jang WJ, Kim JH, Ryu JS, et al. . Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infec Dis 2005; 11:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Raoult D. Antigenic classification of Rickettsia felis by using monoclonal and polyclonal antibodies. Clin Diagn Lab Immunol 2003; 10:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Kadosaka T, Nitta Y, Ando S, et al. . Rickettsia sp. in Ixodes granulatus ticks, Japan. Emerg Infec Dis 2008; 14:1963–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii SF, Kopp SR, Abdad MY, Thompson MF, et al. . Molecular evidence supports the role of dogs as potential reservoirs for Rickettsia felis. Vector Borne Zoonotic Dis 2011; 11:1007–1012 [DOI] [PubMed] [Google Scholar]
- Hsu YM, Lin CC, Chomel BB, Tsai KH, et al. . Identification of Rickettsia felis in fleas but not ticks on stray cats and dogs and the evidence of Rickettsia rhipicephali only in adult stage of Rhipicephalus sanguineus and Rhipicephalus haemaphysaloides. Comp Immunol Microbiol Infect Dis 2011; 34:513–518 [DOI] [PubMed] [Google Scholar]
- Kim CM, Yi YH, Yu DH, Lee MJ, et al. . Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol 2006; 72:5766–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Huang CL, Wang HC. Identification of potential hosts and vectors of scrub typhus and tick‐borne spotted fever group rickettsiae in eastern Taiwan. Med Vet Entomol 2011; 25:169–177 [DOI] [PubMed] [Google Scholar]
- Kuo CC, Huang JL, Lin TE, Wang HC. Detection of Rickettsia spp. and host and habitat associations of fleas (Siphonaptera) in eastern Taiwan. Med Vet Entomol 2012; 26:341–350 [DOI] [PubMed] [Google Scholar]
- Lee JH, Park HS, Jung KD, Jang WJ, et al. . Identification of the spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol Immunol 2003; 47:301–304 [DOI] [PubMed] [Google Scholar]
- Lee Y, Wang P, Tseng S, Ko C, Teng H. Epidemiology of scrub typhus in eastern Taiwan, 2000–2004. Jpn J Infect Dis 2006; 59:235–238 [PubMed] [Google Scholar]
- Levin ML, Killmaster LF, Zemtsova GE. Domestic dogs (Canis familiaris) as reservoir hosts for Rickettsia conorii. Vector Borne Zoonotic Dis 2012; 12:28–33 [DOI] [PubMed] [Google Scholar]
- Mediannikov O, Matsumoto K, Samoylenko I, Drancourt M, et al. . Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int J Syst Evo Microbiol 2008; 58:1635–1639 [DOI] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Socolovschi C, Labruna MB, et al. . Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbi Rev 2013; 26:657–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Osorio CE, Zavala-Velázquez JE, León JJA, Zavala-Castro JE. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis 2008; 14:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev 1997; 10:694–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif KE, Macaluso KR. Ecology of Rickettsia felis: A review. J Med Entomol 2009; 46:723–736 [DOI] [PubMed] [Google Scholar]
- Takada N, Fujita H, Yano Y, Huang WH, Khamboonruang C. Serosurveys of spotted fever and murine typhus in local residents of Taiwan and Thailand compared with Japan. Southeast Asian J Trop Med Public Health 1993; 24:354–356 [PubMed] [Google Scholar]
- Traub R, Wisseman C. The ecology of murine typhus-a critical review. Trop Dis Bull 1978; 75:237–317 [PubMed] [Google Scholar]
- Tsai KH, Lu HY, Tsai JJ, Yu SK, et al. . Human case of Rickettsia felis infection, Taiwan. Emerg Infect Dis 2008a; 14:1970–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KH, Wang HC, Chen CH, Huang JH, et al. . Isolation and identification of a novel spotted fever group rickettsia, strain IG-1, from Ixodes granulatus ticks collected on Orchid Island (Lanyu), Taiwan. Am J Trop Med Hyg 2008b; 79:256–261 [PubMed] [Google Scholar]
- Tsai KH, Huang CG, Fang CT, Shu PY, et al. . Prevalence of Rickettsia felis and the first identification of Bartonella henselae Fizz/CAL-1 in cat fleas (Siphonaptera: Pulicidae) from Taiwan. J Med Entomol 2011; 48:445–452 [DOI] [PubMed] [Google Scholar]
- Tsui PY, Tsai KH, Weng MH, Hung YW, et al. . Molecular detection and characterization of spotted fever group rickettsiae in Taiwan. Am J Trop Med Hyg 2007; 77:883–890 [PubMed] [Google Scholar]


