Abstract
Epidemiological studies worldwide have reported a high prevalence and a great diversity of Bartonella species, both in rodents and their flea parasites. The interaction among Bartonella, wild rodents, and fleas reflects a high degree of adaptation among these organisms. Vertical and horizontal efficient Bartonella transmission pathways within flea communities and from fleas to rodents have been documented in competence studies, suggesting that fleas are key players in the transmission of Bartonella to rodents. Exploration of the ecological traits of rodents and their fleas may shed light on the mechanisms used by bartonellae to become established in these organisms. The present review explores the interrelations within the Bartonella–rodent–flea system. The role of the latter two components is emphasized.
Key Words: : Rodents, Fleas, Bartonella
Introduction
The family Bartonellaceae represents an ecologically successful group of bacteria that inhabits an immense diversity of mammals and arthropods all over the world (Birtles 2005). Bartonellae are facultative intracellular, fastidious, Gram-negative bacteria, belonging to the alpha-2-Proteobacteria class (Birtles and Raoult 1996). Through an apparent double niche, infecting erythrocytes and endothelial cells, bartonellae establish long-term infections in mammalian reservoirs with a silent strategy that prevents their rapid clearance by the host's immune system (Harms and Dehio 2012). Furthermore, the transmission of bartonellae has been facilitated by bloodsucking arthropod vectors (Kosoy et al. 2012), spreading the bacteria from one animal to another within specific reservoir communities and between different reservoirs.
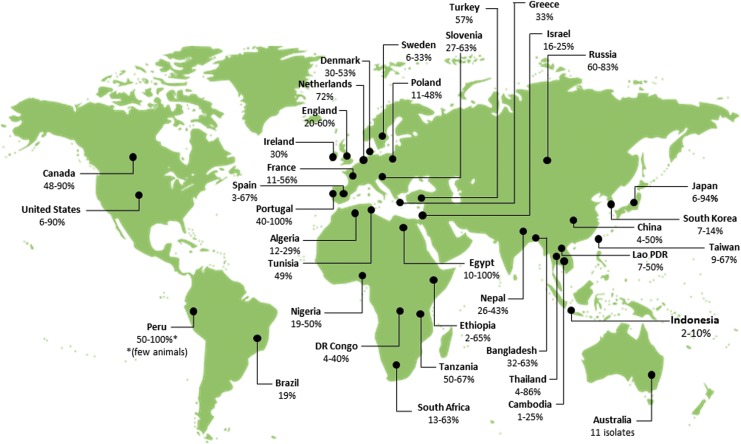
Rodents have been found to be natural reservoirs of many Bartonella species. The association between rodents and bartonellae is of great importance, because the former display persistent and subclinical bacteremia that lasts for months. In addition, many different rodent species have been reported to be infected in high percentages with different Bartonella spp. and variants worldwide (Fig. 1).
FIG. 1.
Range of Bartonella-infection rates in rodents reported worldwide (data from PubMed, January, 2014). References according to continents and countries: Africa, Algeria, Bitam et al. 2009; Egypt, Inoue et al. 2009; Ethiopia, Meheretu et al. 2013; DR Congo and Tanzania, Gundi et al. 2012b; Nigeria, Kamani et al. 2013; South Africa, Pretorius et al. 2004, Brettschneider et al. 2012b, Trataris et al. 2012; Tunisia, Fichet-Calvet et al. 2000; Asia, Bangladesh, Bai et al. 2007b; Cambodia, Lao PRD, and Thailand, Jiyipong et al. 2012; China, Ying et al. 2002, Ye et al. 2009, Inoue et al. 2009, Liu et al. 2010; Indonesia, Winoto et al. 2005; Israel, Harrus et al. 2009, Morick et al. 2009; Japan, Inoue et al. 2008, Kabeya et al. 2011; Lao PDR, Angelakis et al. 2009; Nepal, Gundi et al. 2010; Taiwan, Lin et al. 2008, Hsieh et al. 2010, Tsai et al. 2010, Chae et al. 2008; Russia (Far east), Mediannikov et al. 2005; South Korea, Kim et al. 2005; Thailand, Castle et al. 2004, Bai et al. 2009, Saisongkorh et al. 2009, Inoue et al. 2009; Turkey (Kaman, Kirsehir), Karagöz et al. 2013; America, Brazil, Costa et al. 2014; Canada, Jardine et al. 2005, Jardine et al. 2006b; Peru, Birtles et al. 1999; United States, Kosoy et al. 1997, Ellis et al. 1999, Bown et al. 2002, Kosoy et al. 2003, 2004a, Bai, et al. 2007a, 2008b, Morway et al. 2008, Matsumoto et al. 2010, Bai et al. 2011, Gundi, et al. 2012a; Europe, Denmark, Engbaek and Lawson 2004; England, Birtles et al. 2001, Bown et al. 2002, Telfer et al. 2007a, France, Gundi et al. 2004, Buffet et al. 2012, 2013b; Greece, Tea et al. 2004; Ireland, Harrison et al. 2012; Netherlands, Holmberg et al. 2003; Poland, Welc-Faleciak et al. 2008, 2010, Paziewska et al. 2012a, Hildebrand et al. 2013; Portugal, Ellis et al. 1999; Slovenia, Knap et al. 2007; Spain, Marquez et al. 2008, Gil et al. 2010; Oceania, Australia, Gundi et al. 2009. Color images available online at www.liebertpub.com/vbz
Fleas are considered key players in the Bartonella cycle, because they harbor a high diversity of Bartonella spp. and strains and demonstrate great efficiency in the transmission of these bacteria among rodents (Brinkerhoff et al. 2010, Morick et al. 2013a). Therefore, fleas were suggested not to serve solely as vectors, but to represent additional reservoirs for these bacteria (Birtles 2005, Deng et al. 2012).
Several rodent-associated Bartonella spp. and variants have been implicated as the causative agents of human clinical manifestations, including endocarditis (Daly et al. 1993, Fenollar et al. 2005), myocarditis (Kosoy et al. 2003), fever and neurologic disorders (Welch et al. 1999), intraocular neuroretinitis (Kerkhoff et al. 1999), meningitis (Probert et al. 2009), splenomegaly (Eremeeva et al. 2007), and lymphadenopathy (Oksi et al. 2013). These findings make rodents and their fleas a relevant natural system for the study of ecological pathways of vector-borne pathogens and emerging diseases of human importance.
This review attempts to summarize and bridge some knowledge gaps in the transmission and distribution routes and in the dynamics and composition of Bartonella infection in rodents and their flea parasites. Many studies and reviews have focused on the bacterial component within the organism–host–vector triangle (Saenz et al. 2007, Chomel et al. 2009, Vayssier-Taussat et al. 2010, Engel et al. 2011, Deng et al. 2012, Harms and Dehio 2012, Buffet et al. 2013a, Buffet et al. 2013b). In this review, we focus on the particular ecological traits of the other two components, the rodent and the flea, that may explain the apparent co-adaptation of these organisms.
Bartonella in Rodents and Their Associated Fleas
Rodents infected with Bartonella spp. have been described since the mid-twentieth century (Baker 1946, Kosoy 2010), but the majority of reports on Bartonella-infected rodents have significantly accumulated after the expansion of the Bartonella genus in 1993 (Kosoy 2010). Bartonella spp. have been virtually detected from rodents worldwide (Fig. 1). Pioneering studies were carried out in North America and Europe, and later in Asia, Africa, Latin America, and Oceania (Fig. 1). Extremely high Bartonella infection rates were reported in rodent communities reaching up to 90.4% in the northern grasshopper mice (Onychomys leucogaster) from Kansas (Bai et al. 2007a) and 82.4% in the deer mice (Peromyscus maniculatus) from Colorado (Bai et al. 2011), suggesting a mutual adaptation between the bacteria and their reservoirs. Yet, some species have shown to be less susceptible or completely resistant for infection. For instance, only seven of 14 rodent species captured in grasslands of four different states in the United States were found to be infected (Bai et al. 2007a).
Rodents harbor the greatest diversity of bartonellae described to date. Numerous isolations of Bartonella spp. and variants have been obtained from more than 98 rodent species belonging to at least seven families. About 22 rodent-associated Bartonella spp. and subspecies have been described, some of which were initially isolated from human cases (Buffet et al. 2013a). The chronological succession of the description of these Bartonella spp. is: B. elizabethae and B. vinsonii subsp. vinsonii (Brenner et al. 1993), B. doshiae, B. grahamii, and B. taylorii (Birtles et al. 1995), B. tribocorum (Heller et al. 1998), B. vinsonii subsp. arupensis (Welch et al. 1999), B. birtlesii (Bermond et al. 2000), B. washoensis (Kosoy et al. 2003), B. phoceensis and B. rattimassiliensis (Gundi et al. 2004), B. rochalimae (Lin et al. 2008), B. tamiae (Kosoy et al. 2008), B. rattaustraliani, B. queenslandensis, and B. coopersplainsensis (Gundi et al. 2009), B. japonica and B. silvatica (Inoue et al. 2010), and B. jaculi, B. callosciuri, B. pachyuromydis, and B. acomydis (Sato et al. 2013). Other Bartonella isolated from rodents have been proposed as new species or subspecies, including Candidatus Bartonella washoensis subsp. cynomysii (Bai et al. 2008a), Candidatus Bartonella volans, Candidatus Bartonella durdenii, and Candidatus Bartonella monaxi (Breitschwerdt et al. 2009). Phylogenetic analysis of the characterized species has shown that the majority of Bartonella spp. are clustered in a common lineage, with the exception of B. rochalimae and B. tamiae (Buffet et al. 2013a), evidencing the occurrence of an adaptive evolution of these bacteria in rodents (Engel et al. 2011). Moreover, several molecular studies have found a wider variety of Bartonella genotypes that have challenged the current taxonomic classification (Bai et al. 2009, Harrus et al. 2009, Inoue et al. 2009). To clarify the vast rodent–bartonellae diversity, the use of alternative taxonomic classification according to species complexes has been proposed (Kosoy et al. 2012). The recent elevated number of reported new Bartonella spp. and genotypes from rodents can be explained by a potential accelerated evolution of the Bartonella genus in rodents as a result of frequent recombination events, horizontal gene acquisitions, and accumulation of mutations (Berglund et al. 2010, Guy et al. 2013). Increased Bartonella-oriented research can also contribute to this phenomenon.
Bartonella infection in rodents can be composed of more than one Bartonella sp. or genetic variants in the same rodent (i.e., co-infection) (Morick et al. 2011). The potential interaction between the co-existing Bartonella variants could be the source of the recombination events and the diversity revealed in rodent-associated bartonellae (Berglund et al. 2009, Berglund et al. 2010, Paziewska et al. 2011, Paziewska et al. 2012b). Different rodent or flea species can play a greater role in the occurrence of such events (Paziewska et al. 2012a). In addition, evidence for the presence of the same Bartonella sp. in two different rodent species has been accumulated and described as the “spillover” phenomenon (Ying et al. 2002, Castle et al. 2004, Kosoy et al. 2004b, Jardine et al. 2006a, Bai et al. 2007a, Telfer et al. 2007b). This phenomenon can be driven by the act of ectoparasites exchanged between different rodent species or even genera, or by close interspecies and/or intergeneric interaction between rodents.
Among the ectoparasites infesting rodents, fleas are considered major vectors of bartonellae (Billeter et al. 2008, Tsai et al. 2011). Various fleas have been demonstrated to acquire and transmit Bartonella spp. and strains under experimental conditions, probably serving as competent vectors of these bacteria in the wild (Krampitz 1962, Bown et al. 2004, Morick et al. 2011, Morick et al. 2013a). Bartonellae have been shown to be dominant members of the bacterial communities of several rodent associated fleas (Jones et al. 2008, Hawlena et al. 2013). Moreover, many rodent-associated fleas have been shown to be naturally infected with Bartonella closely related to zoonotic species (Stevenson et al. 2003, Marie et al. 2006, Jones et al. 2008, Morick et al. 2010, Billeter et al. 2011, Kabeya et al. 2011, Billeter et al. 2013, Kim et al. 2013). The frequent feeding of fleas and their ability to move from one host to another might explain the high prevalence and diversity of Bartonella spp. infection found in rodents and fleas (Kosoy et al. 2012). Moreover, adaptation between bartonellae and fleas was evidenced through the experimental infection of Xenopsylla ramesis fleas with Bartonella sp. OE 1-1, a strain closely related to B. elizabethae. Infection with this Bartonella variant did not affect the metabolic rate, blood consumption, life span, fertility, or fecundity of female fleas, nor the developmental time, the life span, or sex ratio of their offspring fleas (Morick et al. 2013b). Interestingly, bartonellae DNA has been detected in other rodent ectoparasites, including ticks, mites, and lice (Durden et al. 2004, Kim et al. 2005, Reeves et al. 2006). A recent study has demonstrated the competency of Ixodes ricinus ticks to transmit the rodent-associated B. birtlesii (Reis et al. 2011). However, the biological role of ticks in transmission of Bartonella in nature is still under debate, and their epidemiological role has been considered secondary (Matsumoto et al. 2010, Harrison et al. 2012). In this review, the role of fleas in the ecology of bartonellae in rodents is emphasized, although alternative vectors may also play a role in the transmission cycle.
Ecological Insights of the Rodent-Flea-Bartonella Triangle
Better understanding of rodent ecological traits and the dynamics of flea infestation may shed light on the pathways that enabled bartonellae to be established in rodent hosts and flea vectors. Rodents' habitat, behavior, and flea parasitism may represent crucial variables influencing the transmission and establishment of bartonellae, and these typically vary across species and geographical areas.
The rodent habitat can represent an important variable in the efficiency of the transmission cycle and in the selection of Bartonella spp. repertoire to which rodents are exposed. The burrow habitat can directly affect the flea cycle, because immature stages live off-host and exhibit certain microclimatic preferences (Krasnov et al. 2001). Therefore, if the conditions are not favorable for flea development, vectorial transmission of bartonellae may not occur. The habitat location and geographic conditions (e.g., rainy versus dry seasons) can also influence the feeding performance of certain rodents and can lead to an increased vulnerability for bacterial infections (Beldomenico and Begon 2010), especially for those who base their nutrition on green vegetation. Visit of burrows or invasion to the territories of other rodent species can represent an important source of flea interchange (Krasnov and Khokhlova 2001). Therefore, the geographical and/or habitat traits of rodents and their fleas may enhance or restrict the diversity of bartonellae observed in those animal communities (Jardine et al. 2006a, Morick et al. 2010).
Rodent behavior can directly influence the transmission of bartonellae between members of the same community. Physical contact between individual rodents may promote the transmission of bartonellae. Grooming can facilitate the acquisition of bartonellae by disrupting the skin barrier (e.g., by aggressive grooming) or by removal and interchange of ectoparasites between rodents (e.g., social grooming) (Krasnov and Khokhlova 2001, Stopka and Graciasova 2001). Parental behavior (carrying, licking, and huddling of young animals) (Lonstein and De Vries 2000) can lead to an early exposure and transmission of bartonellae among rodents. In addition, mobility, spatial behavior, and seasonality of the rodents (e.g., for reproductive purposes or emergence of juveniles from burrows) are traits that can contribute to the risk of bartonellae acquisition in wild rodents by influencing the rate of rodent–rodent interactions (Krasnov et al. 2005). In fact, during reproductive periods, male rodents tend to increase their mobility and may suffer immunosuppression due to effects of sex hormones (Krasnov et al. 2005) that can lead to a major susceptibility to bacterial infections. Moreover, hibernation can also play a role in the persistence or clearance of infection in rodent communities across seasons (Jardine et al. 2006b). Nevertheless, all of these particular behaviors can vary across rodent species and may reflect the different host adaptability to bartonellae of certain rodents.
Flea parasitism likely plays a fundamental role in the transmission and acquisition of bartonellae being mediated by the level of flea's host specificity, flea exchange between rodents, and flea abundance. The host specificity of fleas is an important trait that can influence the introduction or the restriction of new Bartonella spp. and strains to new hosts. Fleas vary greatly in the degree of their host specificity, from being highly host specific to host opportunistic (Krasnov et al. 2004). The latter will underline the potential success of exchanging fleas between rodents. In fact, the exchange of fleas can likely occur between conspecific rodents or even across rodent species in the wild (Krasnov and Khokhlova 2001). The flea abundance (number of fleas per host) might directly influence the chances of bartonellae infection establishment in the host. It is noteworthy that the abundance of fleas varies across rodent species, flea species habitats, and seasons (Krasnov et al. 1997, Krasnov et al. 2005, Kim et al. 2013).
Acquisition and Transmission Pathways of Bartonella
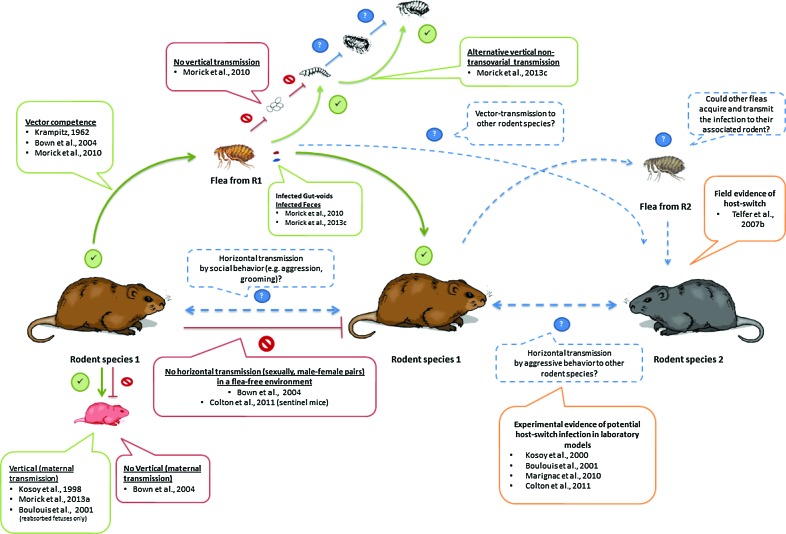
Investigation of the transmission routes and dispersal dynamics of bartonellae among animals and their vectors is crucial in elucidating the ways they are being maintained in nature. Microorganisms are transmitted via vertical and/or horizontal pathways to spread within host populations. Thereby, competence studies have contributed greatly to the comprehension of rodent-associated bartonellae cycles (Fig. 2).
FIG. 2.
Experimentally demonstrated pathways and potential transmission routes of bartonellae in wild rodents and their fleas. (Green lines) Experimentally demonstrated pathways; (red lines) investigated routes that failed to be demonstrated; (dashed blue lines) proposed transmission pathways. Boxes include the corresponding reference studies. Yellow-lined boxes indicate indirect evidence for the suggested pathways. Color images available online at www.liebertpub.com/vbz
Various studies have demonstrated the capability of fleas to acquire and transmit Bartonella strains under experimental conditions. Xenopsylla cheopis fleas were reported as competent vectors of an unidentified Bartonella sp. on Myodes glareolus voles through a pioneer study (Krampitz 1962). More recently, wild-collected Ctenophthalmus nobilis demonstrated the ability to transmit B. grahamii and B. taylorii to captive-bred M. glareolus voles (Bown et al. 2004) and Xenopsylla ramesis has been shown to be a competent vector of Bartonellae sp. OE 1-1 to Meriones crassus jirds (Morick et al. 2011, Morick et al. 2013a). The latter studies demonstrated a remarkable efficiency of fleas in acquiring and transmitting Bartonella. About 69%–100% of the fleas acquired the Bartonella sp. within a period of 72 h, and a similar period was sufficient for the fleas to infect naïve jirds. It is acknowledged that this transmission route from arthropod to mammal is done through the gastrointestinal content, mainly feces (stercoraria) (Birtles 2005). Supporting evidence for this route was initially demonstrated in cats infected through the inoculation of cat-flea (Ctenocephalides felis) feces (Foil et al. 1998). As stercoraria may seem an inefficient infection route (Birtles 2005, Marignac et al. 2010), further studies are required to investigate the occurrence of stercoraria, determine possible fecal components that may promote entry and establishment of bartonellae in the intradermal compartment, calculate the amount of fecal depositions in host skin per a specific time period, and ultimately define the minimal bacterial load required for infection efficiency.
Alternative vertical or horizontal transmission pathways of Bartonella infection among rodents, without the mediation of an arthropod, have been studied under natural and laboratory conditions. First, vertical transmission in wild-captured Sigmodon hispidus and Peromyscus leucopus rodents was observed by isolating Bartonella spp. from the placental tissues of the pregnant animals, embryos, and from neonatal pups (Kosoy et al. 1998). Later, Boulouis et al. (2001) explored the vertical transmission of B. birtlesii in BALB/c mice. The authors obtained similar results in the transplacental transmission of B. birtlesii in bacteremic pregnant BALB/c mice. However, isolation of the bacteria was possible only from reabsorbed fetuses, but not from dead or viable ones. Contrary to those studies, no transmission was detected from infected females to their offspring in bank voles (M. glareolus) (Bown et al. 2004). Recent studies on a desert rodent, M. crassus, demonstrated the presence of Bartonella DNA in one of 15 pups born to experimental infected jirds (Morick et al. 2013a). Altogether, these studies suggest that this maternal transmission route is feasible, although it seems to have a minor role in the Bartonella cycle in the wild. Horizontal transmission between male and female rodents was also investigated, where rodents were kept under an arthropod-free environment, resulting in no transmission of Bartonella from the experimentally infected to the naïve rodents (Bown et al. 2004). To fill the knowledge gaps in arthropod-free transmission routes, studies evaluating the potential interaction (e.g., social or aggressive interactions) between rodents from the same sex and between different species and genera are required (Fig. 2).
Arthropods may serve as reservoirs for bacteria in nature. To accomplish such role, the arthropod needs to allow the transmission of the bacteria from one generation to another through vertical mechanisms, such as transstadial, transovarial, co-feeding, or even sexual mechanisms (Parola and Raoult 2001). The vertical transmission of Bartonella among arthropods has been explored in various studies (Halos et al. 2004, Cotte et al. 2008, Morick et al. 2011, Morick et al. 2013c). The detection of Bartonella DNA in the reproductive tissues of Cediopsylla inaequalis, Oropsylla hirsuta, Aetheca wagneri, and Orchopeas leucopus (Brinkerhoff et al. 2010), and Ctenophthalmus congener truncus and Neopsylla sasai (Kabeya et al. 2011) fleas, highlighted the potential transovarial transmission of Bartonella in fleas. However, X. ramesis–infected fleas did not show a transovarial transmission to their offspring (Morick et al. 2011). It has to be acknowledged that flea interspecies differences might be involved in such contrasting results; thus, this route cannot be ruled out for all flea species. Additionally, in the latter study, the presence of Bartonella DNA in gut voids from infected fleas suggested an alternative non-transovarial transmission route, since it is known that underdigested blood regurgitated by parent fleas can serve as food supply for flea larvae (Krasnov 2008, Khokhlova et al. 2010). This route was later demonstrated through the acquisition of Bartonella infection by immature flea stages that were exposed to gut voids and/or feces deposited by infected adult fleas (Morick et al. 2013c). Similarly, other arthropods have shown this non-transovarial transmission route: The stinkbug Riptortus clavatus were shown to acquire its Burkholderia sp. gut symbiont from the environment on nymph stages, allowing the transfer of the symbiont every generation through a postnatal mechanism (Kikuchi et al. 2007). This transmission route gives extra significance to the microhabitat in burrows of rodents for the continuous infection of bartonellae among fleas. It can be hypothetized that old and abandonned rodent burrows can still serve as potential temporal reservoirs of arthropods for bartonellae infection through the presence of flea feces or gut voids that can serve as food for newly hatched flea larvae. Nevertheless, further questions, such as the time length that these gut contents remain infective, have to be answered to verify this hypothesis.
Alternative routes of infection have been explored in mammals. Guptill et al. (1999) reported infection through oral inoculation of B. henselae in cats. Foil et al. (1998) explored additional infection strategies of B. henselae in cats, including bacteremic blood injections. Similarly, alternative routes were tested in rodents, including oral and ocular routes (Marignac et al. 2010). These experiments investigated the potential acquisition of Bartonella infection by ingestion of fleas by the rodents (e.g., as a result of grooming) or the direct inoculation of the bacteria into the eyes (e.g., as a result of aggression behavior) that could prompt these membranes as alternative entry pathways. Nevertheless, these routes showed to be less efficient and required higher doses to produce bacteremia.
Bartonella Infection Dynamics: From Experimental Infection to Field Studies
Experimental infection
The infection dynamics of rodent-associated bartonellae have been extensively explored and partially elucidated in wild-captured and laboratory rodent models (Kosoy et al. 1999, Kosoy et al. 2000, Boulouis et al. 2001, Koesling et al. 2001, Schulein et al. 2001, Marignac et al. 2010, Colton et al. 2011, Colton and Kosoy 2012, Morick et al. 2013a). The first phase of the Bartonella infection, spanning from the inoculation of the flea infected-contents (e.g., feces and gut voids) on the wounded skin of the mammal to the appearance of the bacteremia still remains obscure (Chomel et al. 2009, Harms and Dehio 2012). An incubation period has been evidenced after rodents were inoculated with bartonellae by intravenous, subcutaneous, intradermal, or flea-infected challenges (Schulein et al. 2001, Marignac et al. 2010, Morick et al. 2013a). Thus, a primary niche for the infection has been proposed (Chomel et al. 2009, Harms and Dehio 2012). Although, nonconclusive in vivo evidence of this niche or the potential cells involved have been published to date, the vascular endothelial cells seem to be the major candidate for this niche (Deng et al. 2012). Once the bartonellae reach the bloodstream, they infect their final target cells, the erythrocytes. Schulein et al. (2001) observed cyclic bacteremic waves during the course of infection after intravenous challenge, supporting the role of the proposed niche in reseeding the bacteria into the bloodstream.
In a recent study, bacteremic waves were observed in naturally infected jirds, and in jirds challenged with infected fleas or subcutaneous inoculation, raising supporting evidence that this phenomenon occurs in the wild (Morick et al. 2013a). It has been proposed that these cyclic bacteremic waves are the result of an apparent clearance of the bacteria by the immune system, followed by a new bacterial input from the primary niche to the bloodstream (Harms and Dehio 2012). However, because infection with Bartonella does not cause hemolysis or reduced erythrocytes life span, it is likely that those reinfection waves are a product of lytic cycles of the primary niche (Schulein et al. 2001). Interestingly, bacteremia relapses were not reproducible in outbred and inbred heterologous mice models, even after induction of immunosuppression (Marignac et al. 2010). Therefore, if the primary niche is playing the proposed role, this may occur only in highly adapted hosts (natural reservoir). Through this unique infection strategy, bartonellae have evolved in a way that permits hematophagous arthropods to efficiently acquire the bacteria and successfully transmit it to other hosts (Schulein et al. 2001, Chomel et al. 2009).
Infection dynamic studies using murine models infected with adapted bartonellae have reproduced long-lasting bacteremia under laboratory conditions. Cotton rats (S. hispidus) infected with three Bartonella strains isolated from the same rodent species, reproduced bacteremia for up to 15 weeks (Kosoy et al. 1999). Notably, two Sundevall's jirds (M. crassus) naturally infected with Bartonella sp. OE 1-1, kept under laboratory conditions, remained bacteremic for 24 and 30 weeks, respectively. Additionally, subcutaneously and flea-challenged naïve rodents showed infection persistence of up to 23 weeks (Morick et al. 2013a). These results in rodent models mirrored the patterns observed in other natural reservoirs, such as cats infected with B. henselae (Kordick et al. 1995).
To evaluate the course of Bartonella infection in accidental hosts, murine laboratory animals were challenged with Bartonella strains isolated from other rodent species. Interestingly, these studies have raised contradicting results. Through an extensive challenge study, using cotton rats (S. hispidus), white-footed mice (P. leucopus), BALB/c (Mus musculus), and Wistar rats (Rattus norvegicus) inoculated with 14 Bartonella strains (including human isolates), Kosoy et al. (2000) observed that bacteremia could be reproduced only in cotton rats and white-footed mice infected with Bartonella strains isolated from the same species or from congenic rodents. In contrast, other studies have reproduced long-lasting bacteremia (up to 11 weeks postinfection [p.i.]) in laboratory mice (M. musculus inbred strains) with B. birtlesii and B. grahamii, isolated from Apodemus sp. mouse and Microtus agrestis vole, respectively (Boulouis et al. 2001, Koesling et al. 2001, Marignac et al. 2010). A recent study reported a sustained bacteremia (11 weeks) in M. musculus with two B. tribocorum strains isolated from Mus caroli and Mus cervicolor, evidencing the congenic potential transmission of bartonellae (Colton and Kosoy 2012). Similarly, Swiss Webster mice inoculated with a Bartonella strain closely related to B. coopersplainensis isolated from R. norvegicus became bacteremic during a period that lasted from 4 to 8 weeks (Colton et al. 2011). Nevertheless, in the same study, bacteremia was not obtained when three other rat-associated bartonellae strains were used. Therefore, experimental evidences raised a dual scenario of what might be happening in nature: On one hand, some bartonellae may have a limited host specificity range, whereas certain spillover of other bartonellae between animals may occur. The reasons for this dichotomy must rely on the Bartonella strain and its armament of virulence factors that provide or limit its capability to “jump” from one host species to another, as described in B. grahamii (Berglund et al. 2009).
When murine challenge studies have applied to more phylogenetically distant Bartonella spp., such as B. henselae from cats, mimicking natural incidental infections, pathological manifestations were observed. It is noteworthy in these cases that bacteremia was not established (Regnath et al. 1998, Kunz et al. 2008).
Bacterial dose, required to generate an effective infection, is an important variable that should be taken into consideration in the study of Bartonella infections. In this regard, contrasting results have been obtained under experimental challenges. In one study, more mice became infected when higher doses were inoculated (Colton et al. 2011). Conversely, longer duration and higher bacteremia levels have been obtained with low inoculum doses (∼103 colony-forming units [CFU]) (Kosoy et al. 1999, Marignac et al. 2010). Interestingly, Boulouis et al. (2001) did not find significant differences in the bacteremia level with inoculum doses above 1.5×103 CFU. In host communities, rodents would likely be exposed to low doses of bartonellae by vector transmission. Notwithstanding, the number of fleas infesting each rodent and the rate of blood feeding of each flea are extra variables that can contribute to the real dose and exposure to bartonellae, as discussed before. Therefore, quantification of the bartonellae load in fleas and their gastrointestinal contents and tissues is crucial. In a pioneering study, bartonellae loads in C. felis feces were determined, reaching up to 5.3×103 B. henselae CFU/mg of feces (Finkelstein et al. 2002). More recently, through a kinetics study of B. henselae infection in cat fleas fed on an artificial feeding system, a potential replication and prolonged persistence of the Bartonella in fleas and their feces was suggested (Bouhsira et al. 2013). In addition, the determination of potential substances that could enhance the bartonellae replication or establishment of the infection in the host skin should be explored as they may reveal important features.
Field studies: Natural dynamics of bartonellae infection
To describe the temporal dynamics of Bartonella infection in rodent communities, longitudinal field studies have been carried out (Fichet-Calvet et al. 2000, Birtles et al. 2001, Kosoy et al. 2004a, Telfer et al. 2007a, 2007b, Bai et al. 2008b, Welc-Faleciak et al. 2010, Bai et al. 2011). These studies have shown interesting variations in the pattern of Bartonella infections among seasons, rodent age cohorts, rodent sexes, in dependence of the level of flea parasitism, persistence of infection, and other factors in different rodent species and communities.
As rodents' density and activity vary across seasons (Telfer et al. 2007b), the exposition and prevalence of microbial infections can also be affected by this seasonality (Bai et al. 2008b). Microbial prevalence in hosts can vary due to changes in infection resistance, association between other microbial species, and changes in the activity of their vectors (Telfer et al. 2007b). Accordingly, it has been observed that Bartonella prevalence varies significantly during the year and across years (Bai et al. 2008b, Welc-Faleciak et al. 2010). Peaks of higher Bartonella prevalence have been reported from summer to fall in most of the rodent species worldwide (Fichet-Calvet et al. 2000, Jardine et al. 2006b, Telfer et al. 2007a, Bai et al. 2008b, Paziewska et al. 2012a). Even in cotton rats (S. hispidus) from Georgia in the United States that maintain a high prevalence all over the year, a peak during early summer to mid-fall was evident (Kosoy et al. 2004a). Increasing prevalence of Bartonella infection in those warmer periods, correlates with the emerging of juvenile rodents from their burrows (Jardine et al. 2006b), higher activity of rodents, peak of the vector activity, or higher infestation by seasonal fleas (Krasnov et al. 2002, 2005). Interestingly, bank voles and wood mice from the United Kingdom demonstrated two patterns of seasonality depending on the Bartonella sp. involved (Telfer et al. 2007b). Additionally, the survival rates after a critical period (e.g., winter or summers) is a relevant variable for the Bartonella cycle, as this may remodel the host population demographics (Fichet-Calvet et al. 2000, Welc-Faleciak et al. 2010).
Age- or body mass–dependence patterns in the prevalence of Bartonella infection have been found in wild rodents. Generally, juvenile and subadult animals tend to be more affected than adults in the wild (Fichet-Calvet et al. 2000, Kosoy et al. 2004a, Jardine et al. 2006b, Telfer et al. 2007a, Bai et al. 2008b, Morway et al. 2008). This age bias has been associated with several factors, such as a tendency of juvenile rodents to be more mobile than territorial adults (higher exposure in the formers), higher risk of acquiring the infection as a result of its immature immune system, or the fact that older rodents have the chance to clear the infection during their lifetime (Fichet-Calvet et al. 2000, Welc-Faleciak et al. 2010). An acquired humoral immunity was previously proposed for the lower infection prevalence in older rodents (Fichet-Calvet et al. 2000), but low titers of antibodies observed in cotton rats have challenged this explanation (Kosoy et al. 2004a). The latter authors suggested a potential immune tolerance as an outcome of exposure to the bartonellae antigens in utero or antibody sequestration as explanation of the nondetection of antibodies, but this can be true only for those host species that present vertical transmission. Bai et al. (2008b) observed that juvenile black-tailed prairie dogs became infected shortly after emerging from their burrows, but once they reached a body mass above 300 grams, the infection prevalence declined. The authors suggested that a mass threshold (>700 grams) can represent a dramatic adjustment of their immune system linked to the clearance of the infection (Bai et al. 2008b). Furthermore, a natural selection for noninfected animals could explain the reduced prevalence of Bartonella in older animals (Jardine et al. 2006b). Nevertheless, no specific rule for Bartonella infection can be concluded, since no correlation between age groups and weight-dependent prevalence patterns were observed both in deer mice from Colorado (Bai et al. 2011) and bank voles from France (Buffet et al. 2012).
Despite sex differences in rodent ecology, contradictory results of the role of rodent sex in Bartonella infection have been observed. No difference between infection rates of males and female rodents has been observed in many studies (Fichet-Calvet et al. 2000, Kosoy et al. 2004a, Bai et al. 2008b, Morway et al. 2008, Bai et al. 2011). However, differences in the Bartonella prevalence according to sex was reported in Apodemus sylvaticus and A. flavicollis, in which males presented higher infection prevalence in comparison to female rodents (Welc-Faleciak et al. 2010, Harrison et al. 2012).
The correlation between flea abundance and bartonellae infection in rodents has shown some variation across studies. In certain cases, the seasonality of the flea positively correlated to the bartonellae prevalence in rodents (Jardine et al. 2006b, Welc-Faleciak et al. 2010). For instance, it was noticed that the higher flea abundance on wild rodents from Poland in 2006 correlated with a higher overall Bartonella infection compared to 2004 (Welc-Faleciak et al. 2010). On the contrary, differences in infestation rates of R. rattus and R. norvegicus from South Africa did not explain the observed difference in Bartonella infection prevalence (24% versus 5%) between these rat species (Brettschneider et al. 2012a). The Bartonella sp. involved can also determine the final effect of fleas on the Bartonella prevalence, as was reported on field voles (M. agrestis) in which the presence of fleas had only a positive effect on the infection probability of a Bartonella sp. (i.e., BGA strain) (Telfer et al. 2007a). Moreover, the role of fleas as intermediaries of the natural cycle was proposed for B. taylorii in wood mice (A. sylvaticus) and B. doshiae in field voles (M. agrestis), since a “delayed effect” of host densities was noticed (i.e., fleas buffering the timing of transmission) (Telfer et al. 2007a, 2007b).
Variations in the persistence of Bartonella infection in wild conditions have also been observed. Some studies on rodent populations from Europe and United States have reported long-persistent bacteremia of several months (Kosoy et al. 2004a, Bai et al. 2011). Through sequential screenings of tagged individual rodents, the Bartonella infection has shown to be very dynamic. Some rodents demonstrated a clearance of the infection in a short time (Birtles et al. 2001), whereas others remained infected for periods as long as 9 months (Kosoy et al. 2004a). In addition, reappearance of bacteremia after an apparent infection clearance has also been observed in wild rodents (Kosoy et al. 2004b, Jardine et al. 2006a, Bai et al. 2011).
Many rodent populations exhibit certain features that may play a role in the Bartonella prevalence and distribution. The northern grasshopper mouse (O. leucogaster) routinely invades burrows of other rodents and predates on their owners (McCarty 1978). This behavior can be associated with the remarkable prevalence of Bartonella infection in O. leucogaster, one of the highest detected to date (Bai et al. 2007a). On the contrary, there are cases in which ecological characteristics can hardly be associated with the Bartonella infection. Two closely related Neotoma spp. demonstrated a different Bartonella prevalence, despite the fact that their habitat, diet, behavior, and parasitizing flea species are virtually the same (Morway et al. 2008). Similarly, the difference in the Bartonella persistence on M. glareolus and A. flavicollis seems to lie in the bacteria–rodent interaction rather than ecological differences between these rodents (Paziewska et al. 2012a).
Bartonella Infection Composition
Infection with more than one Bartonella spp. or variant is a well-acknowledged phenomenon in rodents and fleas (Kosoy et al. 2004b, Abbot et al. 2007, Telfer et al. 2007a, Brinkerhoff et al. 2010, Morick et al. 2011). The determination of the infection composition in the host and vectors can help understanding the infection fluctuations observed in longitudinal studies. Kosoy et al (2004b) isolated up to three different genotypes from a single cotton rat blood sample. The authors detected co-infection in 21.3% of 408 cotton rat samples holding an overall of 26 combinations of mixed strain infections. In a recent study, we observed that multiple variants can be distributed in rodents and their fleas in an expanded repertoire of infection compositions (Gutiérrez et al. 2014). Single carriers (rodent and fleas) harbored infection composed of multiple closely and distantly phylogenetically related Bartonella genotypes, which circulated under a potential intergenotype competition and reflected a tendency to dominate a particular carrier type (i.e., rodent or flea). It seems that traditional diagnostic methods could bias the real picture of Bartonella infection structures in reservoir animals.
Longitudinal studies have shown that in sequential screenings a Bartonella genotype can be repetitively detected or replaced by a close or distinct phylogenetic genotype, even after a nonbacteremic period (Birtles et al. 2001, Kosoy et al. 2004b, Jardine et al. 2006a). New bacteremic periods of a previously detected genotype could be the result of a new vector transmission event or a reseeding event of the hidden genotype from the potential primary niche (as previously discussed) (Schulein et al. 2001, Kosoy et al. 2004b, Bai et al. 2011). An alternative explanation might be that the infection has never been cleared from the blood, but its level decreased below detectable levels (Bai et al. 2011). Thus, this fluctuation in Bartonella in wild rodents (alternating bacteremic and nonbacteremic periods) has questioned the effectiveness of the host immune system.
Co-infection with more than one Bartonella species in wild rodents might reveal an additional scenario associated with the cyclic bacteremia phenomenon. In a previous study, two different Bartonella strains were isolated at different sampling dates from the blood of naturally infected rodents (M. crassus and Gerbillus nanus) kept under laboratory conditions (i.e., no external source of infection) (Morick et al. 2011). Similarly, different B. henselae variants were isolated from different bacteremic peaks in naturally infected cats (Kabeya et al. 2002). These findings suggest that two co-existing Bartonella variants could alternate between the primary niche and the bloodstream, producing individual bacteremic waves. Another option is that both variants can co-exist in the blood, but one dominates the infection and can obscure the presence or detection of other variants, as observed in the Bartonella infection composition in wild rodents and their fleas (Gutiérrez et al. 2014). Nevertheless, Chan and Kosoy (2010) explored the frequencies of acquiring new variants in the cotton rats, and concluded that some cross-immunity exists and plays a filtering role, limiting the reinfection of some variants that are closely related. It seems evident that in sequential determinations either the different variant infections are intercalating in a way that a long persistent infection is maintained (yet heterogeneous), or a multi-infection with different variants is occurring more frequently than expected, and the determination of the predominant variant in a given time obscures the others (Paziewska et al. 2012a).
Conclusions
Bartonellae have shown an outstanding adaptation within rodents and their flea parasites. The diversity of Bartonella spp. and genotypes discovered in Rodentia compared to other mammals is the highest to date. Additionally, spillover of bartonellae between rodent species and genera, and the interactions between co-existing Bartonella variants in the same host, might play a substantial role in the generation of diversity in these bacteria. Investigation of the ecology of populations and communities of rodents and fleas can help understand the dynamics of Bartonella infections, but generalizations are not apparent across rodent–flea systems. Much contrasting evidence has raised a puzzle of ecological traits affecting the Bartonella cycle between the rodent populations studied to date. It appears that the interactions within the bartonellae–flea–rodent triangle are specific and result in particular traits for each system. Thus, the elucidation of each Bartonella cycle must be evaluated individually.
Acknowledgments
This research was supported by the Israel Science Foundation (grant number 30/11 to Shimon Harrus), the Ministerio de Ciencia y Tecnologia (MICIT), and the Consejo Nacional para Investigaciones Cientificas y Tecnologicas (CONICIT), Costa Rica. This is publication no. 835 of the Mitrani Department of Desert Ecology.
Author Disclosure Statement
No competing financial interests exist.
References
- Abbot P, Aviles AE, Eller L, Durden LA. Mixed infections, cryptic diversity, and vector-borne pathogens: Evidence from Polygenis fleas and Bartonella species. Appl Environ Microbiol 2007; 73:6045–6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E, Khamphoukeo K, Grice D, Newton PN, et al. Molecular detection of Bartonella species in rodents from the Lao PDR. Clin Microbiol Infect 2009; 15(Suppl 2):95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Kosoy MY, Cully JF, Bala T, et al. Acquisition of nonspecific Bartonella strains by the northern grasshopper mouse (Onychomys leucogaster). FEMS Microbiol Ecol 2007a; 61:438–448 [DOI] [PubMed] [Google Scholar]
- Bai Y, Montgomery SP, Sheff KW, Chowdhury MA, et al. Bartonella strains in small mammals from Dhaka, Bangladesh, related to Bartonella in America and Europe. Am J Trop Med Hyg 2007b; 77:567–570 [PubMed] [Google Scholar]
- Bai Y, Kosoy M, Martin A, Ray C, et al. Characterization of Bartonella strains isolated from black-tailed prairie dogs (Cynomys ludovicianus). Vector Borne Zoonotic Dis 2008a; 8:1–5 [DOI] [PubMed] [Google Scholar]
- Bai Y, Kosoy MY, Ray C, Brinkerhoff RJ, et al. Temporal and spatial patterns of Bartonella infection in black-tailed prairie dogs (Cynomys ludovicianus). Microb Ecol 2008b; 56:373–382 [DOI] [PubMed] [Google Scholar]
- Bai Y, Kosoy MY, Lerdthusnee K, Peruski LF, et al. Prevalence and genetic heterogeneity of Bartonella strains cultured from rodents from 17 provinces in Thailand. Am J Trop Med Hyg 2009; 81:811–816 [DOI] [PubMed] [Google Scholar]
- Bai Y, Calisher CH, Kosoy MY, Root JJ, et al. Persistent infection or successive reinfection of deer mice with Bartonella vinsonii subsp. arupensis. Appl Environ Microbiol 2011; 77:1728–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JA. A rickettsial infection in Canadian voles. J Exp Med 1946; 84:37–50 [PubMed] [Google Scholar]
- Beldomenico PM, Begon M. Disease spread, susceptibility and infection intensity: Vicious circles? Trends Ecol Evol 2010; 25:21–27 [DOI] [PubMed] [Google Scholar]
- Berglund EC, Frank AC, Calteau A, Vinnere Pettersson O, et al. Run-off replication of host-adaptability genes is associated with gene transfer agents in the genome of mouse-infecting Bartonella grahamii. PLoS Genet 2009; 5:e1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund EC, Ellegaard K, Granberg F, Xie Z, et al. Rapid diversification by recombination in Bartonella grahamii from wild rodents in Asia contrasts with low levels of genomic divergence in Northern Europe and America. Mol Ecol 2010; 19:2241–2255 [DOI] [PubMed] [Google Scholar]
- Bermond D, Heller R, Barrat F, Delacour G, et al. Bartonella birtlesii sp. nov., isolated from small mammals (Apodemus spp.). Int J Syst Evol Microbiol 2000; 50(Pt 6):1973–1979 [DOI] [PubMed] [Google Scholar]
- Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol 2008; 22:1–15 [DOI] [PubMed] [Google Scholar]
- Billeter SA, Gundi VA, Rood MP, Kosoy MY. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvegicus rats in Los Angeles, California. Appl Environ Microbiol 2011; 77:7850–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter SA, Colton L, Sangmaneedet S, Suksawat F, et al. Molecular detection and identification of Bartonella species in rat fleas from northeastern Thailand. Am J Trop Med Hyg 2013; 89:462–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtles RJ. Bartonellae as elegant hemotropic parasites. Ann NY Acad Sci 2005; 1063:270–279 [DOI] [PubMed] [Google Scholar]
- Birtles RJ, Harrison TG, Saunders NA, Molyneux DH. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol 1995; 45:1–8 [DOI] [PubMed] [Google Scholar]
- Birtles RJ, Raoult D. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol 1996; 46:891–897 [DOI] [PubMed] [Google Scholar]
- Birtles RJ, Canales J, Ventosilla P, Alvarez E, et al. Survey of Bartonella species infecting intradomicillary animals in the Huayllacallan Valley, Ancash, Peru, a region endemic for human bartonellosis. Am J Trop Med Hyg 1999; 60:799–805 [DOI] [PubMed] [Google Scholar]
- Birtles RJ, Hazel SM, Bennett M, Bown K, et al. Longitudinal monitoring of the dynamics of infections due to Bartonella species in UK woodland rodents. Epidemiol Infect 2001; 126:323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitam I, Rolain JM, Kernif T, Baziz B, et al. Bartonella species detected in rodents and hedgehogs from Algeria. Clin Microbiol Infect 2009; 15(Suppl 2):102–103 [DOI] [PubMed] [Google Scholar]
- Bouhsira E, Ferrandez Y, Liu MF, Franc M, et al. Ctenocephalides felis an in vitro potential vector for five Bartonella species. Comp Immunol Microbiol Infect Dis 2013; 36:105–111 [DOI] [PubMed] [Google Scholar]
- Boulouis HJ, Barrat F, Bermond D, Bernex F, et al. Kinetics of Bartonella birtlesii infection in experimentally infected mice and pathogenic effect on reproductive functions. Infect Immun 2001; 69:5313–5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown KJ, Ellis BA, Birtles RJ, Durden LA, et al. New world origins for haemoparasites infecting United Kingdom grey squirrels (Sciurus carolinensis), as revealed by phylogenetic analysis of Bartonella infecting squirrel populations in England and the United States. Epidemiol Infect 2002; 129:647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown KJ, Bennet M, Begon M. Flea-borne Bartonella grahamii and Bartonella taylorii in bank voles. Emerg Infect Dis 2004; 10:684–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Cadenas MB, de Paiva Diniz PP. A groundhog, a novel Bartonella sequence, and my father's death. Emerg Infect Dis 2009; 15:2080–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner DJ, O'Connor SP, Winkler HH, Steigerwalt AG. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol 1993; 43:777–786 [DOI] [PubMed] [Google Scholar]
- Brettschneider H, Anguelov R, Chimimba CT, Bastos AD. A mathematical epidemiological model of gram-negative Bartonella bacteria: Does differential ectoparasite load fully explain the differences in infection prevalence of Rattus rattus and Rattus norvegicus? J Biol Dyn 2012a; 6:763–781 [DOI] [PubMed] [Google Scholar]
- Brettschneider H, Bennett NC, Chimimba CT, Bastos AD. Bartonellae of the Namaqua rock mouse, Micaelamys namaquensis (Rodentia: Muridae) from South Africa. Vet Microbiol 2012b; 157:132–136 [DOI] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Kabeya H, Inoue K, Bai Y, et al. Detection of multiple Bartonella species in digestive and reproductive tissues of fleas collected from sympatric mammals. ISME J 2010; 4:955–958 [DOI] [PubMed] [Google Scholar]
- Buffet JP, Marsot M, Vaumourin E, Gasqui P, et al. Co-infection of Borrelia afzelii and Bartonella spp. in bank voles from a suburban forest. Comp Immunol Microbiol Infect Dis 2012; 35:583–589 [DOI] [PubMed] [Google Scholar]
- Buffet JP, Kosoy M, Vayssier-Taussat M. Natural history of Bartonella-infecting rodents in light of new knowledge on genomics, diversity and evolution. Future Microbiol 2013a; 8:1117–1128 [DOI] [PubMed] [Google Scholar]
- Buffet JP, Pisanu B, Brisse S, Roussel S, et al. Deciphering Bartonella diversity, recombination, and host specificity in a rodent community. PLoS One 2013b; 8:e68956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle KT, Kosoy M, Lerdthusnee K, Phelan L, et al. Prevalence and diversity of Bartonella in rodents of northern Thailand: A comparison with Bartonella in rodents from southern China. Am J Trop Med Hyg 2004; 70:429–433 [PubMed] [Google Scholar]
- Chae JS, Yu do H, Shringi S, Klein TA, et al. Microbial pathogens in ticks, rodents and a shrew in northern Gyeonggi-do near the DMZ, Korea. J Vet Sci 2008; 9:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KS, Kosoy M. Analysis of multi-strain Bartonella pathogens in natural host population—do they behave as species or minor genetic variants? Epidemics 2010; 2:165–172 [DOI] [PubMed] [Google Scholar]
- Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, et al. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet Res 2009; 40:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton L, Kosoy M. Experimental infection of laboratory mice with two Bartonella tribocorum strains from wild Mus species: A homologous host-bacteria model system at the genus level. Parasitology 2012; 140:61–68 [DOI] [PubMed] [Google Scholar]
- Colton L, Zeidner N, Kosoy MY. Experimental infection of Swiss webster mice with four rat bartonella strains: Host specificity, bacteremia kinetics, dose dependent response, and histopathology. Comp Immunol Microbiol Infect Dis 2011; 34:465–473 [DOI] [PubMed] [Google Scholar]
- Costa F, Porter FH, Rodrigues G, Farias H, et al. Infections by Leptospira interrogans, Seoul Virus, and Bartonella spp. Among Norway Rats (Rattus norvegicus) from the urban slum environment in Brazil. Vector Borne Zoonotic Dis 2014; 14:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotte V, Bonnet S, Le Rhun D, Le Naour E, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis 2008; 14:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JS, Worthington MG, Brenner DJ, Moss CW, et al. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol 1993; 31:872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Le Rhun D, Buffet JP, Cotte V, et al. Strategies of exploitation of mammalian reservoirs by Bartonella species. Vet Res 2012; 43:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden LA, Ellis BA, Banks CW, Crowe JD, et al. Ectoparasites of gray squirrels in two different habitats and screening of selected ectoparasites for bartonellae. J Parasitol 2004; 90:485–489 [DOI] [PubMed] [Google Scholar]
- Ellis BA, Regnery RL, Beati L, Bacellar F, et al. Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: An Old World origin for a New World disease? J Infect Dis 1999; 180:220–224 [DOI] [PubMed] [Google Scholar]
- Engbaek K, Lawson PA. Identification of Bartonella species in rodents, shrews and cats in Denmark: Detection of two B. henselae variants, one in cats and the other in the long-tailed field mouse. APMIS 2004; 112:336–341 [DOI] [PubMed] [Google Scholar]
- Engel P, Salzburger W, Liesch M, Chang CC, et al. Parallel evolution of a type IV secretion system in radiating lineages of the host-restricted bacterial pathogen Bartonella. PLoS Genet 2011; 7:e1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremeeva ME, Gerns HL, Lydy SL, Goo JS, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med 2007; 356:2381–2387 [DOI] [PubMed] [Google Scholar]
- Fenollar F, Sire S, Raoult D. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J Clin Microbiol 2005; 43:945–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichet-Calvet E, Jomaa I, Ben Ismail R, Ashford RW. Patterns of infection of haemoparasites in the fat sand rat, Psammomys obesus, in Tunisia, and effect on the host. Ann Trop Med Parasitol 2000; 94:55–68 [DOI] [PubMed] [Google Scholar]
- Finkelstein JL, Brown TP, O'Reilly KL, Wedincamp J Jr., et al. Studies on the growth of Bartonella henselae in the cat flea (Siphonaptera: Pulicidae). J Med Entomol 2002; 39:915–919 [DOI] [PubMed] [Google Scholar]
- Foil L, Andress E, Freeland RL, Roy AF, et al. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J Med Entomol 1998; 35:625–628 [DOI] [PubMed] [Google Scholar]
- Gil H, Garcia-Esteban C, Barandika JF, Peig J, et al. Variability of Bartonella genotypes among small mammals in Spain. Appl Environ Microbiol 2010; 76:8062–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundi VA, Davoust B, Khamis A, Boni M, et al. Isolation of Bartonella rattimassiliensis sp. nov. and Bartonella phoceensis sp. nov. from European Rattus norvegicus. J Clin Microbiol 2004; 42:3816–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundi VA, Taylor C, Raoult D, La Scola B. Bartonella rattaustraliani sp. nov., Bartonella queenslandensis sp. nov. and Bartonella coopersplainsensis sp. nov., identified in Australian rats. Int J Syst Evol Microbiol 2009; 59:2956–2961 [DOI] [PubMed] [Google Scholar]
- Gundi VA, Kosoy MY, Myint KS, Shrestha SK, et al. Prevalence and genetic diversity of Bartonella species detected in different tissues of small mammals in Nepal. Appl Environ Microbiol 2010; 76:8247–8254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundi VA, Billeter SA, Rood MP, Kosoy MY. Bartonella spp. in rats and zoonoses, Los Angeles, California, USA. Emerg Infect Dis 2012a; 18:631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundi VA, Kosoy MY, Makundi RH, Laudisoit A. Identification of diverse Bartonella genotypes among small mammals from Democratic Republic of Congo and Tanzania. Am J Trop Med Hyg 2012b; 87:319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptill L, Slater L, Wu CC, Glickman LT, et al. Immune response of neonatal specific pathogen-free cats to experimental infection with Bartonella henselae. Vet Immunol Immunopathol 1999; 71:233–243 [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Morick D, Cohen C, Hawlena H, et al. The effect of ecological and temporal factors on the composition of Bartonella infection in rodents and their fleas. ISME J 2014; doi: 10.1038/ismej.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy L, Nystedt B, Toft C, Zaremba-Niedzwiedzka K, et al. A gene transfer agent and a dynamic repertoire of secretion systems hold the keys to the explosive radiation of the emerging pathogen Bartonella. PLoS Genet 2013; 9:e1003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halos L, Jamal T, Maillard R, Girard B, et al. Role of Hippoboscidae flies as potential vectors of Bartonella spp. infecting wild and domestic ruminants. Appl Environ Microbiol 2004; 70:6302–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A, Dehio C. Intruders below the radar: Molecular pathogenesis of Bartonella spp. Clin Microbiol Rev 2012; 25:42–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, Bown KJ, Montgomery WI, Birtles RJ. Ixodes ricinus is not an epidemiologically relevant vector of Bartonella species in the wood mouse (Apodemus sylvaticus). Vector Borne Zoonotic Dis 2012; 12:366–371 [DOI] [PubMed] [Google Scholar]
- Harrus S, Bar-Gal GK, Golan A, Elazari-Volcani R, et al. Isolation and genetic characterization of a Bartonella strain closely related to Bartonella tribocorum and Bartonella elizabethae in Israeli commensal rats. Am J Trop Med Hyg 2009; 81:55–58 [PubMed] [Google Scholar]
- Hawlena H, Rynkiewicz E, Toh E, Alfred A, et al. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J 2013; 7:221–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R, Riegel P, Hansmann Y, Delacour G, et al. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int J Syst Bacteriol 1998; 48(Pt 4):1333–1339 [DOI] [PubMed] [Google Scholar]
- Hildebrand J, Paziewska-Harris A, Zalesny G, Harris PD. PCR characterization suggests that an unusual range of Bartonella species infect the striped field mouse (Apodemus agrarius) in Central Europe. Appl Environ Microbiol 2013; 79:5082–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg M, Mills JN, McGill S, Benjamin G, et al. Bartonella infection in sylvatic small mammals of central Sweden. Epidemiol Infect 2003; 130:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JW, Tung KC, Chen WC, Lin JW, et al. Epidemiology of Bartonella infection in rodents and shrews in Taiwan. Zoonoses Public Health 2010; 57:439–446 [DOI] [PubMed] [Google Scholar]
- Inoue K, Maruyama S, Kabeya H, Yamada N, et al. Prevalence and genetic diversity of Bartonella species isolated from wild rodents in Japan. Appl Environ Microbiol 2008; 74:5086–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Maruyama S, Kabeya H, Hagiya K, et al. Exotic small mammals as potential reservoirs of zoonotic Bartonella spp. Emerg Infect Dis 2009; 15:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Kabeya H, Shiratori H, Ueda K, et al. Bartonella japonica sp. nov. and Bartonella silvatica sp. nov., isolated from Apodemus mice. Int J Syst Evol Microbiol 2010; 60:759–763 [DOI] [PubMed] [Google Scholar]
- Jardine C, Appleyard G, Kosoy MY, McColl D, et al. Rodent-associated Bartonella in Saskatchewan, Canada. Vector Borne Zoonotic Dis 2005; 5:402–409 [DOI] [PubMed] [Google Scholar]
- Jardine C, McColl D, Wobeser G, Leighton FA. Diversity of Bartonella genotypes in Richardson's ground squirrel populations. Vector Borne Zoonotic Dis 2006a; 6:395–403 [DOI] [PubMed] [Google Scholar]
- Jardine C, Waldner C, Wobeser G, Leighton FA. Demographic features of Bartonella infections in Richardson's ground squirrels (Spermophilus richardsonii). J Wildl Dis 2006b; 42:739–749 [DOI] [PubMed] [Google Scholar]
- Jiyipong T, Jittapalapong S, Morand S, Raoult D, et al. Prevalence and genetic diversity of Bartonella spp. in small mammals from Southeastern Asia. Appl Environ Microbiol 2012; 78:8463–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT, McCormick KF, Martin AP. Bacterial communities of Bartonella-positive fleas: Diversity and community assembly patterns. Appl Environ Microbiol 2008; 74:1667–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya H, Maruyama S, Irei M, Takahashi R, et al. Genomic variations among Bartonella henselae isolates derived from naturally infected cats. Vet Microbiol 2002; 89:211–221 [DOI] [PubMed] [Google Scholar]
- Kabeya H, Inoue K, Izumi Y, Morita T, et al. Bartonella species in wild rodents and fleas from them in Japan. J Vet Med Sci 2011; 73:1561–1567 [DOI] [PubMed] [Google Scholar]
- Kamani J, Morick D, Mumcuoglu KY, Harrus S. Prevalence and diversity of Bartonella species in commensal rodents and ectoparasites from Nigeria, West Africa. PLoS Negl Trop Dis 2013; 7:e2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagöz A, Çelebi B, Şimşek H, Taner M, et al. Detection of Bartonella spp. in field mice (Microtus socialis) by culture and PCR. Ankara Üniv Vet Fak Derg 2013; 60:235–239 [Google Scholar]
- Kerkhoff FT, Bergmans AM, van Der Zee A, Rothova A. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J Clin Microbiol 1999; 37:4034–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlova IS, Hovhanyan A, Degen AA, Krasnov BR. The effect of larval density on pre-imaginal development in two species of desert fleas. Parasitology 2010; 137:1925–1935 [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Hosokawa T, Fukatsu T. Insect-microbe mutualism without vertical transmission: A stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 2007; 73:4308–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Kim SJ, Kang JG, Ko S, et al. First report for the seasonal and annual prevalence of flea-borne Bartonella from rodents and soricomorphs in the republic of Korea. Vector Borne Zoonotic Dis 2013; 13:457–467 [DOI] [PubMed] [Google Scholar]
- Kim CM, Kim JY, Yi YH, Lee MJ, et al. Detection of Bartonella species from ticks, mites and small mammals in Korea. J Vet Sci 2005; 6:327–334 [PubMed] [Google Scholar]
- Knap N, Duh D, Birtles R, Trilar T, et al. Molecular detection of Bartonella species infecting rodents in Slovenia. FEMS Immunol Med Microbiol 2007; 50:45–50 [DOI] [PubMed] [Google Scholar]
- Koesling J, Aebischer T, Falch C, Schulein R, et al. Cutting edge: Antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen Bartonella grahamii. J Immunol 2001; 167:11–14 [DOI] [PubMed] [Google Scholar]
- Kordick DL, Wilson KH, Sexton DJ, Hadfield TL, et al. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J Clin Microbiol 1995; 33:3245–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy MY. Ecological Associations between bacteria of the genus Bartonella and mammals. Biol Bull 2010; 37:716–724 [Google Scholar]
- Kosoy MY, Regnery RL, Tzianabos T, Marston EL, et al. Distribution, diversity, and host specificity of Bartonella in rodents from the Southeastern United States. Am J Trop Med Hyg 1997; 57:578–588 [DOI] [PubMed] [Google Scholar]
- Kosoy MY, Regnery RL, Kosaya OI, Jones DC, et al. Isolation of Bartonella spp. from embryos and neonates of naturally infected rodents. J Wildl Dis 1998; 34:305–309 [DOI] [PubMed] [Google Scholar]
- Kosoy MY, Regnery RL, Kosaya OI, Childs JE. Experimental infection of cotton rats with three naturally occurring Bartonella species. J Wildl Dis 1999; 35:275–284 [DOI] [PubMed] [Google Scholar]
- Kosoy MY, Saito EK, Green D, Marston EL, et al. Experimental evidence of host specificity of Bartonella infection in rodents. Comp Immunol Microbiol Infect Dis 2000; 23:221–238 [DOI] [PubMed] [Google Scholar]
- Kosoy M, Murray M, Gilmore RD, Jr., Bai Y, et al. Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. J Clin Microbiol 2003; 41:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M, Mandel E, Green D, Marston E, et al. Prospective studies of Bartonella of rodents. Part I. Demographic and temporal patterns in population dynamics. Vector Borne Zoonotic Dis 2004a; 4:285–295 [DOI] [PubMed] [Google Scholar]
- Kosoy M, Mandel E, Green D, Marston E, et al. Prospective studies of Bartonella of rodents. Part II. Diverse infections in a single rodent community. Vector Borne Zoonotic Dis 2004b; 4:296–305 [DOI] [PubMed] [Google Scholar]
- Kosoy M, Morway C, Sheff KW, Bai Y, et al. Bartonella tamiae sp. nov., a newly recognized pathogen isolated from three human patients from Thailand. J Clin Microbiol 2008; 46:772–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M, Hayman DT, Chan KS. Bartonella bacteria in nature: Where does population variability end and a species start? Infect Genet Evol 2012; 12:894–904 [DOI] [PubMed] [Google Scholar]
- Krampitz HE. [Further studies on Grahamella Brumpt 1911]. Z Tropenmed Parasitol 1962; 13:34–53 [PubMed] [Google Scholar]
- Krasnov BR, Khokhlova IS. The effect of behavioural interactions on the transfer of fleas (Siphonaptera) between two rodent species. J Vector Ecol 2001; 26:181–190 [PubMed] [Google Scholar]
- Krasnov BR, Shenbrot GI, Medvedev SG, Vatschenok VS, et al. Host-habitat relations as an important determinant of spatial distribution of flea assemblages (Siphonaptera) on rodents in the Negev Desert. Parasitology 1997; 114( Pt 2):159–173 [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV. Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J Med Entomol 2001; 38:629–637 [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Burdelova NV, Shenbrot GI, Khokhlova IS. Annual cycles of four flea species in the central Negev desert. Med Vet Entomol 2002; 16:266–276 [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Poulin R, Shenbrot GI, Mouillot D, et al. Ectoparasitic “jacks-of-all-trades”: Relationship between abundance and host specificity in fleas (Siphonaptera) parasitic on small mammals. Am Nat 2004; 164:506–516 [DOI] [PubMed] [Google Scholar]
- Krasnov BR, Morand S, Hawlena H, Khokhlova IS, et al. Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia 2005; 146:209–217 [DOI] [PubMed] [Google Scholar]
- Krasnov BR. Functional and Evolutionary Ecology of Fleas: A Model for Ecological Parasitology. Cambridge, UK, New York: Cambridge University Press, 2008 [Google Scholar]
- Kunz S, Oberle K, Sander A, Bogdan C, et al. Lymphadenopathy in a novel mouse model of Bartonella-induced cat scratch disease results from lymphocyte immigration and proliferation and is regulated by interferon-alpha/beta. Am J Pathol 2008; 172:1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Chen CY, Chen WC, Chomel BB, et al. Isolation of Bartonella species from rodents in Taiwan including a strain closely related to 'Bartonella rochalimae' from Rattus norvegicus. J Med Microbiol 2008; 57:1496–1501 [DOI] [PubMed] [Google Scholar]
- Liu Q, Sun J, Lu L, Fu G, et al. Detection of Bartonella species in small mammals from Zhejiang Province, China. J Wildl Dis 2010; 46:179–185 [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev 2000; 24:669–686 [DOI] [PubMed] [Google Scholar]
- Marie JL, Fournier PE, Rolain JM, Briolant S, et al. Molecular detection of Bartonella quintana, B. Elizabethae, B. Koehlerae, B. Doshiae, B. Taylorii, and Rickettsia felis in rodent fleas collected in Kabul, Afghanistan. Am J Trop Med Hyg 2006; 74:436–439 [PubMed] [Google Scholar]
- Marignac G, Barrat F, Chomel B, Vayssier-Taussat M, et al. Murine model for Bartonella birtlesii infection: New aspects. Comp Immunol Microbiol Infect Dis 2010; 33:95–107 [DOI] [PubMed] [Google Scholar]
- Marquez FJ, Rodriguez-Liebana JJ, Pachon-Ibanez ME, Docobo-Perez F, et al. Molecular screening of Bartonella species in rodents from south western Spain. Vector Borne Zoonotic Dis 2008; 8:695–700 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Cook JA, Goethert HK, Telford SR., 3rd.Bartonella sp. Infection of voles trapped from an interior Alaskan site where ticks are absent. J Wildl Dis 2010; 46:173–178 [DOI] [PubMed] [Google Scholar]
- McCarty R. Onychomys leucogaster. Mammalian Species; 1978; 87:6 pp [Google Scholar]
- Mediannikov O, Ivanov L, Zdanovskaya N, Vysochina N, et al. Molecular screening of Bartonella species in rodents from the Russian Far East. Ann NY Acad Sci 2005; 1063:308–311 [DOI] [PubMed] [Google Scholar]
- Meheretu Y, Leirs H, Welegerima K, Breno M, et al. Bartonella prevalence and genetic diversity in small mammals from Ethiopia. Vector Borne Zoonotic Dis 2013; 13:164–175 [DOI] [PubMed] [Google Scholar]
- Morick D, Baneth G, Avidor B, Kosoy MY, et al. Detection of Bartonella spp. in wild rodents in Israel using HRM real-time PCR. Vet Microbiol 2009; 139:293–297 [DOI] [PubMed] [Google Scholar]
- Morick D, Krasnov BR, Khokhlova IS, Shenbrot GI, et al. Bartonella genotypes in fleas (Insecta: Siphonaptera) collected from rodents in the Negev desert, Israel. Appl Environ Microbiol 2010; 76:6864–6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morick D, Krasnov BR, Khokhlova IS, Gottlieb Y, et al. Investigation of Bartonella acquisition and transmission in Xenopsylla ramesis fleas (Siphonaptera: Pulicidae). Mol Ecol 2011; 20:2864–2870 [DOI] [PubMed] [Google Scholar]
- Morick D, Krasnov BR, Khokhlova IS, Gottlieb Y, et al. Transmission dynamics of Bartonella sp. strain OE 1-1 in Sundevall's jirds (Meriones crassus). Appl Environ Microbiol 2013a; 79:1258–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morick D, Krasnov BR, Khokhlova IS, Gutiérrez R, et al. Effects of Bartonella spp. on flea feeding and reproductive performance. Appl Environ Microbiol 2013b; 79:3438–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morick D, Krasnov BR, Khokhlova IS, Gutiérrez R, et al. Vertical nontransovarial transmission of Bartonella in fleas. Mol Ecol 2013c; 22:4747–4752 [DOI] [PubMed] [Google Scholar]
- Morway C, Kosoy M, Eisen R, Montenieri J, et al. A longitudinal study of Bartonella infection in populations of woodrats and their fleas. J Vector Ecol 2008; 33:353–364 [DOI] [PubMed] [Google Scholar]
- Oksi J, Rantala S, Kilpinen S, Silvennoinen R, et al. Cat scratch disease caused by Bartonella grahamii in an immunocompromised patient. J Clin Microbiol 2013; 51:2781–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin Infect Dis 2001; 32:897–928 [DOI] [PubMed] [Google Scholar]
- Paziewska A, Harris PD, Zwolinska L, Bajer A, et al. Recombination within and between species of the alpha proteobacterium Bartonella infecting rodents. Microb Ecol 2011; 61:134–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paziewska A, Harris PD, Zwolinska L, Bajer A, et al. Differences in the ecology of Bartonella infections of Apodemus flavicollis and Myodes glareolus in a boreal forest. Parasitology 2012a; 139:881–893 [DOI] [PubMed] [Google Scholar]
- Paziewska A, Sinski E, Harris PD. Recombination, diversity and allele sharing of infectivity proteins between Bartonella species from rodents. Microb Ecol 2012b; 64:525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius AM, Beati L, Birtles RJ. Diversity of bartonellae associated with small mammals inhabiting Free State province, South Africa. Int J Syst Evol Microbiol 2004; 54:1959–1967 [DOI] [PubMed] [Google Scholar]
- Probert W, Louie JK, Tucker JR, Longoria R, et al. Meningitis due to a “Bartonella washoensis”-like human pathogen. J Clin Microbiol 2009; 47:2332–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WK, Szumlas DE, Moriarity JR, Loftis AD, et al. Louse-borne bacterial pathogens in lice (Phthiraptera) of rodents and cattle from Egypt. J Parasitol 2006; 92:313–318 [DOI] [PubMed] [Google Scholar]
- Regnath T, Mielke ME, Arvand M, Hahn H. Murine model of Bartonella henselae infection in the immunocompetent host. Infect Immun 1998; 66:5534–5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis C, Cote M, Le Rhun D, Lecuelle B, et al. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl Trop Dis 2011; 5:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz HL, Engel P, Stoeckli MC, Lanz C, et al. Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat Genet 2007; 39:1469–1476 [DOI] [PubMed] [Google Scholar]
- Saisongkorh W, Wootta W, Sawanpanyalert P, Raoult D, et al. “Candidatus Bartonella thailandensis”: A new genotype of Bartonella identified from rodents. Vet Microbiol 2009; 139:197–201 [DOI] [PubMed] [Google Scholar]
- Sato S, Kabeya H, Fujinaga Y, Inoue K, et al. Bartonella jaculi sp. nov., Bartonella callosciuri sp. nov., Bartonella pachyuromydis sp. nov., and Bartonella acomydis sp. nov. isolated from wild Rodentia. Int J Syst Evol Microbiol 2013; 63:1734–1740 [DOI] [PubMed] [Google Scholar]
- Schulein R, Seubert A, Gille C, Lanz C, et al. Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J Exp Med 2001; 193:1077–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson HL, Bai Y, Kosoy MY, Montenieri JA, et al. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae) using multiplex polymerase chain reaction. J Med Entomol 2003; 40:329–337 [DOI] [PubMed] [Google Scholar]
- Stopka P, Graciasova R. Conditional allogrooming in the herb-field mouse. Behav Ecol 2001; 12:584–589 [Google Scholar]
- Tea A, Alexiou-Daniel S, Papoutsi A, Papa A, et al. Bartonella species isolated from rodents, Greece. Emerg Infect Dis 2004; 10:963–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer S, Begon M, Bennett M, Bown KJ, et al. Contrasting dynamics of Bartonella spp. in cyclic field vole populations: The impact of vector and host dynamics. Parasitology 2007a; 134:413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer S, Clough HE, Birtles LR, Bennett M, et al. Ecological differences and coexistence in a guild of microparasites: Bartonella in wild rodents. Ecology 2007b; 88:1841–1849 [DOI] [PubMed] [Google Scholar]
- Trataris AN, Rossouw J, Arntzen L, Karstaedt A, et al. Bartonella spp. in human and animal populations in Gauteng, South Africa, from 2007 to 2009. Onderstepoort J Vet Res 2012; 79:E1–E8 [DOI] [PubMed] [Google Scholar]
- Tsai YL, Chuang ST, Chang CC, Kass PH, et al. Bartonella species in small mammals and their ectoparasites in Taiwan. Am J Trop Med Hyg 2010; 83:917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YL, Chang CC, Chuang ST, Chomel BB. Bartonella species and their ectoparasites: Selective host adaptation or strain selection between the vector and the mammalian host? Comp Immunol Microbiol Infect Dis 2011; 34:299–314 [DOI] [PubMed] [Google Scholar]
- Vayssier-Taussat M, Le Rhun D, Deng HK, Biville F, et al. The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog 2010; 6:e1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welc-Faleciak R, Paziewska A, Bajer A, Behnke JM, et al. Bartonella spp. infection in rodents from different habitats in the Mazury Lake District, Northeast Poland. Vector Borne Zoonotic Dis 2008; 8:467–474 [DOI] [PubMed] [Google Scholar]
- Welc-Faleciak R, Bajer A, Behnke JM, Sinski E. The ecology of Bartonella spp. infections in two rodent communities in the Mazury Lake District region of Poland. Parasitology 2010; 137:1069–1077 [DOI] [PubMed] [Google Scholar]
- Welch DF, Carroll KC, Hofmeister EK, Persing DH, et al. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: Identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol 1999; 37:2598–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto IL, Goethert H, Ibrahim IN, Yuniherlina I, et al. Bartonella species in rodents and shrews in the greater Jakarta area. Southeast Asian J Trop Med Public Health 2005; 36:1523–1529 [PubMed] [Google Scholar]
- Ye X, Li GW, Yao ML, Luo W, et al. [Study on the prevalence and genotypes of Bartonella species in rodent hosts from Fujian coastal regions]. Zhonghua Liu Xing Bing Xue Za Zhi 2009; 30:989–992 [PubMed] [Google Scholar]
- Ying B, Kosoy MY, Maupin GO, Tsuchiya KR, et al. Genetic and ecologic characteristics of Bartonella communities in rodents in southern China. Am J Trop Med Hyg 2002; 66:622–627 [DOI] [PubMed] [Google Scholar]