Abstract
Aims: Cystathionine β-synthase (CBS) catalyzes the first and rate-limiting step in the two-step trans-sulfuration pathway that converts homocysteine to cysteine. It is also one of three major enzymes responsible for the biogenesis of H2S, a signaling molecule. We have previously demonstrated that CBS is activated in cells challenged by oxidative stress, but the underlying molecular mechanism of this regulation has remained unclear. Results: Here, we demonstrate that S-glutathionylation of CBS enhances its activity ∼2-fold in vitro. Loss of this post-translational modification in the presence of dithiothreitol results in reversal to basal activity. Cys346 was identified as the site for S-glutathionylation by a combination of mass spectrometric, mutagenesis, and activity analyses. To test the physiological relevance of S-glutathionylation-dependent regulation of CBS, HEK293 cells were oxidatively challenged with peroxide, which is known to enhance the trans-sulfuration flux. Under these conditions, CBS glutathionylation levels increased and were correlated with a ∼3-fold increase in CBS activity. Innovation: Collectively, our results reveal a novel post-translational modification of CBS, that is, glutathionylation, which functions as an allosteric activator under oxidative stress conditions permitting enhanced synthesis of both cysteine and H2S. Conclusions: Our study elucidates a molecular mechanism for increased cysteine and therefore glutathione, synthesis via glutathionylation of CBS. They also demonstrate the potential for increased H2S production under oxidative stress conditions, particularly in tissues where CBS is a major source of H2S. Antioxid. Redox Signal. 22, 350–361.
Introduction
Cystathionine β-synthase (CBS) is a pyridoxal 5′-phosphate (PLP)-dependent hemeprotein (2, 33) which catalyzes the first steps in the trans-sulfuration pathway that converts homocysteine to cysteine, the limiting reagent in the synthesis of the antioxidant, glutathione (GSH). Alternatively, CBS can catalyze the condensation of cysteine and homocysteine or the condensation of two moles of cysteine to produce hydrogen sulfide (H2S) (6, 45), a signaling molecule with cytoprotective effects (19–21, 24). Mutations in CBS represent the single most common cause of severe hereditary hyperhomocysteinemia and lead to a number of complications affecting the cardiovascular, ocular, skeletal, and central nervous systems (26).
Innovation.
The flux of sulfur to cysteine, the limiting reagent for glutathione biogenesis, increases under oxidative stress conditions and serves to replenish depleted glutathione levels. We demonstrate that glutathionylation of human cystathionine β-synthase under oxidizing conditions increases its activity, thereby supporting increased cysteine synthesis.
Human CBS is a modular protein comprising three functional domains (50). The N-terminal domain binds the heme cofactor, which functions as an allosteric regulator (44), inhibiting CBS activity in response to binding of CO or NO (3, 47) or during reduction of nitrite (14). Nitration of tryptophan residues in CBS by peroxynitrite results in alterations in the heme pocket, leading to loss of cysteinate coordination by the heme ligand Cys52 and concomitant inactivation of CBS (5). While a structural role for the heme in stabilizing the CBS structure has been invoked (29), homologous CBSs from lower organisms, for example, yeast, are stable without this cofactor (18). The central catalytic domain of CBS contains the PLP cofactor essential for catalysis and a CXXC motif, whose function, if any, has yet to be determined (30, 48). The C-terminal regulatory domain contains a tandem repeat of “CBS domains” that bind S-adenosylmethionine (AdoMet), an allosteric activator (11, 39). The regulatory domain can be cleaved to yield a truncated dimeric enzyme, which is more active than the full-length enzyme but unresponsive to AdoMet (23).
The trans-sulfuration pathway is a quantitatively significant route for cysteine, the limiting substrate for GSH synthesis (36). GSH is an abundant antioxidant in mammalian cells and plays an important role in intracellular redox homeostasis and in cellular defense against xenobiotic stress (27). It is, therefore, not surprising that a number of enzymes in the sulfur metabolic nexus display sensitivity to redox changes. Indeed, metabolic labeling studies have revealed that flux through the trans-sulfuration pathway is increased in response to oxidative stress (36). Thus, a threefold increase in the level of cystathionine formation catalyzed by CBS was observed when HepG2 cells were exposed to 200 μM tertiary butyl hydroperoxide (tBuOOH).
Since CBS is the committing enzyme in the trans-sulfuration pathway, we focused on it as a locus for potential regulation in response to exogenous oxidants. However, since no changes were seen in CBS protein levels in oxidatively challenged cells (36), we hypothesized that the activity of CBS might be regulated in response to oxidative stress via reversible post-translational modification of one or more cysteine residues. Human CBS has 11 cysteine residues, including two in the CXXC motif, which have been observed in the oxidized disulfide and reduced dithiol states (30, 48) and were the initial focus of our study to elucidate how the activity of CBS is upregulated under oxidative conditions. However, as reported here, our studies led us to the unexpected discovery that the activity of CBS is regulated via glutathionylation, which occurs at a cysteine residue that does not reside within the CXXC motif. We demonstrate that glutathionylation of CBS results in an ∼2-fold increase in enzyme activity in vitro. Furthermore, glutathionylation of CBS is also observed in cultured cells subjected to oxidative stress and is correlated with an ∼3-fold increase in CBS activity. These results suggest a model for an auto-corrective cellular response to GSH pools compromised under oxidative stress conditions via glutathionylation of CBS, which increases flux through the trans-sulfuration pathway, thereby increasing availability of cysteine, the limiting reagent for GSH synthesis.
Results
CBS is S-glutathionylated in vitro
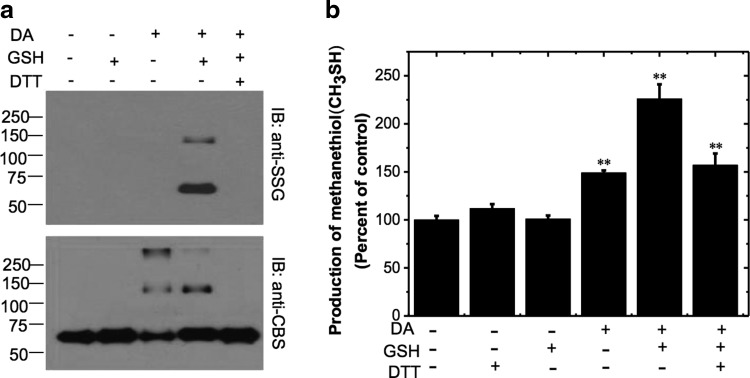
To test whether CBS can be glutathionylated, the protein was initially treated with dithiothreitol (DTT) to reduce all accessible cysteines to thiols. After removal of DTT, we then determined the effect of different concentrations of diamide on glutathionylation of CBS. For this, the GSH concentration was fixed at 1 mM and the diamide concentrations were varied from 0 to 1 mM. The minimum diamide concentration that was required for glutathionylation detectable by Western blot analysis was 0.7 mM (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). Hence, in subsequent experiments, CBS was oxidized with 1 mM diamide and then treated with an excess of GSH (1.5 mM) to oxidize exposed cysteines and to subsequently glutathionylate one or more susceptible thiols. Formation of S-glutathionylated CBS was detected by Western blot analysis using an anti-SSG antibody (Fig. 1a, top panel). Two bands cross-reacted with the anti-SSG antibody and corresponded to the CBS monomer and dimer with molecular masses of ∼63 and ∼130 kDa, respectively (lane 4). Addition of DTT to the glutathionylated sample resulted in loss of the cross-reacting bands, which is consistent with reduction of the mixed disulfide bond between GSH and CBS (lane 5). Diamide treatment of CBS led to intersubunit cross-linking and in Western blots using CBS antibody (Fig. 1a, lower panel), bands corresponding to the monomeric, dimeric, and multimeric (>250 kDa) species were observed (lanes 3 and 4), which were resolved into the monomeric species only on DTT treatment (lane 5). S-glutathionylation was not observed in control samples in which CBS was treated with diamide or GSH alone (top panel, lanes 2 and 3).
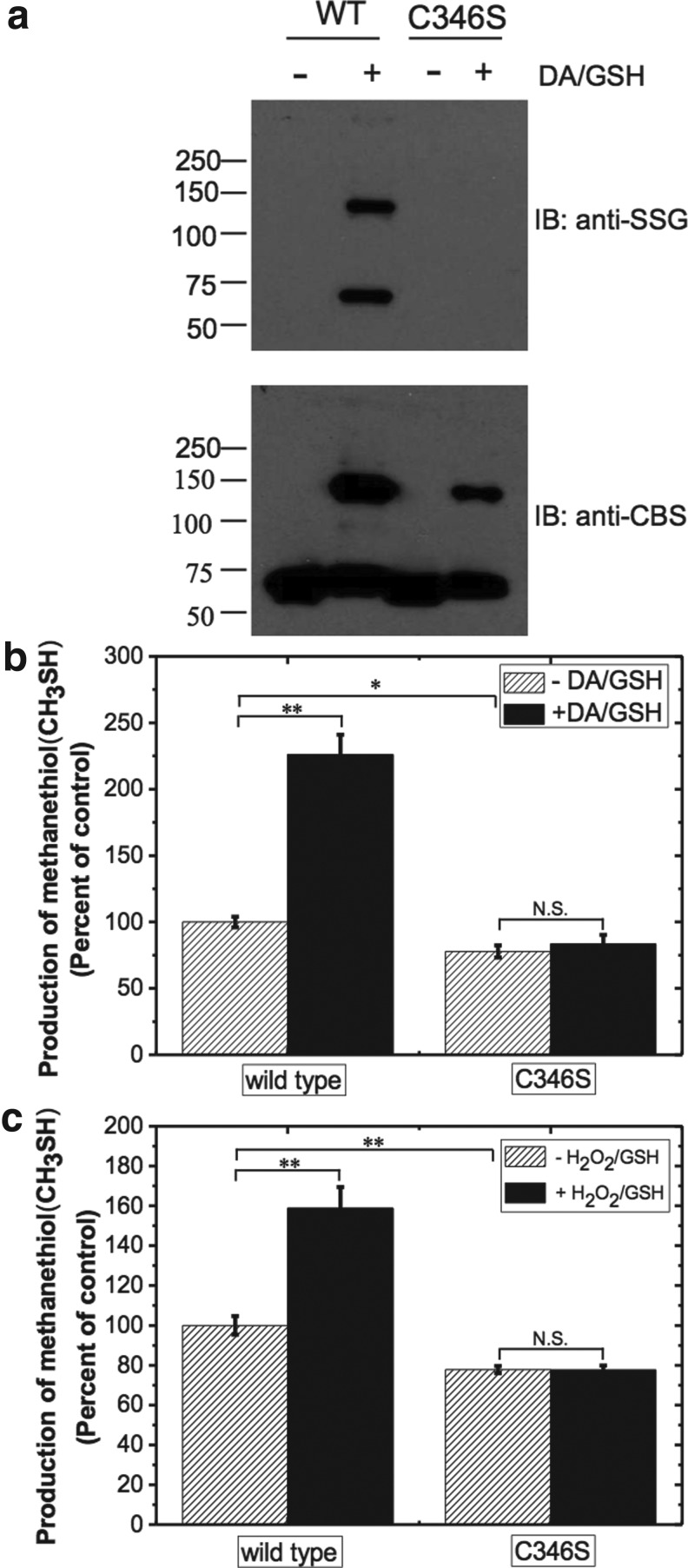
FIG. 1.
S-glutathionylation of CBS in vitro and its effect on activity. (a) Purified recombinant CBS (0.5 mg/ml) was incubated with 1 mM DA and 1.5 mM GSH followed by treatment with 10 mM DTT in indicated samples as described under the “Materials and Methods” section. The protein samples (25 ng) were separated by nonreducing SDS-PAGE followed by Western blot analysis using anti-SSG antibody (top) or anti-CBS antibody (bottom). Molecular masses of protein standards in kDa are shown on the left. The data are representative of at least three independent experiments. IB denotes immunoblotting with either the anti-glutathione (anti-SSG) or anti-CBS antibody. (b) Effect of glutathionylation on CBS activity. Recombinant wild-type CBS (0.5 mg/ml) was incubated with 1 mM DA and 1.5 mM GSH, followed by 10 mM DTT as indicated, and then the samples were dialyzed to remove DA, GSH, and DTT. CBS activity was measured using methylcysteine (10 mM) as a substrate in the 100 mM HEPES buffer, pH 7 .4. The graph represents the relative activity of samples compared with untreated controls and is the mean±SD (n=3), **p<0.01 versus control. The specific activity of the control sample in the methylcysteine assay (using DTNB detection) was 2.0±0.1 μmol methanethiol h−1 mg protein−1. CBS, cystathionine β-synthase; DA, diamide; DTNB, 5,5′-dithiobis-2-nitrobenzoic acid; DTT, dithiothreitol; GSH, glutathione; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Proportion of CBS that is glutathionylated
Native CBS has 11 cysteine residues, and the number of accessible thiols was determined using Ellman's reagent (5,5′-dithiobis-2-nitrobenzoic acid [DTNB]). A total of 4.7±0.3 cysteines were modified per CBS monomer and the number decreased to 3.5±0.25 after diamide (1 mM)+GSH (1.5 mM) treatment. This result indicated that one of the five accessible cysteines in the untreated sample was oxidized and/or modified after diamide+GSH treatment. Previously, two cysteines, Cys15 and Cys431 in the heme and regulatory domains, respectively were reported to be solvent accessible in CBS, using the DTNB assay (12). The basis for the discrepancy between these two data sets is not known but could result from differences in handling of proteins samples given the propensity of full-length CBS to aggregate (41). To determine what proportion of the protein was glutathionylated, untreated and diamide-oxidized CBS were treated with biotinylated glutathione ethyl ester (BioGEE). Approximately 80%±4% of the protein was retained on the streptavidin-agarose resin corresponding to the proportion of protein that was glutathionylated.
Glutathionylation increases CBS activity
We next investigated the effect of glutathionylation on CBS activity. The standard in vitro assay for CBS is not suitable for measuring the activity of glutathionylated CBS, as homocysteine is used as a substrate and can reduce the mixed disulfide between GSH and CBS. Hence, we used a modified assay in which S-methylcysteine is employed as a substrate. The product of this reaction is methanethiol, which is detected by UV-visible absorption or fluorescence spectroscopy after treatment with DTNB or Thiostar, respectively. Glutathionylation increased the activity of CBS to 226%±15% compared with untreated control samples (Fig. 1b). Interestingly, glutathionylated CBS was not further activated in the presence of 1 mM AdoMet, an allosteric activator. Unexpectedly, diamide alone (1 mM) also increased the activity of CBS to 149%±2% of the control value. Addition of DTT (10 mM) partially reversed the activity of glutathionylated CBS to 157%±12% of the control value. However, the basal activity level was not completely recovered.
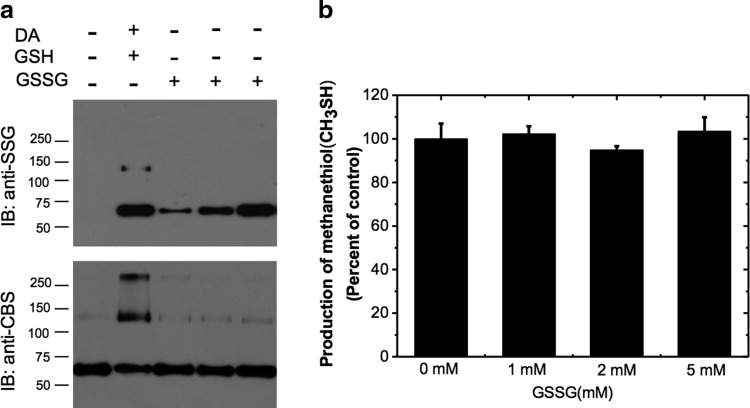
We next determined whether the physiologically relevant oxidants glutathione disulfide (GSSG) or H2O2 activate CBS. Under oxidative stress conditions, the GSSG pool size increases and this can promote protein glutathionylation. Treatment of reduced CBS with 1–5 mM GSSG showed increasing glutathionylation of the CBS monomer but not of the cross-linked dimer as observed with diamide+GSH (Fig. 2a). However, CBS treated with GSSG did not show a detectable change in activity even in the presence of 5 mM GSSG (Fig. 2b), suggesting that the proportion of glutathionylation with this reagent was significantly lower than that observed with GSH. This was confirmed by thiol quantitation of CBS treated with 2 mM GSSG, which revealed that 4.8±0.35 cysteines were reduced and accessible for modification by DTNB, which is identical to the value obtained in the untreated control (4.7±0.3). Hence, the high sensitivity of the Western blot method enables detection of the very low proportion of CBS that is glutathionylated in the presence of GSSG.
FIG. 2.
Effect of GSSG on CBS. (a) S-glutathionylation was carried out by treating CBS [0.5 mg/ml with 1 mM DA/1.5 mM GSH (lane 2), 1 mM GSSG (lane 3), 2 mM GSSG (lane 4), or 5 mM GSSG (lane 5) followed by nonreducing SDS-PAGE and Western blot analysis] with anti-SSG antibody (top) or anti-CBS antibody (bottom). (b) Effect of GSSG on CBS activity. CBS (0.5 mg/ml) was incubated with the indicated concentrations of GSSG for 1 h at room temperature, and then the samples were dialyzed to remove GSSG. CBS activity was measured using the methylcysteine assay. The graph represents the treated compared with untreated controls and is the average±SD (n=3). GSSG, glutathione disulfide.
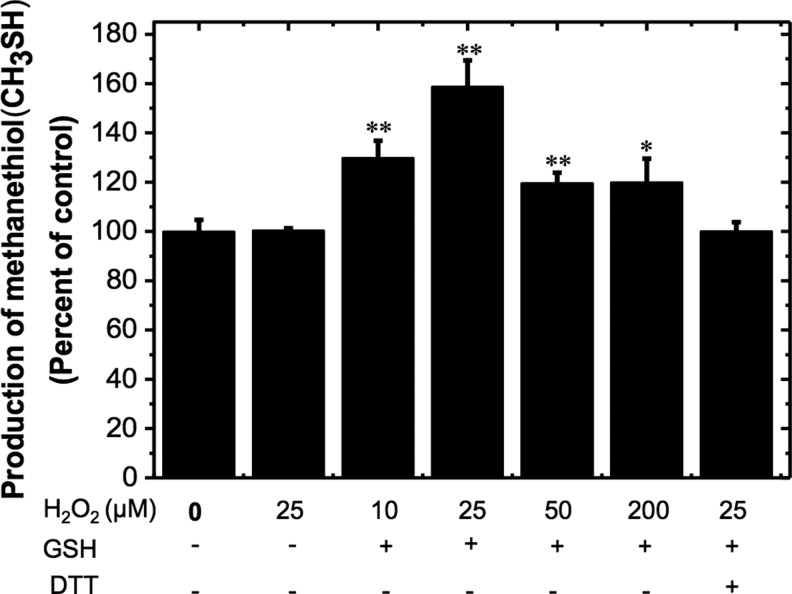
In contrast to GSSG treatment, the activity of CBS increased when treated with different concentrations of H2O2 (10–200 μM) for 30 min and then exposed to GSH, peaking at 25 μM H2O2, where the activity was 159%±11% of the control value (Fig. 3). Beyond this concentration of H2O2, the activity of CBS decreased. Unlike diamide treatment, which only results in formation of disulfide bonds, treatment with H2O2 can generate sulfeninc, sulfinic, and sulfonic acids in addition to disulfides. Of these, the overoxidized sulfinic and sulfonic acids cannot be modified by GSH. Hence, the proportion of glutathionylated CBS is expected to be lower in H2O2 versus diamide-treated samples. To test this possibility, the extent of BioGEE modification of H2O2-oxidized CBS samples was determined after affinity purification using a streptavidin column. In comparison to the 80%±4% modification observed in diamide-treated samples, 64%±3% of CBS was glutathionylated in H2O2-treated samples. Treatment of CBS with 25 μM H2O2 alone did not affect its activity and in the presence of 10 mM DTT, basal CBS activity was fully recovered (Fig. 3).
FIG. 3.
S-glutathionylation of H2O2-oxidized CBS and its effect on activity. Recombinant wild-type CBS (0.5 mg/ml) was incubated with different concentrations of H2O2 and GSH, followed by 10 mM DTT treatment as indicated. The samples were then dialyzed, and CBS activity was measured using the methylcysteine assay as described in Figure 1 legend. The data represent the activity of samples compared with untreated controls and are the average±SD (n=3), *p<0.05 or **p<0.01 versus control.
CBS is glutathionylated at Cys346
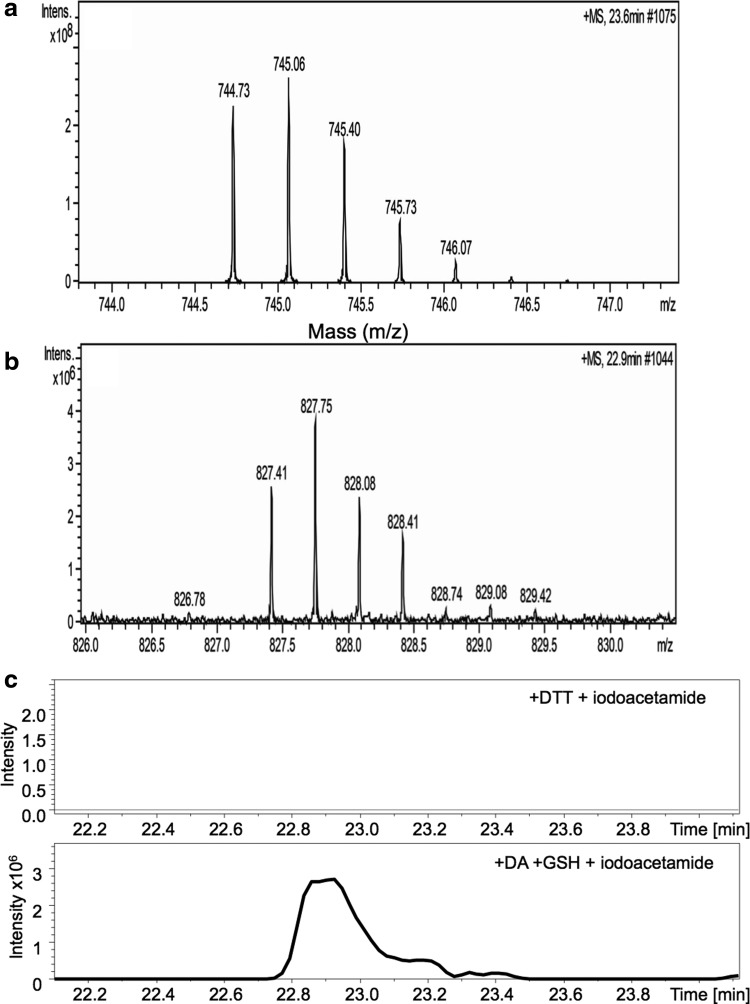
The site(s) of glutathionylation on CBS were identified by liquid chromatography–tandem mass spectrometry (LC-MS/MS) after tryptic digestion of glutathionylated CBS (generated in the presence of diamide+GSH) (Fig. 4 and Supplementary Fig. S2). Before trypsin digestion, CBS was denatured and the free thiol groups were blocked with iodoacetamide. The experiment was repeated thrice, and MS analysis identified a single modified peptide (MLIAQEGLLCGGSAGSTVAVAVK) and Cys346 as the site of glutathionylation (Fig. 4). The difference in the monoisotopic masses of the triply charged glutathionylated (827.41 Da) and control iodoacetamide-derivatized (744.73 Da) peptides is 248.04 Da (3×82.68 Da), which corresponds to the expected mass difference between GSH (306.09 Da) and iodoacetamide (58.05 Da) modification of the cysteine residue. The modified M337-K359 peptide had an observed molecular mass of 2479.21 Da in excellent agreement with a mass of 2479.23 Da calculated for the presence of a single glutathione-Cys346 mixed disulfide.
FIG. 4.
Mass spectroscopic identification of the glutathionylation site in CBS. CBS (100 μM) was treated with±2 mM diamide and 3 mM GSH for 30 min at room temperature and then denatured with 6 M guanidine hydrochloride. Free thiol groups were derivatized with 10 mM iodoacetamide, followed by tryptic digestion for LC-MS/MS analysis. Mass spectra of a peptide from untreated (a) and glutathionylated (b) CBS are compared. Triple-charged masses (M+3H)3+ of the glutathionylated and iodoacetamide-derivatized peptide (MLIAQEGLLCGGSAGSTVAVAVK) are 827.41 or 744.73 Da, respectively. The data are representative of three independent experiments. (c) Traces showing that the glutathionylated CBS peak (827.41 Da) is unique for diamide/GSH-treated samples (lower trace) and not seen in the control DTT-treated sample (upper trace). LC-MS/MS, liquid chromatography–tandem mass spectrometry.
Mutation of Cys346 leads to loss of glutathionylation
To validate Cys346 as the site of glutathioylation in CBS, we generated the Cys346Ser mutant. The purity of the mutant CBS was judged to be >90% by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis and comparable to that of the wild-type protein (not shown). However, the yield of the purified Cys346Ser mutant (∼1.0 mg/L of culture) was ∼7-fold lower than that of wild-type CBS. The activities of the wild-type and mutant Cys346Ser CBS were assessed in 100 mM HEPES buffer pH 7.4, and in the presence or absence of 1 mM AdoMet using the standard radioactive and lead acetate assays as described under the “Materials and Methods” section. The basal activity of Cys346Ser CBS was ∼1.3-fold lower than wild-type CBS in both assays.
We next determined the effect of the Cys346Ser mutation on glutathionylation of CBS by Western blot analysis. In contrast to wild-type CBS, which exhibited strong signal intensity due to glutathionylation, this modification was not observed with the Cys346Ser mutant (Fig. 5a). Unlike wild-type CBS, no change in Cys346Ser CBS activity was observed before and after treatment with diamide+GSH (Fig. 5b). Similarly, pretreatment with 25 μM H2O2 followed by GSH did not affect the activity of the mutant CBS (Fig. 5c). Collectively, these results are consistent with Cys346 being the site of glutathionylation, which is correlated with increased CBS activity.
FIG. 5.
Mutation of Cys346 abolishes glutathionylation of CBS. (a) Wild-type and Cys346Ser CBS were treated with 1 mM DA and 1.5 mM GSH for 30 min at room temperature, subjected to nonreducing SDS-PAGE followed by immunoblotting with anti-SSG antibody (top) or anti-CBS antibody (bottom). The CBS monomer (63 kDa) and a dimeric species were detected with anti-SSG antibody in the DA+GSH-treated wild type but not in Cys346Ser CBS. In contrast, anti-CBS antibody was observed in both the monomeric and cross-linked dimeric species in samples that were oxidized with DA. Wild-type and Cys346Ser CBS were treated with±1 mM DA and 1.5 mM GSH for 30 min at room temperature (b) or with±25 μM H2O2 for 30 min followed by 125 μM GSH (c). The samples were then dialyzed, and CBS activity was measured using the methylcysteine assay as described in Figure 1 legend. *p<0.05 or **p<0.01 versus controls. N.S., not significant.
In addition to Cys346, we also mutated the residues in the CXXC motif to generate the double mutant, Cys272Ala/Cys275Ser. Similar to wild-type CBS, the activity of the double mutant was increased by treatment with diamide+GSH (data not shown). These data rule out the cysteines in the CXXC motif as sites for glutathionylation and are consistent with the mass spectrometric data.
H2O2 induces S-glutathionylation of CBS in cultured cells and enhances CBS activity
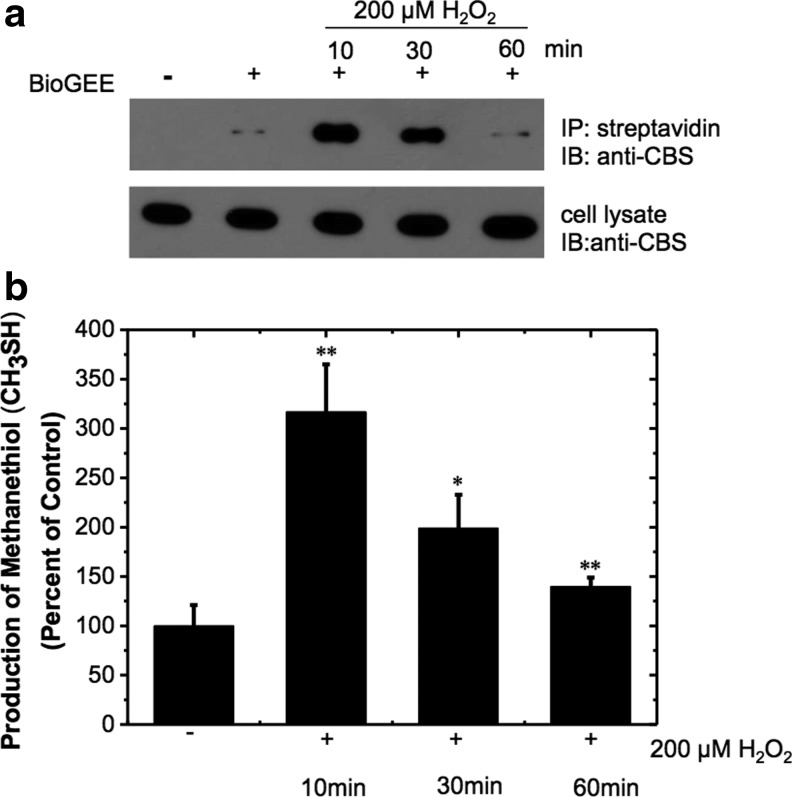
To assess the physiological relevance of CBS glutathionylation, we examined whether this modification is observed in cells and is responsive to oxidative stress conditions induced by H2O2 treatment. For this, HEK293 cells were incubated with 250 μM BioGEE for 1 h, followed by 10, 30, and 60 min exposure to H2O2 (200 μM) as described in the “Materials and Methods” section. While 100 μM H2O2 is unlikely to be physiologically relevant, such concentrations have been previously used and are necessary to observe detectable changes in metabolite pools (36). Incorporation of BioGEE into CBS enables it to be pulled down by streptavidin-agarose beads, followed by detection of the modification by Western blot analysis using CBS antibodies. Exposure to 200 μM H2O2 induced a significant increase in glutathionylated CBS within 10 min (Fig. 6a). Prolonged exposure to H2O2 resulted in a gradual decline in the CBS glutathionylation level. After 60 min of H2O2 exposure, the level of glutathionylated CBS was similar to that seen in untreated cells (Fig. 6a, lanes 2 and 5). In the absence of BioGEE treatment, glutathionylation of CBS could not be detected as expected (lane 1). The dimeric form of CBS seen under in vitro conditions using the strong oxidant, diamide, was not seen in the cell culture experiments (data not shown). These results indicate that a low level of CBS glutathionylation exists under steady-state conditions and that it increases transiently and rapidly after an oxidative challenge.
FIG. 6.
Oxidative stress increases CBS S-glutathionylation and activity in HEK293 cells. (a) HEK293 cells were preincubated±250 μM BioGEE for 1 h and subsequently exposed to 200 μM H2O2 for 10, 30, and 60 min. Biotin-bound proteins were extracted using streptavidin affinity resin and eluted with 10 mM DTT. The eluents (top) and whole cell extracts (10 μg protein/lane) were subjected to nonreducing SDS-PAGE and probed with anti-CBS antibody. (b) Cell lysates from HEK293 cells exposed to 200 μM H2O2 for 10, 30, and 60 min were used to assay for CBS activity using methylcysteine in the presence of the CSE inhibitor, 2.5 mM PPG. CH3SH production was measured by gas chromatography coupled to a sulfur chemiluminescence detector as described under the “Materials and Methods” section. The data are representative of at least three independent experiments. *p<0.05 or **p<0.01 versus control. BioGEE, biotinylated glutathione ethyl ester; CSE, cystathionine-γ-lyase; PPG, propargylglycine.
The activity of CBS was also measured in HEK293 cell lysates from control and H2O2-treated cultures. Since both CBS and cystathionine-γ-lyase (CSE) can, in principle, utilize methylcysteine to produce methanethiol, a suicide inactivator of CSE, propargylglycine (PPG) (1) was added to the reaction mixture. Formation of methanethiol was monitored by gas chromatography coupled to a sulfur chemiluminescence detector as described under the “Materials and Methods” section. CBS activity increased to 317%±48% of the control value when cells were incubated with 200 μM H2O2 for 10 min (Fig. 6b). Increasing exposure to H2O2 (30 and 60 min) resulted in a gradual decrease in CBS activity to 199%±34% and 140%±9% of the control value, respectively, which is qualitatively similar to the results from the Western blot analysis (Fig. 6a). These results are consistent with a transient increase in glutathionylation and activation of CBS under oxidative stress conditions in HEK293 cells.
Discussion
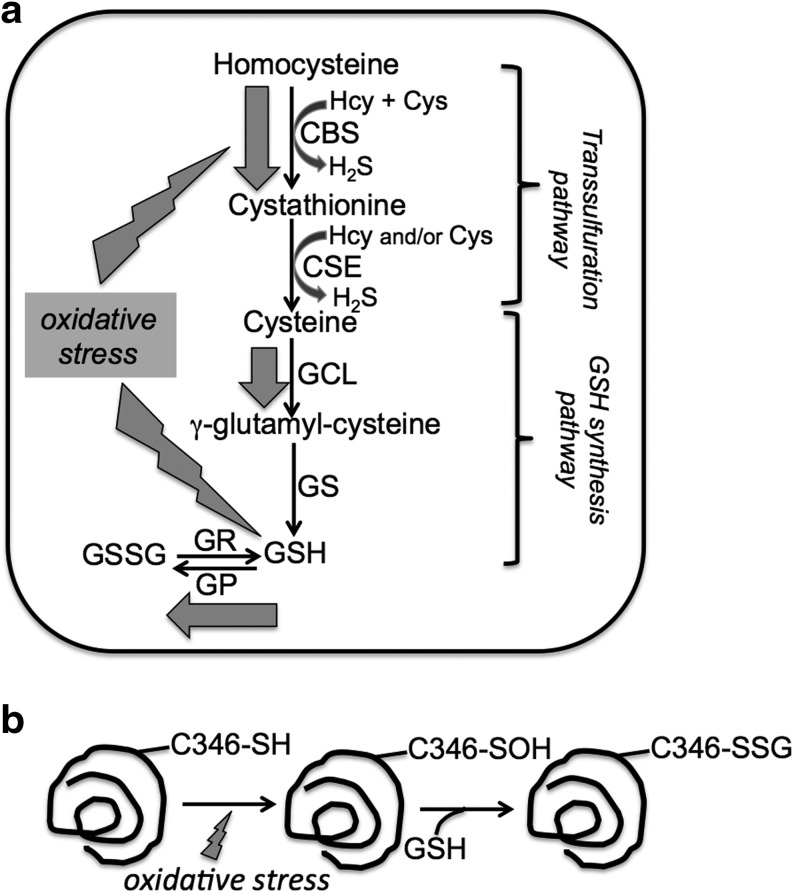
GSH is an abundant peptide antioxidant that is maintained at concentrations varying from ∼1 to 10 mM depending on the cell type. Its synthesis is limited by cysteine, and the rate-limiting enzyme in the GSH synthesis pathway is γ-glutamylcysteine ligase (Fig. 7). The trans-sulfuration pathway is an important determinant of intracellular cysteine concentration and accounts for ∼40%–50% of the cysteine that is tied up in the GSH pool in some cell types (36, 54). An important role of GSH is the removal of H2O2 catalyzed by GSH peroxidase. The product of the reaction is GSSG, which builds up initially under oxidative stress conditions, thus diminishing the GSH pool size (Fig. 7). The cellular autocorrective response for normalizing GSH levels under these conditions includes upregulation of γ-glutamylcysteine ligase (42, 43, 56) and as we had previously shown, increased trans-sulfuration flux (36). While regulation of γ-glutamylcysteine ligase occurs at the transcriptional level (34, 35, 37), the mechanism of increased trans-sulfuration flux was not known. Upregulation of the trans-sulfuration flux is expected to involve CBS, which catalyzes the rate-limiting step in the two-step pathway. Since Western blot analysis had revealed that CBS protein levels were unchanged in cell lysates from oxidatively challenged cells versus untreated controls, a post-translational mechanism for upregulation of the trans-sulfuration flux was implicated (36). In this study, we report that S-glutathionylation of CBS represents a mechanism for enhancing CBS activity under oxidative stress conditions.
FIG. 7.
Model for CBS glutathionylation-dependent increase in the trans-sulfuration flux and H2S production under oxidative stress conditions. (a) The trans-sulfuration enzymes, CBS and CSE also produce H2S from cysteine and homocysteine. Under oxidative stress conditions (denoted by gray block arrows), GSH levels are initially decreased by increased oxidation of GSH, for example, by GP. Under these conditions, glutathionylation of CBS increases its activity, leading to increased cysteine and H2S production. Cysteine is the limiting substrate for GSH synthesis catalyzed by GCL and GS. Increased trans-sulfuration flux leads to restoration of the GSH pool compromised under oxidative stress conditions. GR denotes glutaredoxin. (b) A potential mechanism for glutathionylation of CBS. Under oxidative stress conditions, Cys346 in CBS is oxidized to a sulfenic acid, which is then modified by gluthionylation, which could be catalyzed, for example, by glutaredoxin. GCL, γ-glutamylcysteine ligase; GP, glutathione peroxidase; GS, GSH synthetase.
S-glutathionylation is a reversible post-translational modification of proteins, which is important in redox signaling and metabolism, cytoskeletal assembly, and ion homeostasis (8, 9, 32, 51). Glutathionylation frequently inhibits enzyme activity by targeting an active site cysteine; for example, in protein tyrosine phosphatase 1B (38) and caspase 3 (17) or a cysteine that impedes access to the active site, for instance, in methionine synthase (16). In contrast, CBS is glutathionylated at Cys346 and this modification activates the enzyme. The mechanism of allosteric activation of CBS by glutathionylation warrants further elucidation. Allosteric modulation by glutathionylation has also been described in thioredoxin, where modification of a cysteine residue that is not in the catalytic CXXC motif results in inhibition of its oxidoreductase activity (4, 31).
Both surface exposure and perturbation of the thiol pKa to lower values are important predictors of the glutathionylation propensity of cysteines in protein structures (46). Typically, electrostatic stabilization of the thiolate anion with positively charged residues in the vicinity enhances the reactivity of cysteines. However, exceptions to these generalizations are known to exist, for example, in cyclophilin A, where the site of glutathionylation is not the cysteine whose side chain exhibits the greatest accessible surface area (15). The structure of full-length human CBS (10) reveals that Cys346 is located near the dimer interface in a hydrophobic pocket and is not particularly well exposed (Fig. 8). Since glutathionylation is only detected at Cys346 (Fig. 4) and lost on its mutagenesis to serine (Fig. 5), protein dynamics could allow access to this reactive cysteine residue. Alternatively, in the cell, glutathionylation of CBS might be catalyzed, for example, by glutaredoxin, which could increase exposure of Cys346 in the inter-protein complex. The relatively buried position of Cys346 might explain why GSSG, which is twice the size of GSH, is unable to modify it efficiently to impact activity under in vitro conditions (Fig. 2). In cells exposed to H2O2, glutathionylation of CBS increased transiently before returning to steady-state levels (Fig. 6).
FIG. 8.
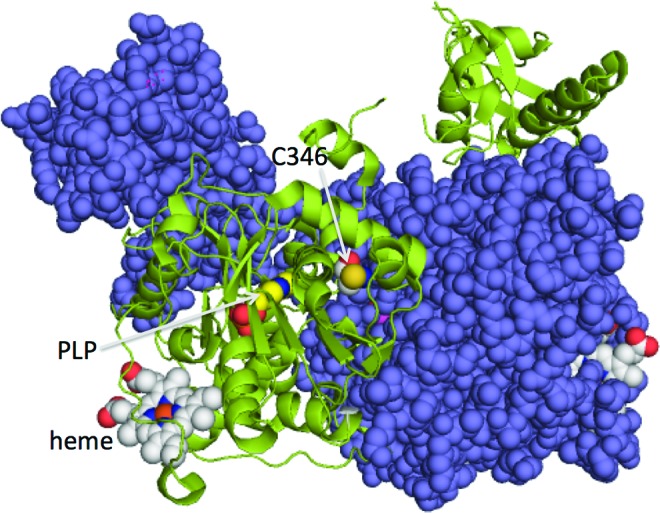
Location of Cys346 in the crystal structure of full-length human CBS. The two subunits of CBS are shown in purple and green, respectively, and the cofactors, PLP and heme, are shown in ball representation. Cys346, also shown in ball representation, is close to the dimeric interface. The figure was made using PDB file 4L28. PLP, pyridoxal 5′-phosphate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Since glutathionylation of CBS is efficient with GSH but not with GSSG, it implies that the thiol of Cys346 should first be oxidized, for example, to a sulfenic acid before being attacked by GSH (Fig. 7b). Hence, Cys346 could function as a redox sensor with its proportion increasing with increasing concentrations of reactive oxygen species under conditions of cellular oxidative stress, which, in turn, sets up S-glutathionylation of CBS. In cells exposed to oxidative challenge, glutathionylation of CBS increased transiently before returning to low steady-state levels and was correlated with an ∼3-fold increase in CBS activity in cell lysates (Fig. 6). It is not known whether or not deglutathionylation of CBS is catalyzed, and this issue needs to be investigated. The expected consequence of CBS glutathionylation is increased synthesis of cysteine, the product of the canonical trans-sulfuration pathway. This model is supported by the previously observed increase in the trans-sulfuration flux as evidenced by increased synthesis of cystathionine (and subsequently GSH) in cells challenged with H2O2 (36). The combined effect of upregulation of the rate-limiting enzymes in the trans-sulfuration and GSH synthesis pathways via glutathionylation of CBS and increased synthesis of γ-glutamylcysteine ligase (42, 43, 56), respectively, is restoration of the GSH pool size, which is initially depleted under oxidative stress conditions.
A second predicted consequence of CBS glutathionylation is the increased production of H2S. In this study, we have employed S-methylcysteine a substrate analog of cysteine, to monitor the effect of glutathionylation on CBS activity. The product of this reaction is methanethiol, an H2S surrogate. Our study demonstrates that glutathionylation increases methanethiol production by CBS, indicating that H2S production is predicted to increase both as a consequence of enhanced CBS activity and greater availability of the H2S substrate, cysteine. H2S is a signaling molecule that is generated by at least three enzymes, CBS, CSE, and mercaptopyruvate sulfurtransferase, whose relative contributions vary in a tissue-specific manner. In tissues such as the brain, where CBS is dominant, enhanced H2S production might be a significant outcome of oxidative stress-induced glutathionylation. H2S has been shown to protect against oxidative stress in brain cells (25, 52) and in HUVECs (55).
In summary, we report a new post-translational modification of CBS that provides a molecular explanation for the old observation that trans-sulfuration flux is increased under oxidative stress conditions (36). The study also demonstrates the potential to increase H2S production under oxidizing conditions via S-glutathionylation of CBS. However, the physiological effects of enhanced H2S remain to be fully elucidated.
Materials and Methods
Materials
[14C]-Serine (164 mCi/mmol) was purchased from Perkin Elmer. Anti-glutathione (anti-SSG) antibody was from Virogen. Thiostar® (thiol fluorescent detection reagent) was purchased from Arbor Assays. BioGEE and streptavidin-agarose were purchased from Invitrogen. All other chemicals were purchased from Sigma or Fisher.
Protein purification
The pGEX4T-1-C346S and pGEX4T-1-C272A/C275S plasmid constructs for expressing mutant CBS were generated by site-directed mutagenesis using the Quikchange II XL site-directed mutagenesis kit (Agilent) with the following mutagenic primers and the corresponding antisense primers containing the opposite sequence:
Cys346Ser: 5′-GCAAGAGGGGCTGCTGAGCGGTGGCAGTGCTGGCAG-3′
Cys272Ala: 5′-CAGGAAGCTGAAGGAGAAGGCTCCTGGATGCAGGATCATTG-3′
Cys275Ser: 5′-GAAGGAGAAGGCTCCTGGAAGCAGGATCATTGGGGTGG-3′
Mutation was confirmed by nucleotide sequence determination at the DNA Sequencing Core Facility (University of Michigan, Ann Arbor).
Wild-type and mutant CBSs were expressed and purified as previously described (49) with the exception that the Cys346Ser mutant was induced in the presence of 3% ethanol to promote correct protein folding (28). In brief, cells obtained from a 6-L culture were resuspended in 500 ml of 1×phosphate-buffered saline (PBS); supplemented with 10 mM β-mercaptoethanol, 10 mg PLP, 50 mg of lysozyme, and a protease inhibitor cocktail tablet (from Roche Diagnostics). The cells were lysed by sonication, and the supernatant was obtained by centrifugation at 15,000 g for 30 min. The supernatant was loaded onto a Glutathione Sepharose 4B column, which was pre-equilibrated with 1×PBS. The column was washed with 1×PBS, and glutathione S-transferase (GST)-fused CBS was eluted with 50 mM Tris, pH 8.0 in the presence of 10 mM GSH. The GST-fused CBS was cleaved with thrombin at 4°C at a final concentration of 2 U/mg protein, and the GST tag was removed on a Q sepharose column (2.5×12 cm) equilibrated with 50 mM Tris buffer, pH 8.0, and then washed with the same buffer. The protein was eluted with a 500 ml gradient ranging from 0 to 500 mM NaCl in 50 mM Tris-HCl, pH 8.0. The fractions of interest were dialyzed, concentrated, and stored at −80°C.
S-glutathionylation of CBS in vitro
Purified CBS (0.5 mg/ml) protein was treated with 1 mM diamide and 1.5 mM GSH in Buffer A (50 mM Tris-HCl, pH 8.0) at room temperature for 30 min. After this reaction, where indicated, 10 mM DTT was added for 30 min at room temperature to induce deglutathionylation and the samples were analyzed using Western blotting. For S-glutathionylation by disulfide exchange with GSSG, recombinant CBS (0.5 mg/ml) protein was incubated for 120 min with 1–5 mM GSSG at room temperature in Buffer A. For S-glutathionylation with H2O2, purified CBS (0.5 mg/ml) protein was treated with 10–200 μM H2O2 at room temperature for 30 min and then, 50–1000 μM GSH (i.e., five equivalents relative to H2O2) was added to the reaction mixture for 30 min.
Determination of the fraction of S-glutathionylated CBS
Titration of free thiol groups with DTNB
Purified wild-type CBS was treated with 10 mM DTT for 30 min at room temperature, and then DTT was removed using a concentrator and washed with 50 mM Tris-HCl, pH 8.0 with six buffer exchanges. For the preparation of S-glutathionylated proteins, CBS was treated with 1 mM diamide and 1.5 mM GSH in Buffer A at room temperature for 30 min, followed by ultrafiltration using a 1 ml spin column.
To examine the number of free thiol groups in CBS, the unmodified and modified CBS (5 μM) was titrated with 0.5 mM DTNB. Control samples contained the same amount of DTNB in the same buffer but were devoid of CBS protein. The final mixtures (200 μl) were incubated for 10 min at room temperature, and the samples were analyzed spectrophotometrically at 412 nm using an extinction coefficient of 13,600 M−1 cm−1 (40).
Separation of S-glutathionylated CBS using streptavidin-agarose resin
Purified CBS was treated with 1 mM diamide and 1.5 mM BioGEE at room temperature for 30 min, after which excess diamide and BioGEE were completely removed using ultrafiltration tubes and 1× PBS buffer. Then, 100 μl CBS (0.5 mg/ml) was added to 100 μl streptavidin-agarose resin pre-equilibrated with 1× PBS buffer and incubated with gentle rotation for 30 min at room temperature. The streptavidin-agarose resin bound glutathionylated CBS and was centrifuged at 1000 g for 5 min. The protein concentration of the supernatant was measured using the Bradford reagent to estimate the proportion of S-glutathionylated CBS. Control reactions in which CBS (0.5 mg/ml) was not treated with diamide and BioGEE were prepared in parallel.
For S-glutathionylation with H2O2, purified CBS (0.5 mg/ml) protein was treated with 25 μM H2O2 at room temperature for 30 min and then 125 μM BioGEE was added to the reaction mixture for 30 min. Excess H2O2 and BioGEE were removed using ultrafiltration tubes and washed with 1× PBS buffer, and the samples were processed as described earlier. Control reactions in which CBS (0.5 mg/ml) was not treated with H2O2 and BioGEE were prepared in parallel.
CBS assay
Enzyme activity was measured using [14C]-serine as previously described (49). Briefly, the 200 μl assay mixture contained 100 mM HEPES, pH 7.4, 0.25 mM PLP, 0.5 mg/ml bovine serum albumin, 30 mM [14C]-serine (∼40,000 dpm), and 20 mM l-homocysteine. The reaction mixture was incubated at 37°C for 5 min, and the reaction was started by the addition of 5.0 μg of purified CBS. After 30 min, the reaction was terminated by the addition of 200 μl of 10% trifluoroacetic acid. AdoMet (1 mM final concentration) was added to the reaction mixture during the preincubation when indicated. One unit of CBS catalyzes the formation of 1 μmol of cystathionine in 1 h at 37°C under standard assay conditions.
H2S production assays
H2S generation using l-cysteine and l-homocysteine as substrates was measured as previously described (7). The reaction mixture containing 100 mM HEPES buffer, pH 7.4, 0.4 mM lead nitrate, 25 mM l-homocysteine, and CBS was preincubated at 37°C for 3 min and the reaction was started by the addition of 25 mM l-cysteine. For testing the effect of AdoMet, 1 mM AdoMet (final concentration) was added to the reaction mixture along with cysteine. An extinction coefficient of 5500 M−1 cm−1 at 390 nm was used for estimation of the lead sulfide concentration.
Activity assay for S-glutathionylated CBS in vitro
The activity of S-glutathionylated CBS was measured using methylcysteine as substrate. Before the start of the assay, S-glutathionylated CBS was centrifuged using a Centricon concentrator to change the buffer using a 1 ml spin column. The reaction mixture containing 100 mM HEPES buffer, pH 7.4, 10 mM methylcysteine, and 50 μmol Thiostar thiol fluorescent detection reagent was added to a 96-well plate, and the reaction was started by the addition of 15 μg of CBS. The 96-well plate was placed in a multifunctional microplate reader, and the kinetics of the enzyme-catalyzed reaction was studied. An excitation wavelength of 400 nm and an emission wavelength of 515 nm were set for measuring the production of the thiol adduct between CH3SH and Thiostar. The activity of unmodified wild-type CBS was set at 100%. Alternatively, DTNB was used in the reaction mixture to detect formation of methanethiol using an extinction coefficient of 13,600 M−1 cm−1 (40).
Western blot analysis
Proteins were blotted onto a polyvinylidenedifluoride membrane, incubated with anti-CBS or anti-SSG primary antibody at 4°C overnight, and then reacted with horseradish-peroxidase-conjugated secondary antibodies for 1 h. Signals were visualized by enhanced chemiluminescence plus reagents.
LC-MS/MS analysis
A 50 μl solution of CBS (100 μM) was reacted with 2 mM diamide and 3 mM GSH for 30 min at room temperature. Untreated CBS was used as a control. The samples were dialyzed against 200 mM Tris-HCl buffer (pH 8.0) and then denatured using 6 M guanidine hydrochloride. At the end of the experiments, the free thiol groups of samples were derivatized with iodoacetamide at a final concentration of 10 mM. The samples were concentrated using a 1 ml Centricon columns, diluted 10-fold with ammonium bicarbonate buffer pH 8.0, and then digested using a protein:trypsin ratio of 20:1 at 37°C for 12 h. The digested samples were acidified with 10% formic and directly used for LC-MS/MS analyses.
All analyses were carried out using an Agilent 1200 Series HPLC, ACE 5 C18-300 column (2.1×100 mm) at a flow rate of 0.1 ml/min, and the injection volume was 6 μl. Mobile phase A and B consisted of 0.1% formic acid in H2O and 0.1% formic acid in acetonitrile, respectively. Separation of peptides was achieved using the following gradient: t=0 min: 5% B; t=1 min: 5% B; t=2 min: 5% B; t=10 min: 15% B; t=38 min: 40% B; t=43 min: 100% B; t=44 min: 100% B; t=45 min: 5% B; and t=60 min: 5% B (column re-equilibration). A high-performance liquid chromatography (HPLC) system was directly coupled to a BrukerSoalrix 70 Hybrid FTMS instrument equipped with an electrospray ionization source. The system was controlled by HyStarv.3.4.8.0 software (BrukerDaltonics). MS data were collected with a resolving power of 110,000 (at m/z 400) in a positive mode using the following conditions: a capillary voltage of 4500 V and an end plate offset of −500 V. The dry temperature was set at 180°C. Dry gas flow was maintained at 4 L/min. Acquisition range was 344–2200 m/z with 0.4 s ion accumulation time. For MS/MS analysis, automated MS/MS spectra were acquired during the run in the data-dependent acquisition MS/MS boost mode with the selection of the most abundant precursor ion and CID fragmentation. Data were deconvoluted and a peak list created by DataAnalysisv.4.0 software (BrukerDaltonics). MS/MS search was performed against the amino-acid sequence of the CBS protein with a mass tolerance of 30 ppm for both the precursor ions and the fragment ions using BioToolsv3.2 software (BrukerDaltonics).
Cell culture and preparation of cell lysates
Human embryonic kidney cells, HEK293, were grown in Dulbecco's modified Eagle's medium containing 10% (v/v) fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were grown at 37°C in an atmosphere of 5% CO2 and 95% air and grown to 80%–90% confluence in six-well plates. To generate whole cell extracts, cells were rinsed thrice with ice-cold PBS and then lysed in RIPA buffer (50 mM Tris-Cl pH 7.4, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, and a protease inhibitor cocktail tablet/50 ml). After centrifugation at 12,000 g for 10 min, the supernatant was collected and the protein concentration was determined by the Bradford method.
Streptavidin pull-down assay
HEK293 cells were preincubated with 250 μM BioGEE, a membrane-permeable analogue of GSH (13) for 1 h, and subsequently exposed to 200 μM H2O2 for 10, 30, and 60 min. Biotin-bound proteins were extracted using streptavidin affinity resin and eluted with 10 mM DTT. The eluent was subjected to nonreducing SDS-PAGE and probed with an antibody that was specific to CBS. Cells were incubated without BioGEE and served as a control. An equal amount of total whole cell extract (10 μg) at the designated time point was then subjected to nonreducing SDS-PAGE and probed with antibodies that were specific to CBS.
Streptavidin-agarose beads were used to pull down the biotinylated GSH-conjugated proteins. Briefly, before the immobilization, the beads were washed thrice with RIPA buffer to remove preservatives. Equal amounts of protein samples from HEK293 cells treated with H2O2 for different time periods were then mixed with 20 μl of beads, and the mixture was incubated at room temperature with gentle rotation for 30 min. The bead-protein complex was separated, and the pellet containing labeled proteins was re-suspended in RIPA buffer, followed by three washes with RIPA buffer. Finally, proteins bound to streptavidin via a mixed disulfide bond were eluted from the beads by incubation for 30 min with 50 mM Tris-Cl pH 7.4 containing 0.1% SDS and 10 mM DTT. Proteins in the eluent were detected by Western blotting.
Activity assay of S-glutathionylated CBS in cell lysates
HEK293 cells treated with 200 μM H2O2 for different time periods were washed thrice with ice-cold PBS buffer and then scraped from 100 mm plates. The harvested cells were transferred to 1.5 ml centrifuge tubes and centrifuged for 5 min. The supernatant was carefully discarded, and the pellet was weighed. HEPES buffer (100 mM, pH 7.4) was added to the tube (15 μl buffer/1 mg cell pellet), and the cell suspension was frozen and thawed thrice using an acetone/dry ice bath. HEK293 cells that were not treated with H2O2 served as a control.
The reaction mixtures (500 μl) containing 100 mM HEPES buffer pH 7.4, cell lysate (400 μl), 10 mM methylcysteine, and 2.5 mM PPG, an inhibitor of CSE, were prepared in polypropylene syringes, as previously described (22). Syringes were sealed with plungers and made anaerobic by flushing the headspace with nitrogen five times using a three-way stopcock, and then left under nitrogen in a final total volume of 5 ml. Syringes were placed in an incubator at 37°C with gentle shaking (75 rpm) for 20 min. Control reactions in which buffer replaced the cell lysate were prepared in parallel. Aliquots of 0.1–0.3 ml from the gas phase of the reaction syringes were collected using gas-tight syringes inserted through a septum attached to the stopcock, and injected in an HP 6890 gas chromatograph (Hewlett Packard) that was equipped with a DB-1 column (30 m×0.53 mm×1.0 μm) as previously described (53). Helium was used as the carrier gas at a flow rate of 1 ml/min, and a temperature gradient ranging from 30°C to 110°C over a 20 min period was used. Methanethiol was detected using a 355 sulfur chemiluminescence detector (Agilent). A methanethiol standard was obtained from Cryogenic Gases. An internal standard of dimethyl sulfide was added to the reaction mixtures.
Statistical analysis
Data from experiments were expressed as mean±SD. Student's t-test was used, where appropriate, for comparing the mean values between control and tested groups. Statistical significance was considered at p<0.05.
Supplementary Material
Abbreviations Used
- AdoMet
S-adenosylmethionine
- BioGEE
biotinylated glutathione ethyl ester
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- DA
diamide
- DTNB
5,5′-dithiobis-2-nitrobenzoic acid
- DTT
dithiothreitol
- GCL
γ-glutamylcysteine ligase
- GP
glutathione peroxidase
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- HPLC
high performance liquid chromatography
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- PBS
phosphate-buffered saline
- PLP
pyridoxal 5′-phosphate
- PPG
propargylglycine
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- tBuOOH
tertiary butyl hydroperoxide
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (HL58984), from the National Natural Science Foundation of China (>20802057), and from the NPU Foundation for Fundamental Research, China (JC201161). The authors thank Dr. Victor Vitvitsky (University of Michigan Medical School) for help with determining the methanethiol levels using the gas chromatography method.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abeles RH. and Walsh C. Acetylenic enzyme inactivators. Inactivation of γ-cystathionase. J Am Chem Soc 95: 6124–6125, 1973 [DOI] [PubMed] [Google Scholar]
- 2.Banerjee R. and Zou CG. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys 433: 144–156, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Carballal S, Cuevasanta E, Marmisolle I, Kabil O, Gherasim C, Ballou DP, Banerjee R, and Alvarez B. Kinetics of reversible reductive carbonylation of heme in human cystathionine beta-synthase. Biochemistry 52: 4553–4562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, and Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci U S A 99: 9745–9749, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celano L, Gil M, Carballal S, Duran R, Denicola A, Banerjee R, and Alvarez B. Inactivation of cystathionine beta-synthase with peroxynitrite. Arch Biochem Biophys 491: 96–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Jhee KH, and Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279: 52082–52086, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, and Banerjee R. H2S biogenesis by cystathionine gamma-lyase leads to the novel sulfur metabolites, lanthionine and homolanthionine, and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, and Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal 10: 445–473, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, and Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci 34: 85–96, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Ereno-Orbea J, Majtan T, Oyenarte I, Kraus JP, and Martinez-Cruz LA. Structural basis of regulation and oligomerization of human cystathionine beta-synthase, the central enzyme of transsulfuration. Proc Natl Acad Sci U S A 110: E3790–E3799, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein JD, Kyle WE, Martin JL, and Pick AM. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem Biophys Res Commun 66: 81–87, 1975 [DOI] [PubMed] [Google Scholar]
- 12.Frank N, Kery V, Maclean KN, and Kraus JP. Solvent-accessible cysteines in human cystathionine beta-synthase: crucial role of cysteine 431 in S-adenosyl L-methionine binding. Biochemistry 45: 11021–11029, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gao XH, Bedhomme M, Veyel D, Zaffagnini M, and Lemaire SD. Methods for analysis of protein glutathionylation and their application to photosynthetic organisms. Mol Plant 2: 218–235, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Gherasim C, Yadav PK, Kabil O, Niu WN, and Banerjee R. Nitrite reductase activity and inhibition of H2S biogenesis by human cystathionine beta-synthase. PLoS One 9: e85544, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghezzi P, Casagrande S, Massignan T, Basso M, Bellacchio E, Mollica L, Biasini E, Tonelli R, Eberini I, Gianazza E, Dai WW, Fratelli M, Salmona M, Sherry B, and Bonetto V. Redox regulation of cyclophilin A by glutathionylation. Proteomics 6: 817–825, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hondorp ER. and Matthews RG. Oxidative stress inactivates cobalamin-independent methionine synthase (MetE) in Escherichia coli. PLoS Biol 2: e336, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Z, Pinto JT, Deng H, and Richie JP., Jr.Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol 75: 2234–2244, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhee KH, McPhie P, and Miles EW. Yeast cystathionine beta-synthase is a pyridoxal phosphate enzyme but, unlike the human enzyme, is not a heme protein. J Biol Chem 275: 11541–11544, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Kabil O. and Banerjee R. The redox biochemistry of hydrogen sulfide. J Biol Chem 285: 21903–21907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabil O. and Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20: 770–782, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabil O, Motl N, and Banerjee R. H2S and its role in redox signaling. Biochim Biophys Acta 1844:1355–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabil O, Vitvitsky V, Xie P, and Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 15: 363–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kery V, Poneleit L, and Kraus JP. Trypsin cleavage of human cystathionine beta-synthase into an evolutionarily conserved active core: structural and functional consequences. Arch Biochem Biophys 355: 222–232, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal 12: 1111–1123, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y, Dargusch R, Schubert D, and Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kraus JP, Janosik M, Kozich V, Mandell R, Shih V, Sperandeo MP, Sebastio G, de Franchis R, Andria G, Kluijtmans LA, Blom H, Boers GH, Gordon RB, Kamoun P, Tsai MY, Kruger WD, Koch HG, Ohura T, and Gaustadnes M. Cystathionine beta-synthase mutations in homocystinuria. Hum Mutat 13: 362–375, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 13: 1169–1183, 1999 [PubMed] [Google Scholar]
- 28.Majtan T, Liu L, Carpenter JF, and Kraus JP. Rescue of cystathionine beta-synthase (CBS) mutants with chemical chaperones: purification and characterization of eight CBS mutant enzymes. J Biol Chem 285: 15866–15873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majtan T, Singh LR, Wang L, Kruger WD, and Kraus JP. Active cystathionine beta-synthase can be expressed in heme-free systems in the presence of metal-substituted porphyrins or a chemical chaperone. J Biol Chem 283: 34588–34595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meier M, Janosik M, Kery V, Kraus JP, and Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J 20: 3910–3916, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelet L, Zaffagnini M, Marchand C, Collin V, Decottignies P, Tsan P, Lancelin JM, Trost P, Miginiac-Maslow M, Noctor G, and Lemaire SD. Glutathionylation of chloroplast thioredoxin f is a redox signaling mechanism in plants. Proc Natl Acad Sci U S A 102: 16478–16483, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, and Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles EW. and Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem 279: 29871–29874, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Morales A, Garcia-Ruiz C, Miranda M, Mari M, Colell A, Ardite E, Fernandez-Checa JC. Tumor necrosis factor increases hepatocellular glutathione by transcriptional regulation of the heavy subunit chain of gamma-glutamylcysteine synthetase. J Biol Chem 272: 30371–30379, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Morales A, Miranda M, Sanchez-Reyes A, Colell A, Biete A, Fernandez-Checa JC. Transcriptional regulation of the heavy subunit chain of gamma-glutamylcysteine synthetase by ionizing radiation. FEBS Lett 427: 15–20, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Mosharov E, Cranford MR, and Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39: 13005–13011, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Rahman I, Smith CA, Antonicelli F, and MacNee W. Characterisation of gamma-glutamylcysteine synthetase-heavy subunit promoter: a critical role for AP-1. FEBS Lett 427: 129–133, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Rinna A, Torres M, and Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med 41: 86–91, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, and Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113: 274–284, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedlak J. and Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192–205, 1968 [DOI] [PubMed] [Google Scholar]
- 41.Sen S. and Banerjee R. A pathogenic linked mutation in the catalytic core of human cystathionine beta-synthase disrupts allosteric regulation and allows kinetic characterization of a full-length dimer. Biochemistry 46: 4110–4116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi MM, Iwamoto T, and Forman HJ. Gamma-glutamylcysteine synthetase and GSH increase in quinone-induced oxidative stress in BPAEC. Am J Physiol 267: L414–L421, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Shi MM, Kugelman A, Iwamoto T, Tian L, and Forman HJ. Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J Biol Chem 269: 26512–26517, 1994 [PubMed] [Google Scholar]
- 44.Singh S, Madzelan P, and Banerjee R. Properties of an unusual heme cofactor in PLP-dependent cystathionine beta-synthase. Nat Prod Rep 24: 631–639, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Singh S, Padovani D, Leslie RA, Chiku T, and Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun C, Shi ZZ, Zhou X, Chen L, and Zhao XM. Prediction of S-glutathionylation sites based on protein sequences. PLoS One 8: e55512, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taoka S. and Banerjee R. Characterization of NO binding to human cystathionine [beta]-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem 87: 245–251, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Taoka S, Lepore BW, Kabil O, Ojha S, Ringe D, and Banerjee R. Human cystathionine beta-synthase is a heme sensor protein. Evidence that the redox sensor is heme and not the vicinal cysteines in the CXXC motif seen in the crystal structure of the truncated enzyme. Biochemistry 41: 10454–10461, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Taoka S, Ohja S, Shan X, Kruger WD, and Banerjee R. Evidence for heme-mediated redox regulation of human cystathionine beta-synthase activity. J Biol Chem 273: 25179–25184, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Taoka S, Widjaja L, and Banerjee R. Assignment of enzymatic functions to specific regions of the PLP-dependent heme protein cystathionine beta-synthase. Biochemistry 38: 13155–13161, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv 7: 313–324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tyagi N, Moshal KS, Sen U, Vacek TP, Kumar M, Hughes WM, Jr., Kundu S, and Tyagi SC. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal 11: 25–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitvitsky V, Kabil O, and Banerjee R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid Red Signal 17: 22–31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, and Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem 281: 35785–35793, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, and Zhu YZ. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One 8: e53147, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamane Y, Furuichi M, Song R, Van NT, Mulcahy RT, Ishikawa T, and Kuo MT. Expression of multidrug resistance protein/GS-X pump and gamma-glutamylcysteine synthetase genes is regulated by oxidative stress. J Biol Chem 273: 31075–31085, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.