Abstract
Abdominal cocoon, or idiopathic sclerosing encapsulating peritonitis, is a rare condition characterised by the presence of a dense fibrocollagenous membrane partially or totally encapsulating the small bowel leading to recurrent intestinal obstructions. We present the case of a patient who has presented for the fourth time with a small bowel obstruction. Previous laparoscopy revealed a plaque-like reactive process encapsulating much of the small bowel and the liver. After initial adhesiolysis, the patient's obstructions continued to reoccur. Further laparotomy was performed in order to excise the entirety of the cocoon membrane and free up loops of small bowel encapsulated by the process, hopefully preventing future obstructions.
Background
Sclerosing encapsulating peritonitis is a rare cause of small bowel obstruction (SBO). The condition is diagnosed by the presence of a dense, greyish-white fibrotic membrane surrounding the small bowel.1–9 The primary aetiology of this condition is idiopathic. The idiopathic form is also known as abdominal cocoon.1 Secondary causes usually occur in patients on peritoneal dialysis.2 The condition is difficult to diagnose preoperatively. Radiological diagnosis can sometimes be made with CT; however, due to its infrequent occurrence, diagnosis is usually made intraoperatively. Surgical excision of the membrane and adhesiolysis is usually curative.1
Case presentation
A 71-year-old Hispanic male was admitted to our hospital with symptoms of abdominal pain. The patient has a history of recurrent SBOs and hypertension. He has presented to our hospital four times over the past 2 years with SBOs. The onset of pain began after eating and was similar to the symptoms he had experienced his last bowel obstruction about 1 month prior. The patient admitted nausea and vomiting. He denied chest pain, shortness of breath and fever. The patient explained that his sister also had a history of recurrent bowel obstructions. She was treated with surgical intervention and her symptoms have greatly improved. Surgical history included a cardiac catheterisation, angioplasty and a hernia repair 30 years ago.
Vital signs were blood pressure 141/76 mm Hg, heart rate 79 bpm, temperature 36.7°C, respiratory rate 18 rpm and body mass index 25.64 kg/m2. The patient had no leukocytosis, haemoglobin was 13.0 g/dL, and the rest of the laboratory tests were within the acceptable range. Physical examination of the abdomen revealed deep tenderness to palpation centrally with no generalised rebound or incarcerated hernias. The rest of the examination was within normal limits. The abdominal pain was relieved on placement of the nasogastric tube.
CT of the abdomen with contrast revealed an SBO similar to the patient's prior imaging studies. The proximal and mid-small bowels were mild to moderately distended. There was fecalisation present in the small bowel of the mid-right abdomen. The small bowel loops beyond this point were decompressed. Small nodules of increased density were seen throughout the wall of the small bowel (figure 1).
Figure 1.
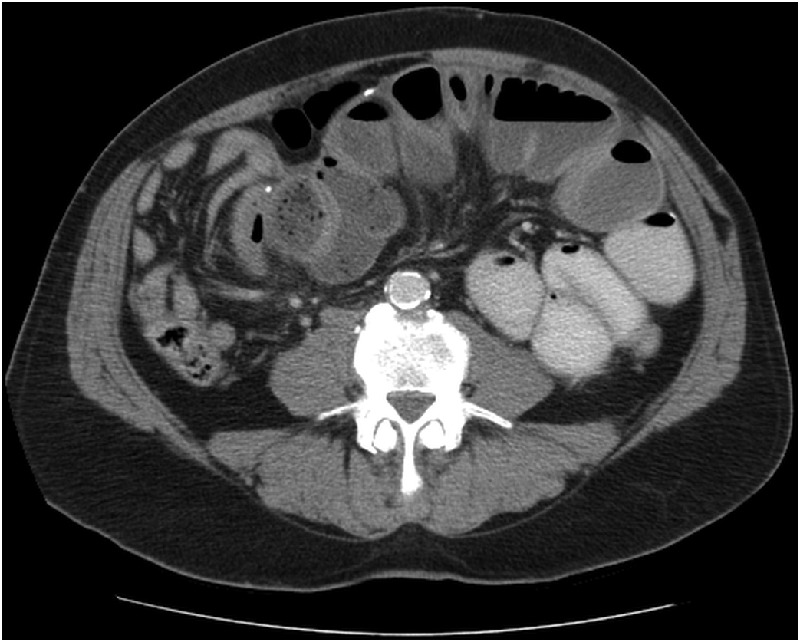
CT of the abdomen showing nodules of calcification along the wall of the bowel. Pathology revealed similar tissue to that of the cocoon process.
The patient had a similar presentation 6 months prior and a laparoscopy was performed in order to free the bowel. During the procedure, a whitish, plaque-like reactive process about the central abdomen encompassing much of the small bowel and the liver was discovered. Adhesions were lysed, and the plaque was incised, freeing up portions of the small bowel and the area of transition.
During this admission, an exploratory laparotomy was performed, as laparoscopy was impossible due to previous adhesions. A cocoon of dense fibrous tissue was found wrapped around three different portions of the small bowel (figure 2). The small bowel loops were visualised by excision of the cocoon process and lysis of adhesions. The underlying bowel was found to be healthy. One portion of the cocoon was densely adherent to the mesentery and required a resection of the small bowel because the blood supply was compromised. Small white nodules were found along the proximal small bowel. Histopathology of the nodules and cocoon showed dense fibrosis with focal necrotic material and dystrophic calcifications (figure 3). Because of the similar pathology, it is possible that these nodules represented the precursor to the abdominal plaque-like cocoon process (figure 1). The same tissue was also found on the bowel that was resected.
Figure 2.
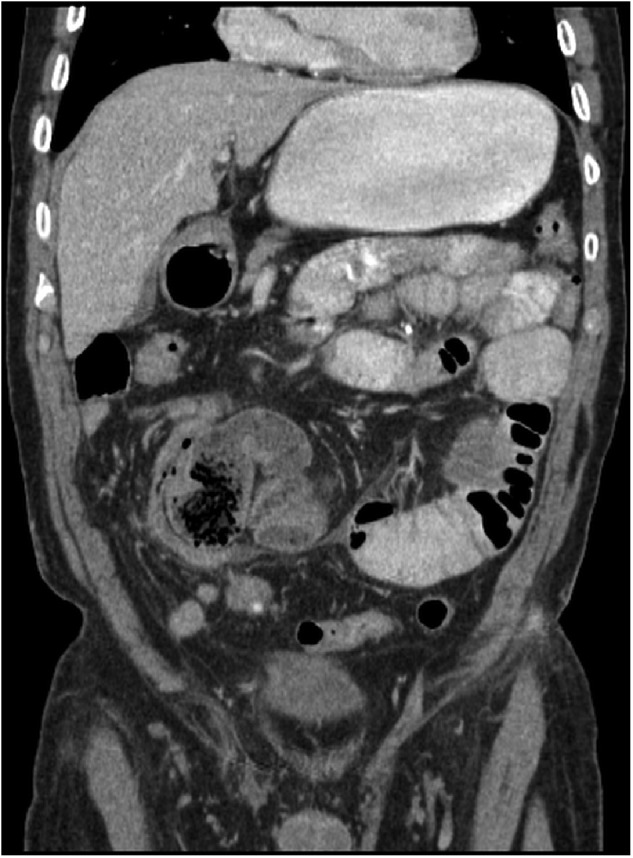
CT of the abdomen showing two of the three areas of the bowel cocoon found on exploratory laparotomy.
Figure 3.
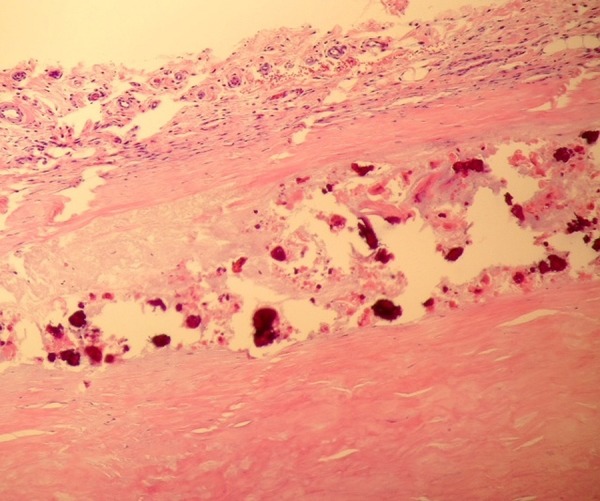
Histopathology of the cocoon process showing a fibrous outer layer with degenerative appearing collagen and dystrophic calcifications.
Outcome and follow-up
Postoperatively, the patient slowly resumed his diet and was discharged after 1 week. On outpatient follow-up, the patient reported remarkable improvement of symptoms and an uneventful recovery. However, considering his symptoms have occurred multiple times despite a previous adhesiolysis, it is possible for reoccurrence.
Discussion
Sclerosing encapsulating peritonitis was first observed in 1907 and termed peritonitis chronica fibrosa encapsulata.1 Most cases have been reported to occur in young adolescent females in tropical and subtropical areas. Many hypotheses have been formulated to try to explain the primary aetiology, including viral peritonitis due to gynecological infection from retrograde menstruation. However, a number of cases have been reported in males and other age groups, which go against the support for these theories.1 3–5 7 Another theory is that the condition is an embryological abnormality. Other developmental malformations such as greater omentum hypoplasia and malformations of mesenteric vessels, have accompanied cases.1 However, there have been cases, including our own, where patients present with symptoms later in life. More investigation still needs to be conducted on these hypotheses.
Most secondary causes have been reported to occur in patients undergoing chronic peritoneal dialysis. Sclerosing encapsulating peritonitis is a rare complication whose incidence increases the longer the patient is receiving dialysis treatment. Other cases have been found to be associated with prior abdominal surgery, practolol (β-blocker) treatment, abdominal tuberculosis, sarcoidosis, cirrhosis and malignancy to name a few.1 6 7 There has also been a case report that describes a patient with a peritoneovenous shunt and diffuse large B-cell lymphoma that later developed sclerosing encapsulating peritonitis.7
Clinically, the patients present with non-specific symptoms, such as nausea, vomiting, recurrent episodes of SBO, abdominal pain, distention and anorexia. Sometimes a non-tender abdominal mass can be found on palpation.1 4 Sixty per cent of SBOs are due to postoperative adhesions. Only 6% of patients present with SBOs due to rare conditions, abdominal cocoon accounting for one of these conditions.1
Radiological findings are consistent with those found in SBOs showing dilated loops of bowel with air fluid levels. Contrast studies may reveal a cluster of loops of small bowel encapsulated by a thickened peritoneum resembling a cauliflower-like appearance. This has been termed the ‘cauliflower-sign’.1 4 5 Our patient had three distinct areas of bowel which where encapsulated by the cocoon process (figure 2). The test of choice is CT. CT scan can reveal loops of small bowel encased in a thick membrane, small bowel tethering, peritoneal thickening and calcification.1 4 In our patient's CT scan, small areas of increased density, likely representing calcifications can be seen scatted along the wall of the small bowel (figure 1). In most cases, the condition cannot be diagnosed until performing diagnostic laparoscopy or laparotomy.
Histopathology of the condition reveals dense fibrocollagenous tissue with or without lymphocytic or plasma cell infiltration.4 In our patient, areas of necrosis and calcifications were also found to be present without any immunological presence. Most have agreed that the management of the condition is surgical dissection and excision of the encapsulating membrane to free the small bowel.1–8 Bowel resection is not usually performed unless the bowel is compromised in some way as it was in our patient.
Learning points.
Sclerosing encapsulating peritonitis is a rare condition; however, it should be considered in the differential diagnosis of patients with recurrent small bowel obstructions of unknown aetiology.
Whether diagnosed preoperatively or intraoperatively, the treatment of sclerosing encapsulating peritonitis is the same. Earlier surgical intervention through preoperative diagnosis will lead to less symptomatic episodes and complications.
More investigation and awareness of the condition can help providers make an earlier diagnosis, provide earlier surgical intervention and prevent further complications.
Acknowledgments
Nova Southeastern University College of Osteopathic Medicine provided the fellowship code in order to submit the article. Memorial Healthcare System is where the patient presented and surgery occurred.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol 2012;18:1999–2004. 10.3748/wjg.v18.i17.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naniwadekar RG, Kulkarin SR, Bane P et al. . Abdominal cocoon: an unusual presentation of small bowel obstruction. J Clin Diagn Res 2014;8:173–4. 10.7860/JCDR/2014/6514.4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awe JA. Abdominal cocoon syndrome (idiopathic sclerosing encapsulating peritonitis): how easy is its diagnosis preoperatively? A case report. Case Rep Surg 2013;2013:604061 10.1155/2013/604061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur R, Chauhan D, Dalal U et al. . Abdominal cocoon with small bowel obstruction: two case reports. Abdom Imaging 2012;37:275–8. 10.1007/s00261-011-9754-5 [DOI] [PubMed] [Google Scholar]
- 5.Shah MY, Gedam BS, Sonarkar R et al. . Abdominal cocoon: an unusual case of subacute intestinal obstruction. Indian J Surg 2013;75:S391–3. 10.1007/s12262-012-0582-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): a report of 5 cases. World J Gastroenterol 2007;13:3649–51. 10.3748/wjg.v13.i26.3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada S, Tanimoto A, Matsuki Y et al. . Sclerosing encapsulating peritonitis (abdominal cocoon) associated with liver cirrhosis and diffuse large B-cell lymphoma: autopsy case. Pathol Int 2009;59:681–6. 10.1111/j.1440-1827.2009.02427.x [DOI] [PubMed] [Google Scholar]
- 8.Caldwell J, Dyer A. Sclerosing encapsulating peritonitis (cocoon bowel) presenting after laparotomy for splenic abscess. J Radiol Case Rep 2013;7:17–23. 10.1186/1752-1947-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamoto H. Encapsulating peritoneal sclerosis—a clinican's approach to diagnosis and medical treatment. Perit Dial Int 2005;25(Suppl 4):S30–8. [PubMed] [Google Scholar]


