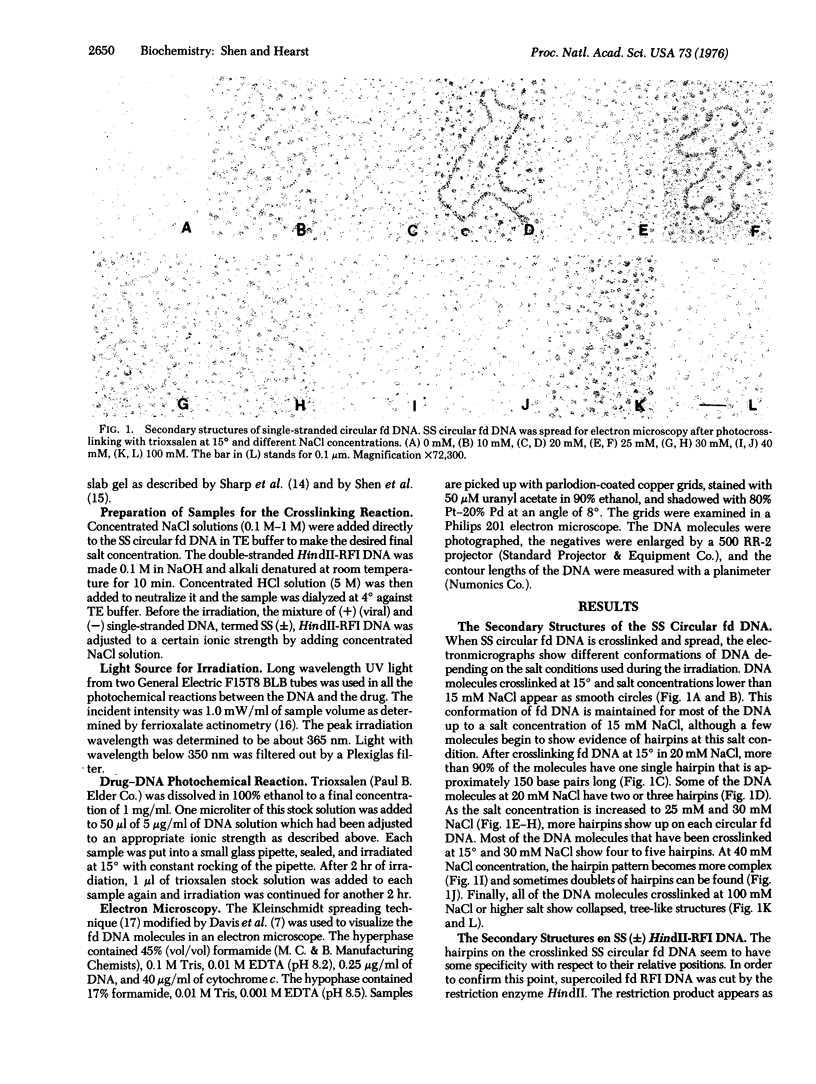
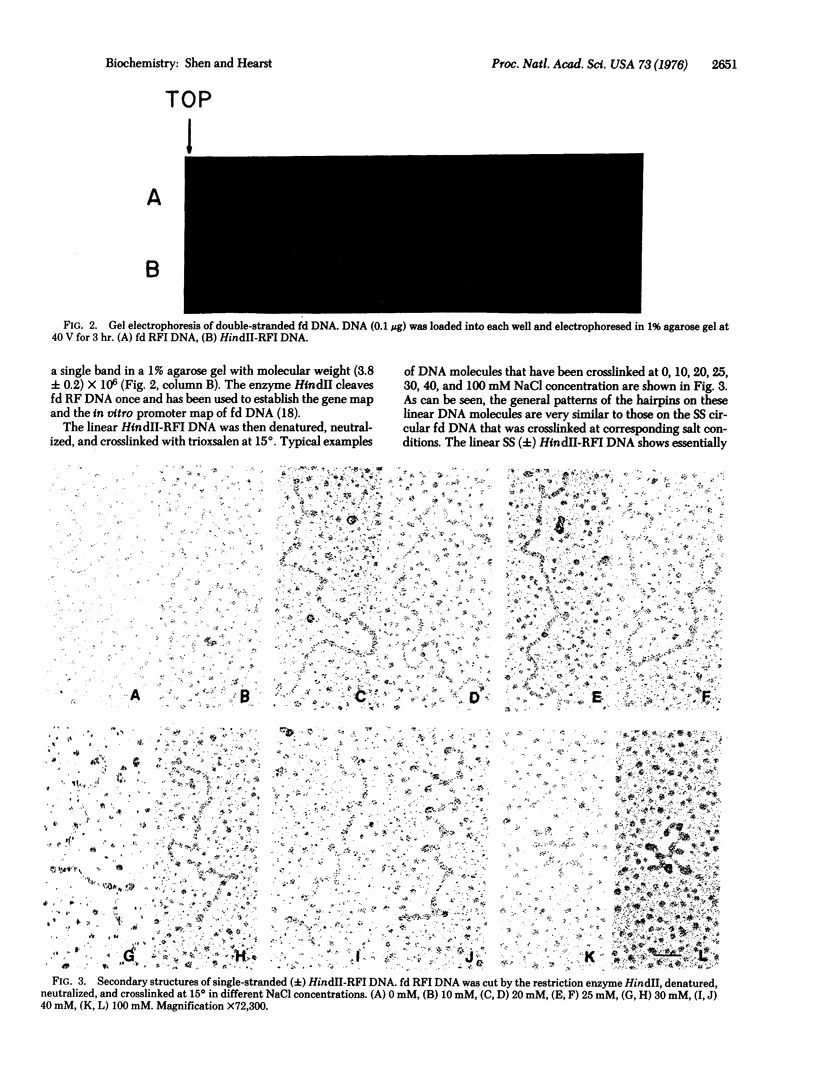
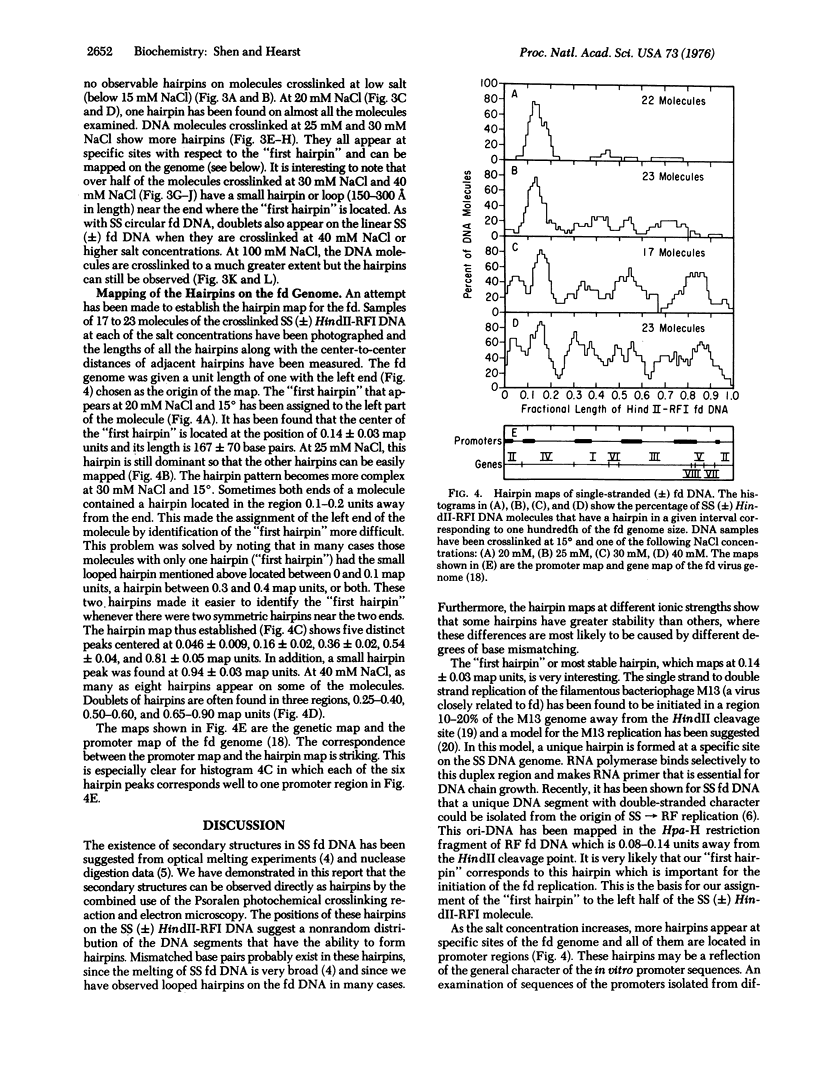
Abstract
The photochemical crosslinking of DNA by 4,5',8-trimethylpsoralen (trioxsalen) has been used to freeze the secondary structures of single-stranded DNA molecules of bacteriophage fd at different ionic strengths. These secondary structures (hairpins or looped hairpins) have been visualized in the electron microscope. Most of the single-stranded circular fd DNA molecules show only one hairpin after irradiation at 15 degrees in 20 mM NaCl in the presence of trioxsalen. As the ionic strength is increased, more hairpins appear on the DNA molecules. To map these secondary structures, double-stranded supercoiled fd DNA (RFI) was cleaved with the restriction enzyme HindII, which makes only one cut on each RFI molecule. After denaturation and crosslinking of the linear single-stranded fd DNA (a mixture of viral and complementary strands), all the hairpins have been mapped on the DNA molecule with respect to this HindII site. The results show that these hairpins occur at specific sites. The most stable hairpin has been assigned to the position where the initiation site for the conversion of single-stranded fd DNA to the double-stranded covalently closed form has been mapped. The remaining hairpins map in or near regions corresponding to in vitro promoter sites on the fd DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz S. A., Day L. A. Molecular weight of single-stranded fd bacteriophage DNA. High speed equilibrium sedimentation and light scattering measurements. Biochemistry. 1974 Nov 5;13(23):4825–4831. doi: 10.1021/bi00720a022. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Vedaldi D., Rodighiero G. Studies on the photoreactions (365 nm) between DNA and some methylpsoralens. Biochim Biophys Acta. 1974 Jul 11;353(3):267–273. doi: 10.1016/0005-2787(74)90019-7. [DOI] [PubMed] [Google Scholar]
- Dhar R., Weissman S. M., Zain B. S., Pan J., Lewis A. M., Jr The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 2. The sequence of the early strand transcript. Nucleic Acids Res. 1974 Apr;1(4):595–611. doi: 10.1093/nar/1.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Kozak M., Nathans D. Translation of the genome of a ribonucleic acid bacteriophage. Bacteriol Rev. 1972 Mar;36(1):109–134. doi: 10.1128/br.36.1.109-134.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Barrell B. G., Donelson J. Sequence of a repressor-binding site in the DNA of bacteriophage lamda. Nature. 1974 Aug 2;250(465):394–397. doi: 10.1038/250394a0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Schaller H. The topology of DNA from the small filamentous bacteriophage fd. J Mol Biol. 1966 Jan;15(1):1–7. doi: 10.1016/s0022-2836(66)80204-8. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Sugiura M., Takanami M. Length of RNA transcribed on the replicative form DNA of coliphage fd. J Mol Biol. 1969 Oct 14;45(1):101–111. doi: 10.1016/0022-2836(69)90213-7. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Voss H., Gucker S. Structure of the DNA of bacteriophage fd. II. Isolation and characterization of a DNA fraction with double strand-like properties. J Mol Biol. 1969 Sep 28;44(3):445–458. doi: 10.1016/0022-2836(69)90372-6. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Sekiua T., Ormondt H. V., Khorana H. G. The nucleotide sequence in the promoter region of the fene for an Escherichia coli tyrosine transfer ribonucleic acid. J Biol Chem. 1975 Feb 10;250(3):1087–1098. [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shen C. J., Wiesehahn G., Hearst J. E. Cleavage patterns of Drosophila melanogaster satellite DNA by restriction enzymes. Nucleic Acids Res. 1976 Apr;3(4):931–951. doi: 10.1093/nar/3.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Okamoto T., Takanami M. Starting nucleotide sequences of RNA synthesized on the replicative form DNA of coliphage fd. J Mol Biol. 1969 Jul 28;43(2):299–315. doi: 10.1016/0022-2836(69)90269-1. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Takanami M. Specific cleavage of coliphage fd DNA by five different restriction endonucleases from Haemophilus genus. FEBS Lett. 1973 Aug 15;34(2):318–322. doi: 10.1016/0014-5793(73)80821-x. [DOI] [PubMed] [Google Scholar]
- Zain B. S., Weissman S. M., Dhar R., Pan J. The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 1. The sequence of the late strand transcript. Nucleic Acids Res. 1974 Apr;1(4):577–594. doi: 10.1093/nar/1.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]