Abstract
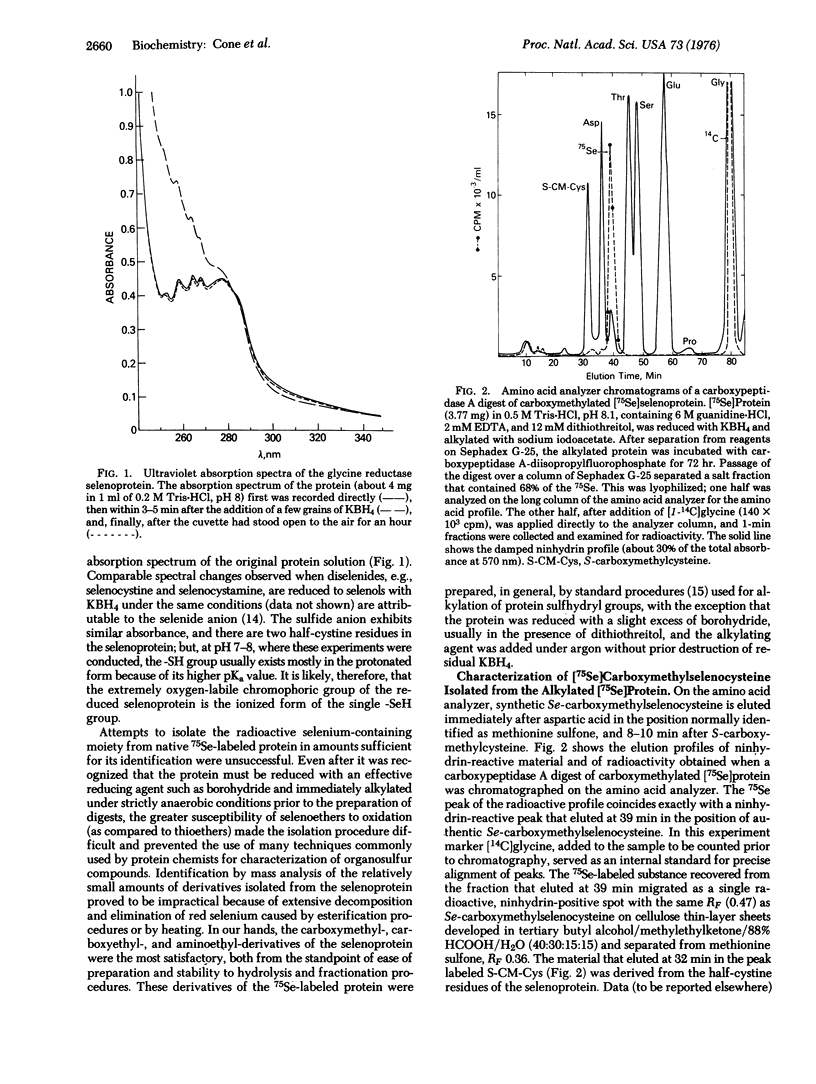
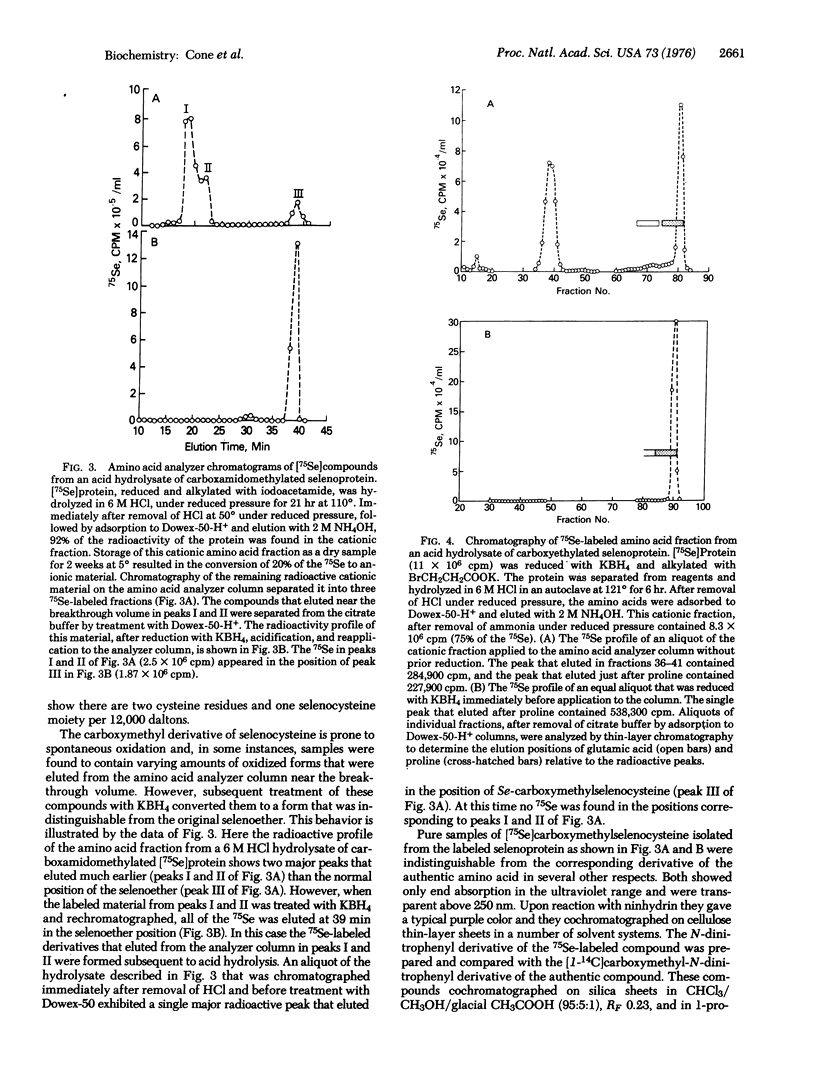
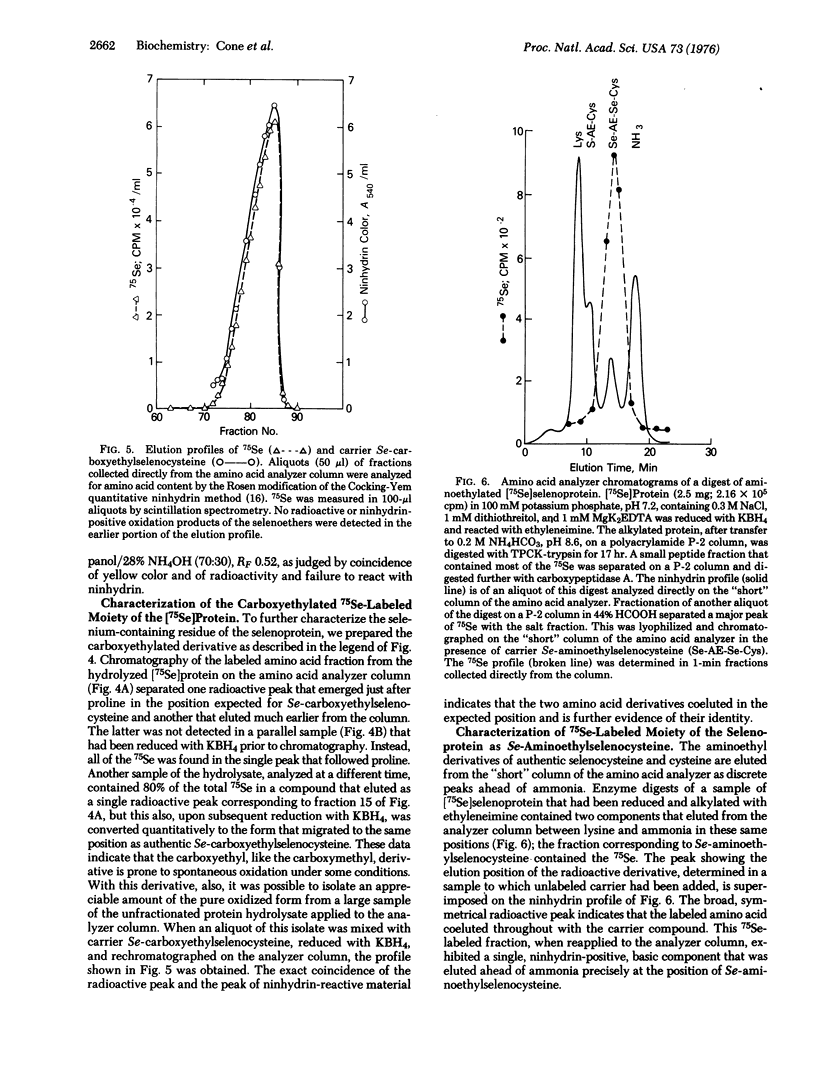
A small, heat-stable selenoprotein, one of the components of the glycine reductase complex, was labeled with 75Se by growth of Clostridium sticklandii in the presence of Na2 75SeO3. The selenium-containing moiety, which is essential for the biological activity of the protein, was shown to be a selenocysteine residue. It was isolated as its Se-carboxymethyl, Se-carboxyethyl, and Se-aminoethyl derivatives from digests of the pure 75Se-labeled protein that had been reduced and treated with the various alkylating agents prior to hydrolysis. In each instance the 75Se-labeled moiety obtained from an alkylated protein sample and the corresponding alkyl derivative of authentic selenocysteine were indistinguishable. Several studies of the native selenoprotein detected a chromophore (UVmax 238nm) that appeared upon reduction of the protein with KBH4 and rapidly disappeared upon exposure to oxygen. This oxygen-labile chromophore is thought to be the ionized -SeH group of the selenocysteine residue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Ljungdahl L. G. Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol. 1973 Nov;116(2):867–873. doi: 10.1128/jb.116.2.867-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973 Mar 25;248(6):2125–2133. [PubMed] [Google Scholar]
- Heizmann C. W., Malencik D. A., Fischer E. H. Generation of parvalbumin-like proteins from troponin. Biochem Biophys Res Commun. 1974 Mar 15;57(1):162–168. doi: 10.1016/s0006-291x(74)80371-2. [DOI] [PubMed] [Google Scholar]
- Huber R. E., Criddle R. S. Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch Biochem Biophys. 1967 Oct;122(1):164–173. doi: 10.1016/0003-9861(67)90136-1. [DOI] [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Cantor A. H., Scott M. L. Mode of action of selenium and vitamin E in prevention of exudative diathesis in chicks. J Nutr. 1973 Oct;103(10):1502–1511. doi: 10.1093/jn/103.10.1502. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Ganther H. E., Hoekstra W. G. Selenium as a component of glutathione periodase isolated from ovine erythrocytes. Biochemistry. 1974 Apr 23;13(9):1825–1829. doi: 10.1021/bi00706a008. [DOI] [PubMed] [Google Scholar]
- PINSENT J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem J. 1954 May;57(1):10–16. doi: 10.1042/bj0570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Glycine reduction to acetate and ammonia: identification of ferredoxin and another low molecular weight acidic protein as components of the reductase system. Arch Biochem Biophys. 1966 Jan;113(1):9–19. doi: 10.1016/0003-9861(66)90151-2. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Science. 1974 Mar 8;183(4128):915–922. doi: 10.1126/science.183.4128.915. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Products of performic acid oxidation and acid hydrolysis of S-carboxymethylcysteine and related compounds. J Biochem. 1973 Dec;74(6):1083–1089. doi: 10.1093/oxfordjournals.jbchem.a130335. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Stadtman T. C. Purification of protein components of the clostridial glycine reductase system and characterization of protein A as a selenoprotein. Arch Biochem Biophys. 1973 Jan;154(1):366–381. doi: 10.1016/0003-9861(73)90069-6. [DOI] [PubMed] [Google Scholar]



