Abstract
Two women underwent stereotactic sampling of mammographically detected lesions with insertion of breast biopsy markers. Localisation of the malignant lesions was performed using iodine 125 seeds, with bracketing of the edges of the larger lesion. The seeds/lesions were located and excised using a γ probe. Liga clips attached to peripheral sutures at the edges of the specimen enabled radiographic orientation. Surgeon and radiologist found the specimen radiographs difficult to interpret. In one case the surgeon thought the lesion had been removed, mistaking the iodine seed for the biopsy marker. The radiologist noted absence of the biopsy marker and marginal calcifications but was concerned the seed was absent. Widening the window level allowed seed identification, revealing a characteristic rectangular radiolucent area in what had been interpreted as a Liga clip. Correct interpretation of the findings helped guide lesion removal, intraoperative margin re-excision and confirmed 125I seed retrieval.
Background
Many breast lesions detected using screening mammography, preoperative image-guided lesion localisation is necessary to guide surgery. Hook-wire insertion is the prevalent lesion localisation method, however, Radioguided-Occult Lesion Localisation using Iodine 125 Seeds (“ROLLIS”) is becoming more common. Specimen radiographs are a vital part of the process of surgical removal of impalpable lesions by confirming (1) the lesion (and iodine seed if used) have been excised and (2) allowing identification of radiographically close or involved margins enabling immediate intra-operative re-excision, potentially avoiding the need for a second operation.1 2 With increasing use of portable specimen X-ray machines, specimen images are now immediately available and the surgeon may choose not to wait for the radiologist's report. It is therefore important that surgeons are aware of the radiographic findings following previous percutaneous biopsy and the details of the localisation procedure so they are aware of what to look for on the specimen radiograph. This paper presents two cases which illustrate these important points.
Case presentation
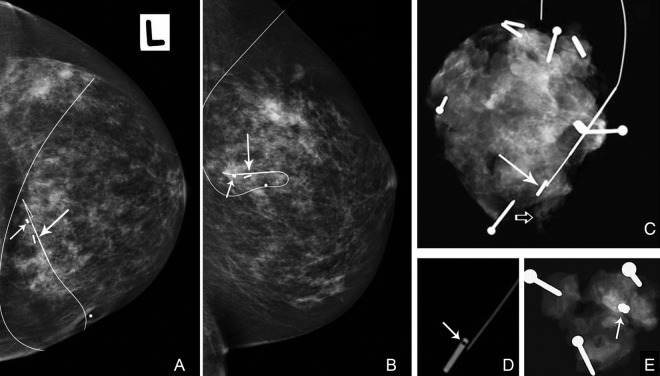
Case 1: Screening mammography in a 57-year-old woman detected a 5 mm cluster of indeterminate microcalcifications in the left breast. Samples containing calcifications were obtained by means of 9G stereotactic core biopsy and a dumbbell-shaped breast biopsy marker inserted. A post procedural mammogram showed satisfactory placement of the marker at the biopsy site. There were very few residual calcifications (figure 1).
Figure 1.
Left cranio-caudal (A) and lateral (B) mammograms following pre-operative image guided localisation using an 125I seed (long arrow) and a hook-wire. Almost all visible micro-calcifications were removed by the core biopsy and the dumbbell-shaped biopsy marker (short arrow) is correctly positioned at the biopsy site. (C) Specimen radiograph (SR). Residual microcalcifications are present at the lateral margin (open arrow). Rectangular metallic structure adjacent to hook-wire tip (arrow) has similar appearances to a liga clip, biopsy marker or 125I seed. (D) Using a high window level, a radiolucent window is seen within, helping to identify the seed. (E) Radiograph of re-excision specimen. The dumbbell-shaped breast biopsy marker is present (arrow).
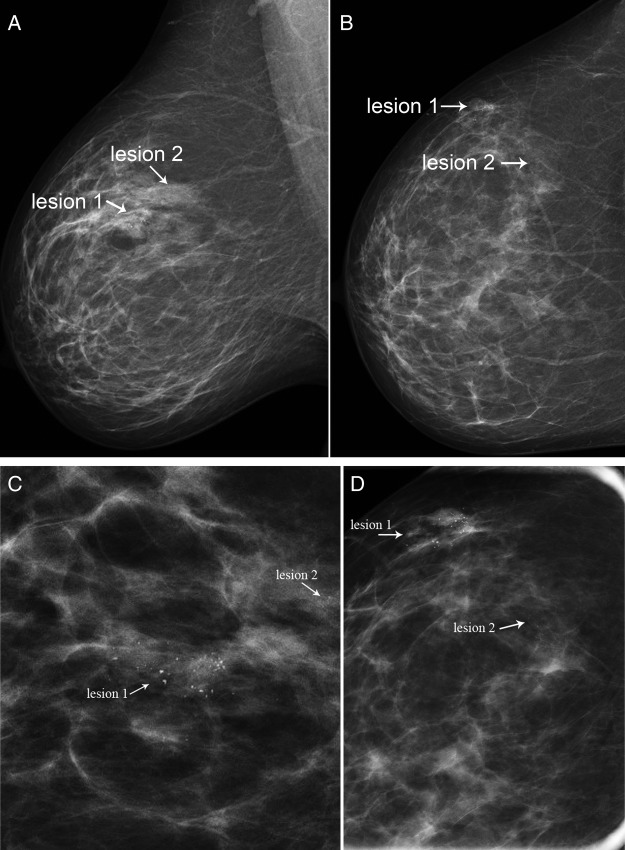
Case 2: Screening mammography in a 58-year-old woman detected two clusters of indeterminate microcalcifications in the right breast, one at the 10 o'clock position measuring 23 mm (lesion 1) and the other at 11 o'clock position measuring 11 mm (lesion 2). Stereotactic 9G core biopsy was performed with accurate placement of a rod-shaped marker at site of lesion 1 and a dumbbell-shaped marker at lesion 2 (figure 2).
Figure 2.
Right MLO and CC mammograms (A,B) and corresponding magnification views (C,D). Two clusters of microcalcification (lesion 1 and lesion 2) are present in the upper outer quadrant of the right breast.
Investigations
Case 1: Histopathology results showed intermediate and high-grade ductal carcinoma in situ (DCIS)
Case 2: Histopathology results showed intermediate grade DCIS for lesion 1 and benign breast change for lesion 2.
Treatment
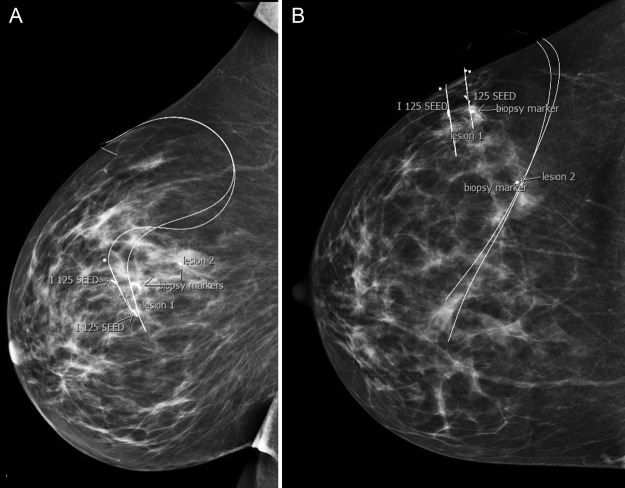
Both patients had given informed consent to participate in pilot studies (ACTRN12611000667910 and ACTRN12611000667910) of pre-operative image-guided localisation of impalpable breast lesions using low-dose iodine 125 seeds (“ROLLIS”). Stereotactic guidance was used for localisation in both cases. As per the study protocol, a hookwire was also inserted for back-up while the surgeons became comfortable with the new technique. In case 1, a single seed/wire was inserted at the site of the biopsy marker (figure 1) and in case 2, two 125I seeds and wires were used to bracket the edges of lesion number 1, a 23 mm cluster of calcifications (figure 3).
Figure 3.
(A and B) Right lateral and craniocaudal mammograms taken after stereotactically-guided insertion of two 125I seeds and modified Kopan's hookwires, bracketing the edges of lesion 1. Note the cylindrical marker at site of prior core biopsy of this lesion. The dumbbell-shaped marker denotes site of lesion 2 (benign core biopsy result).
In theatre, the surgeon used a standard hand held γ probe, set to detect the 27 kev photon emitted by the 125I seed to identify the location of the seeds (lesions), choose the optimal incision site and guide removal. Excision of the seed/lesion was confirmed by absent counts in the surgical bed and high counts within the specimen. The specimen was orientated using the standard technique of insertion of peripheral sutures of varying lengths (long=lateral, short=superior, medium=medial). To facilitate the identification of margins on the specimen radiograph (SR), medium-sized Liga clips were also attached to the sutures (1=superior, 2=medial, 3=lateral). Intra-operative specimen radiography was performed using a portable machine (Trident™, Hologic) in theatre.
In case 1, the surgeon thought the seed and biopsy marker were both visible. The radiologist reported absence of the biopsy marker with residual microcalcifications at the lateral margin (figure 1c). There was initial concern that the 125I seed was not visible and may have become dislodged from the specimen, however high 125I counts were present within the specimen using the probe. The radiologist reviewed the image again using a high window level on the Picture Archiving and Communication System (PACS). This revealed a characteristic radiolucent “window” in what had initially been interpreted as a Liga clip (figure 1d). The findings were discussed with the surgeon. Re-excision of tissue from the deep cavity margin was performed and a further specimen X-ray confirmed successful removal of the biopsy marker (figure 1e).
In case 2, two 125I seeds, the biopsy marker and the cluster of calcifications were all visible on the specimen radiograph (figure 4) Calcifications extended to within 7 mm of the infero-lateral margin prompting intra-operative re-excision of that margin.
Figure 4.
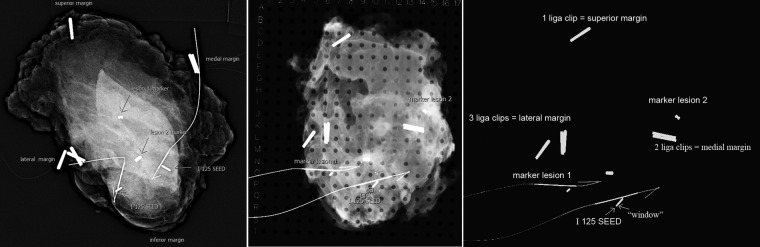
(A) The fresh specimen radiograph taken with a portable X-ray machine in theatre is immediately available to the surgeon. Important structures are labelled. (B) Specimen X-ray taken in a grid after tissue fixation in formalin. The radiographic co-ordinates for the lesion given in the radiologist's report enable the pathologist to concentrate their sections in the region of interest (M-P; 7-12). They also act as a guide as to the location of the 125I seed. Note that while two seeds were present in the initial SR, only one seed is seen in the SR postfixation. Discussion with pathology revealed that the other seed was close to the tissue surface and had been retrieved by the pathologist and sent for safe storage prior to the X-ray. (C) Image viewed using high window level. The 125I seed may be distinguished from the cylindrical biopsy marker and the peripheral Liga clips by presence of a radiolucent “window” which can be detected by increasing the window level on PACS.
Outcome and follow-up
Final histopathology in case 1 showed 20 mm of intermediate to high-grade DCIS extending to the lateral margin and <1 mm from the inferior margin. A 4 mm focus of possible invasive disease was also present at the lateral margin. The remaining radial margins were clear. The extra tissue shave taken from the deep margin at the time of initial surgery (shown to contain the biopsy marker on the SR) was negative.
Re-excision of the infero-lateral margins was performed and a 1.2 mm focus of residual DCIS was found 3 mm from the outer aspect of the new inferior margin. Radiotherapy was not considered indicated and the patient was discharged on tamoxifen. No residual microcalcifications were seen at routine follow-up mammography 12 months later and the patient continues on routine annual surveillance.
In case 2, final pathology showed 32 mm of intermediate grade DCIS, 3 mm from the inferior margin, with a separate 5 mm focus intermediate-grade DCIS within 0.3 mm of the medial margin. Remaining radial margins were clear. The extra infero-lateral margin taken in theatre was clear. The patient was re-admitted for re-excision of the medial margin but no further tumour was found. Post-operative external beam radiotherapy was given. One year follow-up mammography showed no concerning findings.
Discussion
It is important to confirm that a mammographically detected lesion has been successfully removed by identifying it on a specimen X-ray. This completes the investigative loop initiated by the screening mammogram. Finding an abnormality in the specimen on histopathology without this radiographic correlation does not guarantee that this represents the mammographic lesion.
Pre-operative diagnostic vacuum assisted core biopsy of impalpable breast lesions is now common and often removes all visible traces of the sampled lesion on imaging. In this instance, the radiologist usually inserts a breast biopsy marker to act as a visible surrogate for the lesion should preoperative localisation and surgery be needed. Providing the breast biopsy marker lies at the biopsy site, visualisation of the appropriate marker on the specimen radiograph is confirmation that the lesion has (at least in part) been removed. Several different types of radio-opaque breast biopsy markers (varying in size and shape) are now available, and if more than one lesion has been sampled, more than one marker may be present in a patient. It is therefore important that the post biopsy mammogram is reviewed to determine:
If any residual mammographic abnormality is present
The appearances of the marker placed at the site of the sampled lesion and
Whether the marker has migrated from the biopsy site, requiring adjustments to be made both during pre-operative localisation and surgery to ensure excision of the lesion
Radioguided occult lesion localisation using 125I seeds is a promising alternative to the use of hook-wires3 and offers many advantages including:
The ability to insert the seed several days in advance of surgery, thus removing any delays in the theatre list waiting for the localisation procedure.4 5
Shorter operating times.6
The surgeon does not have to follow the wire to the lesion and can thus choose the most direct and cosmetically appropriate incision site.
Unlike hook-wires, which can migrate after insertion, seed migration is rare.5 7
Real-time three dimensional feedback during surgery facilitates centering of the lesion within the excised tissue. Lower re-excision rates have been reported.8 9
Removal of the seed from the patient is confirmed by absent 125I counts within the surgical bed using the intraoperative γ probe. Confirming that the seed lies within the excised tissue is important before the specimen is sent to the Pathology department. Occasionally if the seed is placed superficially within the lesion the surgeon may encounter it on raising the skin flaps during dissection, in which case it may become displaced from the specimen, as noted in 30/1148 (2.6%) of cases by McGhan et al.5 Seeds may also be suctioned into the suction tubing/canister during surgery (3/1148 seeds, 0.3%).5 It is important that this is identified at the time of surgery, and communicated to the other multidisciplinary team members using a seed tracking system to minimise the chances of seed loss. An "on call" medical physicist should be available to provide assistance in locating the seed should any difficulties arise.
High counts within the specimen can obviously confirm presence of the seed within the excised tissue prompting Cox et al10 to suggest that in some cases specimen radiographs may not be necessary to confirm lesion removal. This does not however take into account the importance of the specimen radiograph in enabling immediate intra-operative re-excision of radiographically obvious close or involved margins, which may reduce the need for a second operation.1 2
Confirming the presence of the seed(s) on the specimen radiograph is an important part of the seed tracking process, and helps to minimise the risk of seed loss. The number of seeds inserted at time of localisation should match the number present in the specimen and if (as in case 2) these do not match, immediate efforts to account for this discrepancy must be made. Finding a small radiolucent "window" within metallic structures on the SR using a wide window level on PACS (figures 1D, 4C) may help distinguish the seed from biopsy markers and Liga clips.
Labelling the orientation of the excised tissue is important so that if there is an involved or close margin, re-excision of the correct margin can be performed at the time of the initial surgery (if detected on specimen imaging) or during further surgery if this is not identified until post-operative pathological review. Traditionally margins are labelled by insertion of sutures of varying lengths at the periphery of the specimen. While this works well for the pathologist, correct identification of the sutures on the specimen X-ray is often difficult. Radiopaque labelling markers can be placed next to the edges of the specimen by an assistant before the X-ray is performed, however, labelling errors can be made if sutures are incorrectly identified. If the surgeon attaches Liga clips to the peripheral sutures (figures 1C and 4C) separate labelling becomes unnecessary, removing this potential source of error.
The radiographic assessment of margin adequacy on specimen X-rays has variable accuracy. Graham et al11 reported a positive predictive value of 98% for radiographic evidence of tumour at the specimen margins, however, the negative predictive value of tumour free margins on the radiograph was only 32%. A study by Britton et al12 showed that an 11 mm radiographically clear margin was associated with a ≥5 mm margin on histopathology in 77% of cases, however, if the margin was <11 mm there was a 58% chance the final histological margins would be involved. The variable efficacy of the specimen radiographic findings in predicting presence of clear margins is illustrated in our cases. In case 1, the absence of the biopsy marker and presence of residual microcalcifications adjacent to the lateral margin correlated with presence of DCIS on pathology. Although the appearances on the SR could have prompted intra-operative re-excision of the lateral margin, at second surgery no further disease was found at this margin. While the intra-operative specimen X-ray suggested the inferior margin was clear, the pathologist found DCIS <1 mm from this margin and at re-operation a further focus of DCIS was present 3 mm away.
In case 2, although intra-operative re-excision of the close infero-lateral margin that was noted on the specimen X-ray was performed, unsuspected radiographically occult multifocal DCIS was found at the medial margin on final pathology, requiring a second operation. The presence of DCIS on core biopsy and multifocality are both well known risk factors for positive tumour margins.13 Unfortunately as illustrated by our cases, current imaging techniques are sometimes unable to show the full extent of malignant disease and re-excision may be needed because of this.
In conclusion, specimen radiography is a vital element in the process of surgical removal of impalpable mammographically detected breast lesions. The development of portable specimen radiography machines means that surgeons now have immediate access to these images. Coombs et al14 in a study published in 2006 noted that in most cases suitably trained or experienced surgeons were able to assess specimen radiographs effectively without the need for radiological input. Since this time however, vacuum-assisted biopsy with insertion of biopsy markers and the use of pre-operative image guided125I seed localisation have become more common making interpretation of the specimen radiograph more complex. Surgeons need to be aware of these developments and take them into account when interpreting these images.
Learning points.
Vacuum-assisted core biopsy of small breast lesions may leave the lesion mammographically occult. Insertion of a breast biopsy marker can provide a radiographically visible surrogate target in case future surgery is required.
Identification of the lesion (or the appropriately sited biopsy marker) on the SR is vital to confirm successful removal of impalpable mammographically detected lesions.
Gross tumour margins can be assessed on the SR, however, the correlation with pathological margin width is imperfect and radiographically occult disease remains a significant contributor to re-excision rates.
Attaching Liga clips to the standard specimen orientation sutures facilitates correct identification and immediate re-excision of any radiographically close margins, which may reduce the need for more surgery later.
125I seeds offer many advantages over hook-wires and are being increasingly used for pre-operative lesion localisation. Identification of the seed on the SR is important for seed tracking to help minimise the risk of seed loss.
The seed may be distinguished from Liga clips and breast biopsy markers on the SR by the presence of a characteristic radiolucent ‘window’, visible using a high window level on PACS.
Acknowledgments
Support for the ROLLIS trials has been received from the State Health Research Advisory Committee of Western Australia (WA), the Cancer Council of WA and the Royal Perth Hospital Medical Research Foundation.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dixon J, Sekar OR, Walsh J et al. Specimen-orientated radiography helps define excision margins of malignant lesions detected by breast screening. Br J Surg 1993;80:1001–2. 10.1002/bjs.1800800822 [DOI] [PubMed] [Google Scholar]
- 2.McCormick JT, Keleher AJ, Tikhomirov VB et al. Analysis of the use of specimen mammography in breast conservation therapy. Am J Surg 2004;188:433–6. 10.1016/j.amjsurg.2004.06.030 [DOI] [PubMed] [Google Scholar]
- 3.Dua SM, Gray RJ, Keshtgar M. Strategies for localisation of impalpable breast lesions. Breast 2011;20:246–53. 10.1016/j.breast.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Jakub JW, Gray RJ, Degnim AC et al. Current status of radioactive seed for localization of non palpable breast lesions. Am J Surg 2010;199:522–8. 10.1016/j.amjsurg.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 5.McGhan LJ, McKeever SC, Pockaj BA et al. Radioactive seed localization for nonpalpable breast lesions: review of 1,000 consecutive procedures at a single institution. Ann Surg Oncol 2011;18:3096–101. 10.1245/s10434-011-1910-1 [DOI] [PubMed] [Google Scholar]
- 6.Lovrics PJ, Goldsmith CH, Hodgson N et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol 2011;18:3407–14. 10.1245/s10434-011-1699-y [DOI] [PubMed] [Google Scholar]
- 7.Alderliesten T, Loo CE, Pengel KE et al. Radioactive seed localization of breast lesions: an adequate localization method without seed migration. Breast J 2011;17:594–601. 10.1111/j.1524-4741.2011.01155.x [DOI] [PubMed] [Google Scholar]
- 8.Ahmed M, Douek M. Radioactive seed localisation (RSL) in the treatment of non-palpable breast cancers: Systematic review and meta-analysis. Breast 2013;22:383–8. 10.1016/j.breast.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 9.Lovrics PJ, Cornacchi SD, Vora R et al. Systematic review of radioguided surgery for non-palpable breast cancer. Eur J Surg Oncol 2011;37:388–97. 10.1016/j.ejso.2011.01.018 [DOI] [PubMed] [Google Scholar]
- 10.Cox CE, Furman B, Stowell N et al. Radioactive seed localization breast biopsy and lumpectomy: can specimen radiographs be eliminated? Ann Surg Oncol 2003;10:1039–47. 10.1245/ASO.2003.03.050 [DOI] [PubMed] [Google Scholar]
- 11.Graham RA, Homer MJ, Sigler CJ et al. The efficacy of specimen radiography in evaluating the surgical margins of impalpable breast carcinoma. AJR Am J Roentgenol 1994;162:33–6. 10.2214/ajr.162.1.8273685 [DOI] [PubMed] [Google Scholar]
- 12.Britton P, Sonoda L, Yamamoto A et al. Breast surgical specimen radiographs: How reliable are they? Eur J Radiol 2011;79:245–9. 10.1016/j.ejrad.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 13.Pleijhuis RG, Graafland M, de Vries J et al. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol 2009;16:2717–30. 10.1245/s10434-009-0609-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coombs NJ, Vassallo PP, Parker AJ et al. Radiological review of specimen radiographs after breast localisation biopsy is not always necessary. Eur J Surg Oncol 2006;32:516–19. 10.1016/j.ejso.2006.02.019 [DOI] [PubMed] [Google Scholar]