Abstract
In this study, we report the potential rodent-borne zoonotic helminths in wild-caught murid rodents from four categorized habitats—forest, nonflooded land, irrigated land, and human settlement in seven localities of Thailand, Cambodia, and Lao PDR. Out of 2478 rodent samples, 735 (29.7%) were infected by at least one of the following zoonotic helminth species: Echinostoma malayanum, Echinostoma ilocanum, Plagiorchis muris, Raillietina spp., Hymenolepis diminuta, Hymenolepis nana, Cyclodontostomum purivisi, and Moniliformis moniliformis. Raillietina spp. showed the highest prevalence (13.8%), followed by H. diminuta (8.6%), H. nana (6.7%), and C. purvisi (1.0%). Habitat affected the intensity of helminth infection in murid rodent hosts. Specific habitats favoring each zoonotic helminth species are discussed in relation to the risk of human infection. Season and host maturity influenced intensity of total zoonotic helminths, but there was no influence of host gender. However, in terms of individual helminth species, female rodents were more infected by E. malayanum, E. ilocanum, and C. purvisi than males. Among the rodent species, Rattus tanezumi seems to play the most important role as a reservoir by hosting seven zoonotic heminth species. This rat is ubiquitously found in all types of the habitats, suggesting that it can act as an important bridge species, carrying parasites across different habitats.
Key Words: : Rodent, Zoonosis, Helminth, Habitat, Thailand, Cambodia, Lao PDR
Introduction
Rodents not only act as important reservoirs of microparasites causing substantial human diseases, such as hantaviruses, cowpox, hepatitis E, leptospirosis, plague, murine typhus, toxoplasmosis, bartonellosis, and others, but they also harbor a number of zoonotic macroparasites, e.g., helminthiases (Graczyk and Fried 1998, Spratt 2005, Meerburg et al. 2009, Herbreteau et al. 2012, Bordes et al. 2013).
Helminthiases are considered as neglected diseases, with low public health importance because they produce infections that have low levels of severity in healthy people. Nevertheless, in immunocompromised and immunosuppressive cases (i.e., human immunodeficiency virus [HIV], cancer, organ transplant, radiation sickness, or patients under pharmacological treatment for autoimmune problems, allergy, and inflammatory disorder), helminthiases may cause chronic infection, hyperinfection syndrome, and unusual manifestations in vital organs (Wolday et al. 2002, Delobel et al. 2004, Keiser and Nutman 2004, Syafinaz et al. 2011). In addition, helminth infection in school-aged children significantly impairs physical, nutritional, cognitive, and intellectual development (World Health Organization 1987). Moreover, some zoonotic helminthiases are recognized as important public health concerns, such as echinococcosis, taeniasis, trichinosis, schistosomiasis, filariasis, and opisthorchiasis. Although, these diseases may have less impact compared to the high mortality diseases such as cancer, malaria, tuberculosis, or acquired immunodeficiency syndrome (AIDS), they influence the world population economically and medically.
With respect to the possible role of wild rodents as reservoirs of zoonotic helminthiasis, such studies have been quite limited to date. Potential helminth and rodent reservoir species, as well as the impact of different habitats on the human–rodent–helminth interaction, need to be investigated to better understand their epidemiology and surveillance.
This study focused on rodent-borne helminthiasis of the Indo-Chinese Peninsula countries and aimed to: (1) Reveal potential zoonotic helminth species and their rodent reservoir hosts in this region and (2) analyze the likely habitats of such helminths to evaluate the risk of human contact with these parasites.
Materials and Methods
Rodent samples
During the period 2008 to 2012, rodents were collected from 11 sites across the three countries on the Indochinese Peninsula (Fig. 1): Seven sites in Thailand (Buriram, Chiangrai, Kalasin, Kanchanaburi, Loei, Nan, and Prachuabkirikhan), two in Lao PDR (Luang Prabang and Champasak), and two in Cambodia (Sihanoukville and Mondolkiri). In each trapping site, a 12-day trapping procedure with 100 traps per day (1200 trapping pressure per site) were set within the area of approximately 10×10 km2. Four categorized habitats were selected (applying equal trapping pressure) with regard to human land use or human disturbed habitats, from low to high levels of disturbance: (1) Forest (primary, secondary, or community forests), (2) upland (nonflooded agricultural lands, fields, or fallows), (3) lowland (irrigated cultivation, e.g., rice fields), and (4) human settlements (houses, villages, or cities). Rodent trapping was set twice on each site in relation to the dry (November to April) and wet season (May to October), except for the sites of Chiangrai, Kalasin, Kanchanaburi, and Prachuabkirikhan, for which a single seasonal trapping survey was performed.
FIG. 1.
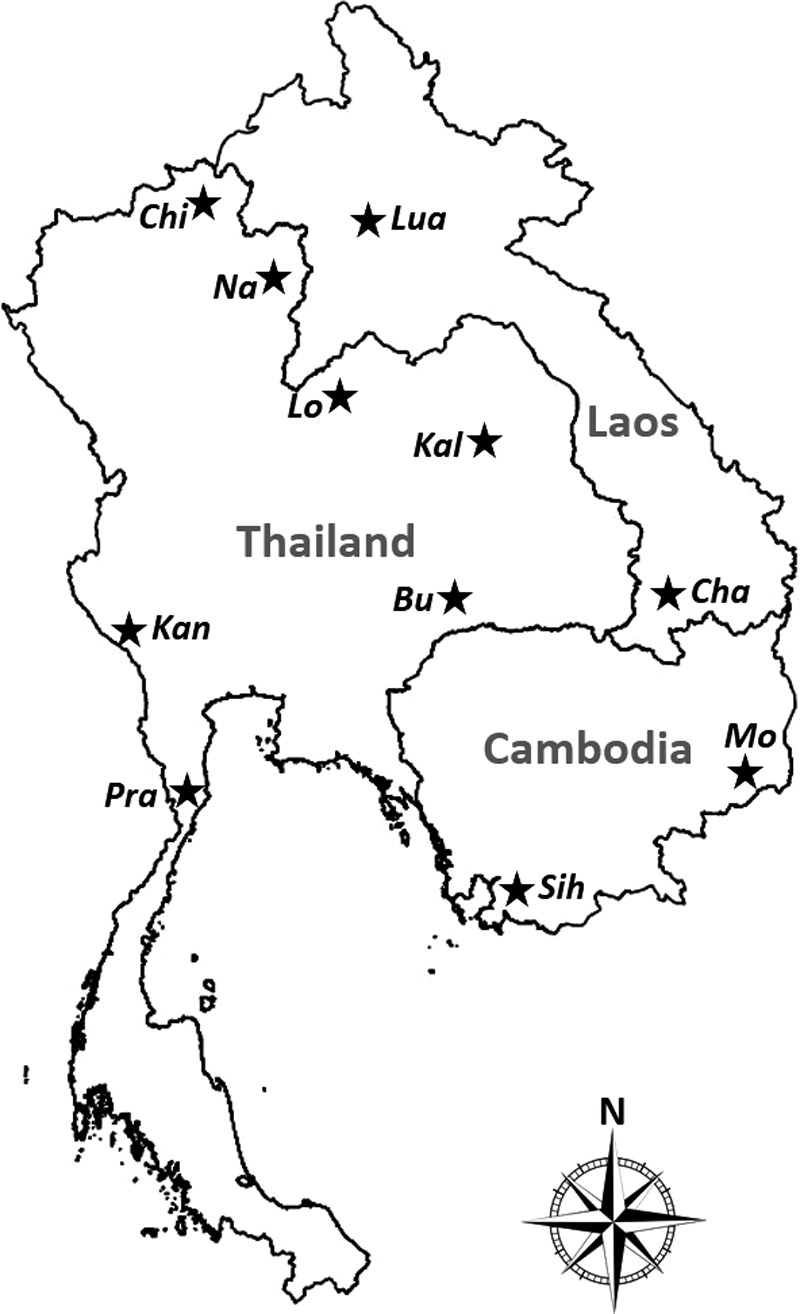
Distribution of rodent sampling localities on Indo-Chinese Peninsula: Thailand (Chi, Chiangrai; Na, Nan; Lo, Loei; Kal, Kalasin; Bu, Buriram; Kan, Kanchanaburi; Pra, Prachuabkirikhan), Lao PDR (Lua, Luang Prabang; Cha, Champasak), and Cambodia (Mo, Mondolkiri; Sih, Sihanoukville).
The captured animals were euthanized and identified morphologically in the field using rodent identification keys (Marshall 1988, Aplin 2003). For problematic or cryptic species, molecular barcoding identification was performed using previously defined, specific primers for the COI gene (www.ceropath.org/barcoding_tool/rodentsea). The gastrointestinal tracts were isolated and preserved individually in 70% alcohol for further helminthological examination.
Animal handling and procedures of specimen collection followed an international standard (American Veterinary Medical Association Council on Research) and the rodent protocols described by Herbreteau et al. (2011).
Potential zoonotic helminth samples
Gastrointestinal tracts were dissected under a stereomicroscope. Worms were isolated and identified on the basis of their morphology following taxonomic identification keys (Yamaguti 1958, Schmidt 1986, Anderson 2000). To obtain quantitative data, each helminth species from individual rodents was counted to determine the infection intensity.
Among overall helminth species, the potential zoonotic species were inferred by searching from previous human case reports and relevant research publications (Table 1). The present study focuses only on the potential zoonotic helminth species, as overall helminth diversity found in these rodents has been reported elsewhere (Chaisiri et al. 2012, Pakdeenarong et al. 2014).
Table 1.
Studies on Rodent-Borne Gastrointestinal Helminthiases in Humans (with an Emphasis on Southeast Asia)
| Zoonotic helminth | Taxon | Country | References |
|---|---|---|---|
| Echinostoma malayanum | Trematoda | India, Indonesia, Lao PDR, Malaysia, Philippines, Thailand, | Lie and Viric 1963, Bhaibulaya et al. 1964, Hadidjaja and Oemijati, 1969, Radomyos et al. 1998, Maji et al. 1993, Belizario et al. 2007, Chai et al. 2012 |
| Echinostoma ilocanum | Trematoda | Cambodia, Indonesia, Philippines, Thailand, | Hilarrio and Wharton 1917, Carney et al. 1980, Radomyos et al. 1982, Cross et al. 1986, Sohn et al. 2011 |
| Plagiorchis murisa | Trematoda | Japan, Korea | Asada et al. 1962, Hong et al. 1996 |
| Hymenolepis diminuta | Cestoda | Worldwide: Argentina, Chile, China, Cuba, Ecuador, Egypt, India, Indonesia, Iran, Italy, Jamaica, Korea, Malaysia, Mexico, Nepal, Panama, Papua New Guinea, Peru, Poland, Rhodesia, Spain, Taiwan, Thailand, United States | Calvo 1951, Castex et al. 1951, Paul and Zaman 1969, Ghadirian and Arfaa 1972, Reyes et al. 1972, Goldsmid 1973, Cutting 1975, Chitchang et al. 1978, Sinniah et al. 1978, Stafford et al. 1980, Kan et al. 1981, Chung et al. 1985, Levi et al. 1987, Cohen 1989, Min 1990, Tena et al. 1998, Marangi et al. 2003, Waloch 2003, Wiwanitkit 2004, Kunwar et al. 2005, Owen 2005, Cordova et al. 2006, El-Shazly et al. 2006, Jacobsen et al. 2007, Watwe and Dardi 2008, Patamia et al. 2010, Alvarez-Fernandez et al. 2012, Rohela et al. 2012 |
| Hymenolepis nana | Cestoda | Worldwide: Cambodia, Honduras, Indonesia, Korea, Lao PDR, Libya, Malaysia, Mexico, Nepal, Papua New Guinea, Peru, Saudi Arabia, Singapore, Taiwan, Thailand, Vietnam | Cheah and Kan 1971, Cross et al. 1976, Chung et al. 1985, Min 1990, Kaminsky 1991, Jongsuksantigul et al. 1992, Sherchand et al. 1996, Toma et al. 1999, Sirivichayakul et al. 2000, El-Sheikh and El-Assouli 2001, Park et al. 2004, Le Hung et al. 2005, Owen 2005, Chhakda et al. 2006, Sithithaworn et al. 2006, Ben-Musa 2007, Chero et al. 2007, Sohn et al. 2011, Syafinaz et al. 2011, Alvarez-Fernandez et al. 2012 |
| Raillietina spp. | Cestoda | China, Costa Rica, French Polynesia, Indonesia, Japan, Thailand | Chandler and Pradatsundarasar 1957, Areekul and Radomyos 1970, Rougier et al. 1981, Brenes et al. 1983, Beaver et al. 1984, Margono 1989 |
| Cyclodontostomum purvisi | Nematoda | Thailand | Bhaibulaya and Indra-ngarm 1975 |
| Moniliformis moniliformisa | Acanthocephala | Australia, Iran, Iraq, Nigeria, United States | Moayedi et al. 1971, Al-Rawas et al. 1977, Counselman et al. 1989, Ikeh et al. 1992, Berenji et al. 2007, Salehabadi et al. 2008 |
Human cases have not been reported in Southeast Asia.
Statistical analysis
Two main descriptors used to quantify parasite number and analyze our data were zoonotic helminth intensity and total zoonotic helminth intensity, where zoonotic helminth intensity is the number of conspecific helminths found in each infected host and total zoonotic helminth intensity is the sum of the number of all zoonotic helminth species found in each infected host. Nonparametric Kruskal–Wallis and multiple pairwise comparison tests were performed to investigate the effects of habitat on zoonotic helminth intensity. In addition, total zoonotic helminth intensities were analyzed as a function of rodent maturity (juvenile and adult), rodent sex, and season (dry and wet) using the nonparametric Mann–Whitney U-test. These tests were performed with SPSS v. 20.0 software (IBM Corp., 2011) applying 95% confidence intervals.
To show the distribution of zoonotic helminth species found in each of the four habitat types, a principal component analysis (PCA) was performed using the package ade4 implemented in R freeware (Team R Core 2010). The PCA was calculated by counting the number of rodents infected by each helminth species across the four categorized habitats. The prevalence, mean abundance, mean intensity, and range of each zoonotic helminth infection were estimated by Quantitative Parasitology software, v. 3.0 (Rozsa et al. 2000).
Results
Among 18 murid rodent species (2478 individuals), eight potential zoonotic helminths were found in 735 rodents (29.66%). Raillietina spp. showed the highest prevalence (13.8%) followed by Hymenolepis diminuta (8.6%), Hymenolepis nana (6.7%), Cyclodontostomum purvisi (1.0%), Plagiorchis muris (0.4%), Echinostoma ilocanum (0.2%), Echinostoma malayanum (0.2%), and Moniliformis moniliformis (0.2%).
Rattus tanezumi harbored the highest number of zoonotic helminth species (six), followed by the other rodents, Bandicota indica, Bandicota savilei, Mus caroli, Mus cervicolor, Mus cookii, Rattus exulans, and Rattus sakaretensis, each of these harboring four helminth species (Table 2).
Table 2.
Prevalence (%), Mean Abundance (MA), Mean Intensity (MI), and Range of Potential Rodent–Borne Zoonotic Helminthes in 18 Rodent Species (n=2478) from Thailand, Cambodia, and Lao PDR (see Materials and Methods)
| Rodent species (n) | Helminth species | Prevalence (%) | MA | MI | Range |
|---|---|---|---|---|---|
| Bandicota indica (287) | Echinostoma ilocanum | 2.1 | 1.66 | 79.17 | 0–293 |
| Raillieina spp. | 43.9 | 3.02 | 6.88 | 0–30 | |
| Hymenolepis diminuta | 0.7 | 0.01 | 1.00 | 0–1 | |
| Cyclodontostomum purvisi | 0.3 | 0.01 | 4.00 | 0–4 | |
| Bandicota savilei (149) | Echinostoma malayanum | 1.3 | 0.07 | 5.50 | 0–10 |
| Raillietina spp. | 29.5 | 0.84 | 2.84 | 0–10 | |
| Hymenolepis diminuta | 10.7 | 0.25 | 2.31 | 0–10 | |
| Cyclodontostomum purvisi | 10.1 | 1.09 | 10.87 | 0–30 | |
| Berylmys berdmorei (52) | Raillietina spp. | 21.2 | 0.40 | 1.91 | 0–5 |
| Hymenolepis diminuta | 7.7 | 0.29 | 3.75 | 0–10 | |
| Berylmys bowersi (37) | Raillietina spp. | 2.7 | 0.03 | 1.00 | 0–1 |
| Hymenolepis diminuta | 8.1 | 0.46 | 5.67 | 0–10 | |
| Leopoldamys edwardsi (16) | Raillietina spp. | 37.5 | 1.56 | 4.17 | 0–10 |
| Hymenolepis diminuta | 37.5 | 5.19 | 13.83 | 0–30 | |
| Cyclodontostomum purvisi | 12.5 | 0.13 | 1.00 | 0–1 | |
| Maxomys surifer (154) | Raillietina spp. | 3.9 | 0.11 | 2.83 | 0–10 |
| Hymenolepis diminuta | 3.2 | 0.08 | 2.60 | 0–4 | |
| Cyclodontostomum purvisi | 2.6 | 0.27 | 10.25 | 0–30 | |
| Mus caroli (135) | Plagiorchis muris | 5.2 | 0.47 | 9.14 | 0–41 |
| Raillietina spp. | 2.2 | 0.03 | 1.33 | 0–2 | |
| Hymenolepis diminuta | 0.7 | 0.01 | 1.00 | 0–1 | |
| Hymenolepis nana | 12.6 | 0.21 | 1.71 | 0–4 | |
| Mus cervicolor (198) | Raillietina spp. | 5.1 | 0.17 | 3.40 | 0–10 |
| Hymenoplepis diminuta | 1.0 | 0.03 | 3.00 | 0–4 | |
| Hymenolepis nana | 28.8 | 1.49 | 5.18 | 0–30 | |
| Cyclodontostomum purvisi | 1.5 | 0.03 | 2.00 | 0–4 | |
| Mus cookii (207) | Plagiorchis muris | 0.5 | <0.01 | 1.00 | 0–1 |
| Raillietina spp. | 1.4 | 0.03 | 2.33 | 0–5 | |
| Hymenolepis diminuta | 3.4 | 0.09 | 2.71 | 0–10 | |
| Hymenolepis nana | 34.8 | 0.87 | 2.5 | 0–30 | |
| Mus pahari (5)a | Hymenolepis nana | 40.0* | 0.80 | 2.00 | 0–3 |
| Niviventer fulvescens (93) | Raillietina spp. | 16.1 | 0.37 | 2.27 | 0–10 |
| Hymenolepis diminuta | 34.4 | 0.83 | 2.41 | 0–10 | |
| Hymenolepis nana | 4.3 | 0.04 | 1.00 | 0–1 | |
| Rattus andamanensis (6)a | Cyclodontostomum purvisi | 16.7* | 3.33 | 20.00 | 0–20 |
| Rattus argentiventer (17) | Hymenolepis diminuta | 5.9 | 0.06 | 1.00 | 0–1 |
| Rattus exulans (545) | Raillietina spp. | 1.5 | 0.03 | 0.38 | 0–10 |
| Hymenolepis diminuta | 13.0 | 0.43 | 3.34 | 0–18 | |
| Hymenolepis nana | 0.4 | 0.01 | 1.50 | 0–2 | |
| Moniliformis moniliformis | 0.5 | 0.01 | 1.67 | 0–3 | |
| Rattus losea (125) | Echinostoma malayanum | 1.6 | 0.03 | 2.00 | 0–3 |
| Raillietina spp. | 16.0 | 0.50 | 3.10 | 0–30 | |
| Hymenolepis diminuta | 0.8 | 0.02 | 2.00 | 0–2 | |
| Hymenolepis nana | 1.6 | 0.09 | 5.50 | 0–10 | |
| Rattus nitidus (14) | Raillietina spp. | 7.1 | 0.14 | 2.00 | 0–2 |
| Hymenolepis diminuta | 21.4 | 0.21 | 1.00 | 0–1 | |
| Rattus norvegicus (17) | Raillietina spp. | 41.2 | 0.41 | 1.00 | 0–1 |
| Hymenolepis diminuta | 23.5 | 0.24 | 1.00 | 0–1 | |
| Rattus tanezumi (421) | Echinostoma malayanum | 0.2 | <0.01 | 1.00 | 0–1 |
| Plagiorchis muris | 0.2 | <0.01 | 2.00 | 0–2 | |
| Raillietina spp. | 19.2 | 0.55 | 2.84 | 0–30 | |
| Hymenolepis diminuta | 13.3 | 0.28 | 2.11 | 0–30 | |
| Hymenolepis nana | 0.2 | <0.01 | 1.00 | 0–1 | |
| Moniliformis moniliformis | 0.3 | 0.01 | 3.00 | 0–3 |
Host sample size <10.
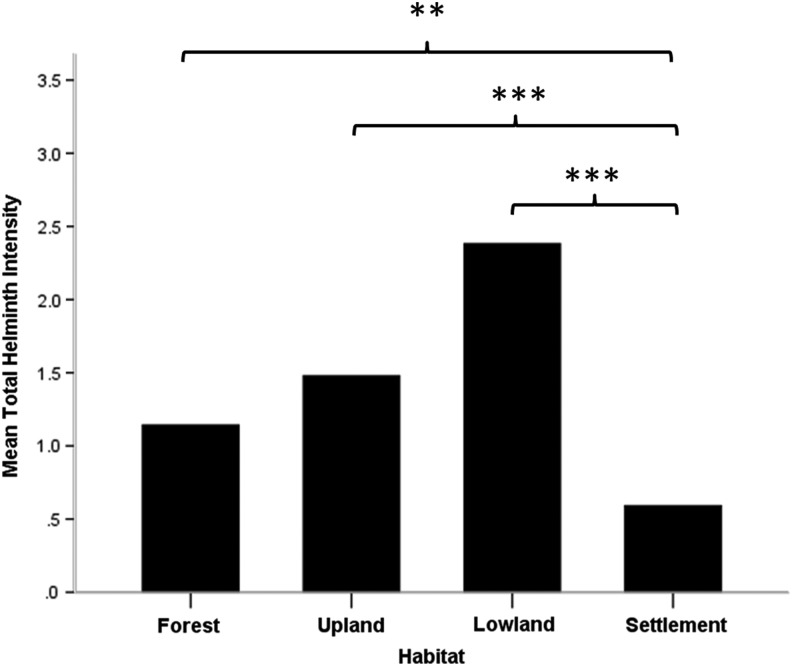
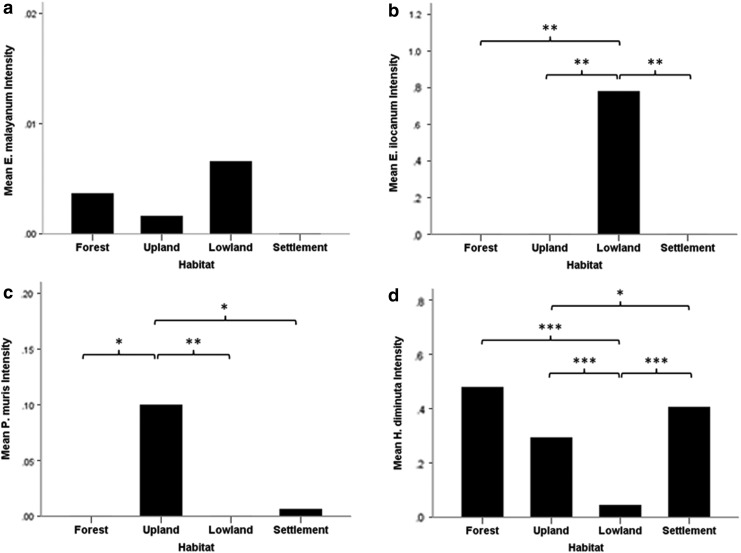
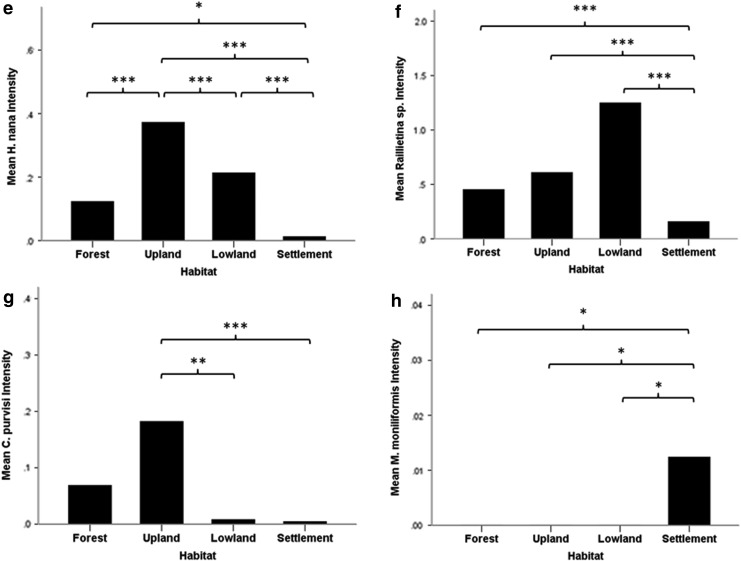
Total zoonotic helminth intensity varied significantly among the four categorized habitats (Kruskal–Wallis test=48.42, p<0.001) (Fig. 2). In addition, most of the species showed significant differences between habitats, such as E. ilocanum (Kruskal–Wallis test=15.32, p=0.002), P. muris (Kruskal–Wallis test=11.18, p=0.011), H. diminuta (Kruskal–Wallis test=60.21, p<0.001), H. nana (Kruskal–Wallis test=66.15, p<0.001), C. purvisi (Kruskal–Wallis test=14.61, p=0.002), and M. moniliformis (Kruskal–Wallis test=9.43, p=0.024), whereas this was not the case for E. malayanum (Kruskal–Wallis test=2.14, p=0.54) (Fig. 3).
FIG. 2.
Analysis of differences in total zoonotic helminth intensity among each habitat with multiple pairwise comparisons after Kruskal–Wallis test. (*) p<0.05, (**) p<0.01, (***) p<0.001.
FIG. 3.
Analysis of differences in zoonotic helminth intensity: (a) Echinostoma malayanum, (b) Echinostoma ilocanum, (c) Plagiorchis muris, (d) Hymenolepis diminuta, (e) Hymenolepis nana, (f) Raillietina spp., (g) Cyclodontostomum purvisi, and (h) Moniliformis moniliformis) among each habitat with multiple pairwise comparisons after Kruskal–Wallis test. (*) p<0.05, (**) p<0.01, (***) p<0.001.
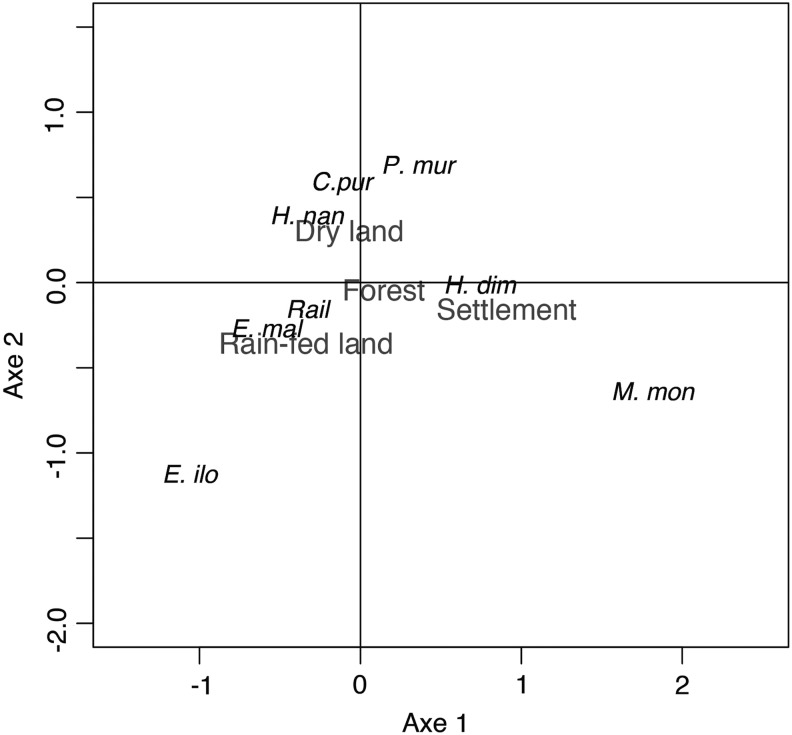
The PCA plot (Fig. 4) shows the associations between the zoonotic helminth species and habitat types, where the first and second dimensions explain 80% of the total variance. Several helminth species seemed to have specialized habitat preferences: M. monoliformis preferred settlements; E. ilocanum and E. malayanum tended to be found in rain-fed fields; and P. muris, C. purvisi, and H. nana had a predilection for nonflooded (or dry) lands. Raillietina spp. and H. diminuta appeared to be more general in habitat preference, as they were found in forests and rain-fed lands, and forest-settlement habitats, respectively.
FIG. 4.
Principal component analysis of helminth species association with the categorized habitats (E. mal, Echinostoma malayanum; E. ilo, Echinostoma ilocanum; P. mur, Plagiorchis muris; H. dim, Hymenolepis diminuta; H. nan, Hymenolepis nana; Rail, Raillietina spp.; C. pur, Cyclodontostomum purvisi; M. mon, Moniliformis moniliformis).
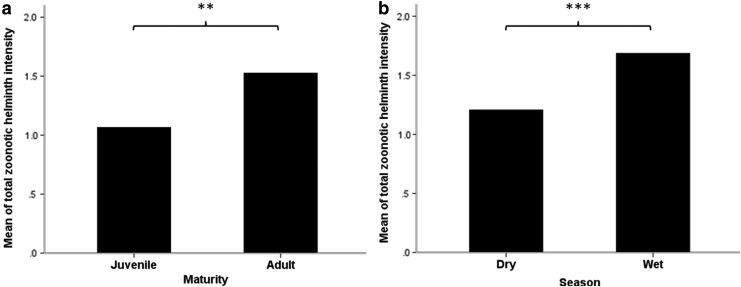
There was no difference in total zoonotic helminth intensity between male and female rodents (Mann–Whitney test=−0.45, p=0.65), but significant differences of individual helminth intensity were found between rodent genders. Female rodents harbored significantly higher intensity in E. malayanum (Mann–Whitney test=2.079, p=0.038), E. ilocanum (Mann–Whitney test=2.278, p=0.023), and C. purvisi (Mann–Whitney test=−2.747, p=0.006), whereas no significantly higher helminth intensity was found in male rodents. Total zoonotic helminth intensity in adult rodents was significantly higher than in juveniles (Mann–Whitney test=−3.13, p=0.002). Also, the seasonal comparison showed that rodents in the wet season harbored significantly higher total zoonotic helminth intensities than the rodents from the dry season (Mann–Whitney test=−4.31, p<0.001).
Discussion
Main rodent-borne zoonotic helminths and their reservoirs in the Indo-Chinese Peninsula
E. malayanum
This fluke is found in small intestine of various animals including humans, rodents, dogs, pigs, and birds (Belizario et al. 2007, Chai et al. 2009). To complete its life cycle, fresh water snails (e.g., Lymnaea, Indoplanorbis, etc.) and a number of aquatic organisms such as other snails, tadpoles, frogs, and fish act as, respectively, first and second intermediate hosts (Sornmani 1969, Rim 1982). Humans become infected by ingestion of encysted metacercaria in the second intermediate hosts, which develop into the adult stage producing ulceration to the intestinal mucosa. In cases of heavy infection, necrosis and infiltration of the mucosa potentially occur, leading to diarrhea (Graczyk and Fried 1998). Previously, E. malayanum has been reported in Rattus argentiventer, R. exulans, R. jalorensis, R. mindanensis mindanensis, R. norvegicus, R. rattus, and R. rattus diardii (Joe 1963, Betterton and Lim 1975, Sinniah 1979, Monzon and Kitikoon 1989, Namue and Wongsawad 1997, Salcedo and Glenn 2006). In the present study, three rodent species: B. savilei from Thailand (Loei), R. tanezumi from Cambodia (Mondolkiri), and R. sakaretensis from Lao PDR (Pakse) were found to be infected and potentially may act as a reservoir of E. malayanum infection in humans.
E. ilocanum
Similar to E. malayanum, E. ilocanum is an intestinal parasitic fluke in mammals and birds. The worm has an indirect life cycle with aquatic snails as intermediate hosts: Gyraulus and Hippeutis are first intermediate hosts, whereas Pila, Viviparous, Thiara, Planorbis, and Lymnaea act as second intermediate hosts. Humans have been reported as an accidental host by ingestion of metacercaria-infected snails. However, the incidence of E. ilocanum infection in humans is quite low, which may be related to the fact that the second intermediate hosts are not consumed by humans (Sirivichayakul et al. 2006). In the present study, E. ilocanum was found only in the small intestine of B. indica from Thailand (Kalasin), although, R. mindanensis mindanensis, R. rattus, and R. norvegicus have been reported as the reservoir of the fluke in Southeast Asia (Cross and Basaca 1986, Namue and Wongsawad 1997, Salcedo and Glenn 2006).
P. muris
This small trematode is found in the small intestine of rats, dogs, and birds. Lymnaeid snails (Stagnicola) were reported as first intermediate hosts, whereas insect larvae and some fresh water fishes are potential second intermediate hosts (Hong et al. 1996, Waikagul and Thairungroj 1997). This fluke has been reported to be capable of infection in humans. In 1937, there was a report of experimental infection in humans by oral ingestion of metacercariae, the infective stage of P. muris, which was isolated from a freshwater snail. Consequently, eggs of the worm were detected in feces on day 9 after infection (McMullen 1937). Thereafter, some human cases were sporadically found in Japan and Korea (Asada et al. 1962, Hong et al. 1996). However, the symptomatology is not well defined, and human cases are yet to be reported from SEA. The present study showed that M. caroli, M. cookii, and R. tanezumi were infected by P. muris. In addition, this trematode was also found in R. rattus from the Philippines (Jueco and Zabala 1990) and Rattus sladeni from Vietnam (Nguyen 1991).
H. diminuta
This cestode is known as the “rat tapeworm” and is commonly found in the small intestine of rats and dogs (Asada 1923, Sirivichayakul et al. 2006). There have been several case reports of infection in humans worldwide (McMillan et al. 1971, Chitchang et al. 1978, Cohen 1989, Marangi et al. 2003, Rohela et al. 2012). Various species of arthropods, including fleas, beetles, caterpillars, and millipedes, were identified as the intermediate hosts (Heicher and Gallati 1978, Andreassen et al. 1999). Humans are infected by accidentally eating the cysticercoid-infected intermediate hosts found in food or, in the case of fleas, on the body. However, this parasite does not generally cause severe symptoms. Indeed, mild to moderate infection may cause no symptoms, whereas heavy infection can be accompanied by dizziness, anorexia, abdominal distress, and diarrhea (Markell et al. 1992, Waikagul and Thairungroj 1997). A number of murid rodent species have been reported as H. diminuta reservoirs in Southeast Asia: Bandicota bengalensis, B. indica, B. savilei, Berylmys berdmorei, Berylmys bowersi, Leopoldamys edwardsi, Leopoldamys sabanus, Maxomys rajah, Maxomys surifer, M. caroli, M. cookii, Niviventer fulvescens, and the rat species Rattus annandalei, R. andamanensis, R. argentiventer, R. diardii, R. exulans, R. losea, R. molliculus, R. nitidus, R. norvegicus, R. rattus, R. tanezumi, and R. tiomanicus (Betterton 1979, Leong et al. 1979, Sinniah 1979, Krishnasamy et al. 1980, Chenchittikul et al. 1983, Roberts 1991; Pham et al. 2001, Syed-Arnez and Mohd Zain 2006, Chaisiri et al. 2012, Pakdeenarong et al. 2014).
H. nana
The cestode H. nana is an intestinal parasite of rats, mice, and other rodents. It also known as the “dwarf tapeworm,” because it has the smallest adult stage of any cestode found in humans (Sedaf et al. 2013). The distribution of human cases is worldwide, but with a high prevalence in warm climates with poor sanitation rather than in colder climatic zones. It is particularly common in children (Voge and Heyneman 1957). Clinically, light infection with H. nana is asymptomatic, whereas heavy infection can produce mild symptoms such as weakness, anal pruritus, abdominal pain, and diarrhea (Sirivichayakul et al. 2000, Chero et al. 2007). This worm produces autoinfection and can directly complete its life cycle without the aid of any intermediate host. However, several species of fleas and beetles are known as facultative intermediate hosts in which the infective cysticercoid stage develops inside the arthropods awaiting ingestion by the definitive host. Humans become infected by direct contamination, by eggs, and less commonly by accidental ingestion of an infected beetle or flea. A high number of rodent species have been reported as reservoirs of this cestode: Hapalomys delacouri, M. surifer, M. caroli, M. cervicolor, M. cookii, N. fulvescens, R. andamanensis, R. annandalei, R. exulans, R. norvegicus, R. diardii, R. losea, R. tanezumi, and R. tiomanicus (Sinniah 1978, Krishnasamy et al. 1980, Chenchittikul et al. 1983, Roberts 1991, Chaisiri et al. 2012, Pakdeenarong et al. 2014), demonstrating that H. nana is very successful at parasitizing a wide range of hosts.
Raillietina spp
This medium-sized tapeworm parasitizes mostly birds and sometimes humans. The parasite requires two intermediate insect hosts, such as beetles, ants, and cockroaches, to complete its life cycle. In Asia, there have been reports of human cases in Indian Ocean countries, China, Japan, and Thailand (Chandler and Pradatsundarasar 1957, Areekul and Radomyos 1970, Beaver et al. 1984). Humans are occasionally infected by ingesting cysticercoid-contaminated intermediate hosts. No symptoms have been recorded to date in humans infected by this cestode. Similar to H. diminuta and H. nana, several rodent species were reported as reservoirs of Raillietina spp. in Southeast Asia: B. indica, B. savilei, B. berdmorei, B. bowersi, L. edwardsi, L. sabanus, M. surifer, M. caroli, M. cervicolor, M. cookii, Niviventer cremoriventer, N. fulvescens, R. losea, R. exulans, R. norvegicus, R. rattus, and R. tanezumi (Areekul and Radomyos 1970, Betterton 1979, Namue and Wongsawad 1997, Chaisiri et al. 2012, Pakdeenarong et al. 2014).
C. purvisi
This nematode is known as the rat hookworm and is commonly found in the cecum of rats from Southeast Asia. This roundworm has a direct life cycle, similar to other hookworm species. The infective form is the third-stage larva that develops in the environment 4–5 days after hatching (Varughese 1973). Humans can be infected by consumption of improperly washed vegetables contaminated by the infective larvae (Sirivichayakul et al. 2006). However, there is only a single human case report (from a Thai man in Saraburi province, the central part of Thailand), and the parasite did not cause any symptoms in this patient (Bhaibulaya and Indra-ngarm 1975). Among rodents, several rat species can harbor C. purvisi infection: B. indica, B. bowersi, L. sabanus, M.s surifer, M. rajah, Maxomys whiteheadi, N. cremoniventer, R. annandalei, R. argentiventer, R. bartelsii, R. hoffmanni, R. molliculus, R.mulleri, R. nitidus, R. sladeni, R. diardii, and R. tiomanicus (Singh and Chee-Hock 1971, Varughese 1973, Wiroreno 1978, Sinniah 1979, Hasegawa and Syafruddin 1994, Chaisiri et al. 2012).
M. moniliformis
This acanthocephalan worm is found worldwide, usually infecting rodents, dogs, foxes, and cats as the definitive hosts. The parasite was clearly found in urban rodent species: Rattus diardii, R. exulans, R. norvegicus, R. rattus, and R. tanezumi (Leong et al. 1979, Chaisiri et al. 2012, Mohd Zain et al. 2012). Nevertheless, rodents from agricultural or periurban areas (B. indica, R. annandalei, R. argentiventer, and R. tiomanicus) were also reported to be infected sporadically (Sinniah 1979). Similar to Macracanthorhynchus hirudinaceus, M. moniliformis is another acanthocephalan that has been reported in humans from the Middle East, Australia, Africa, and America (Moayedi et al. 1971, Al-Rawas et al. 1977, Counselman et al. 1989, Ikeh et al. 1992, Bettiol and Goldsmid 2000, Berenji et al. 2007, Salehabadi et al. 2008), whereas the incidence in Southeast Asia is still unknown. Beetles and cockroaches are recognized as intermediate hosts harboring the infective-stage larvae, the cystacanth. The definitive hosts, including humans, can become infected by consuming cystacanths. Although most infected patients are asymptomatic, some reported symptoms include abdominal pain, dizziness, giddiness, and edema (Berenji et al. 2007, Salehabadi et al. 2008). Although humans infected with M. moniliformis have not been reported in Southeast Asia, there are several other reports of this acanthocephalan infection in rodents from the region, reflecting an important zoonotic risk.
The influence of habitat on the intensity of the zoonotic helminths
Due to the present findings, habitat acts as one of the factors that influence helminth infection in murid rodent hosts. In term of total zoonotic helminth intensity, rodents from human settlement habitat harbored significantly lower than the other habitat types (Fig. 2). This probably could be the effect of urbanization, which reduces biodiversity and parasite survival consequently; the more biodiversity is decreased, the more reduction there is in either the intermediate or definitive host for parasite. In addition, we also found that most of the zoonotic helminth species in the present study showed significant variation in intensity between different habitats (Fig. 3).
Rodents from dry land or nonflooded agricultural areas (e.g., corn fields, cassava fields, orchards, grassland, or fallow) harbored a significantly higher intensity of P. muris, H. nana, and C. purvisi infections than the other habitats (Fig. 3c, e, g). In comparison with the other habitats, insects of agricultural importance might play a role as intermediate hosts for those parasites to complete their life cycle.
Lowlands or irrigated rice fields were the preferred habitat for the two echinostomatid flukes E. malayanum and E. ilocanum, showing significantly higher intensity than in the other habitats (Fig. 3a, b). This could be related to the biology of the gastropod intermediate hosts, which are commonly found in wetlands and rice fields (Lie et al. 1966, Ngoenklan et al. 2010).
Rodents from settlements, cities, or villages showed significantly lower intensity in various helminth species (e.g., H. nana, Raillietina spp., and C. purvisi) than rodents from the other habitats (Fig. 3e, f, g). This may also be related to the low diversity of intermediate hosts in the urban/domestic area. Thus, people living in large villages or cities apparently face a lower risk of these helminth infections. Nevertheless, in the current study or our previous survey (Chaisiri et al. 2012), the acanthocephalan M. moniliformis was clearly found in urban or city rodents (Fig. 3h). These findings suggest that the life cycle of M. moniliformis is maintained by beetle or cockroach intermediate hosts living in domestic areas. Thus, rats in urban habitats appear to be an important reservoir of M. moniliformis infections for humans.
The influence of age, sex, and season on the intensity of zoonotic helminths
A number of researchers have reported the effect of host genders on the parasitic loads (Behnke et al. 2001, Kataranovski et al. 2011). Several publications have reported the trend of male bias in parasite infections, especially mammals (Poulin 1996, Zuk and McKean 1996, Klein 2004), whereas others have revealed the contradiction, with females having higher susceptibility (Behnke et al. 2008, Mohd Zain et al. 2012). There were some factors regarding sex bias in susceptibility to parasitic infection as linked to sex-differences in the production of sexual hormones that affect directly or indirectly to host's immunological response (Foldstad and Karter 1992, Poulin 1996); size difference, i.e, the larger sex (normally male) the more energy investment that reduces efficacy of immune function and tends to be more exposed or attractive for parasites (Moore and Wilson 2002); and sex-specific behavior inducing different exposure, such as a larger home range searching for food or mating that might lead to enhancing exposure to parasites (Tinsley 1989). It is definitely unclear to conclude in a global/broad-scale study that one gender is more susceptible for parasitic infection than another; a smaller-scale study should be better able to emphasize this research assumption. Because of the results from the present study, we found no difference in terms of total helminth intensity between male and female rodents. However, there were significant differences of individual helminth intensity between rodent genders. Female rodents harbored significantly higher intensities in E. malayanum, E. ilocanum, and C. purvisi, whereas no significantly higher helminth intensity was found in male rodents.
In terms of host maturity, total zoonotic helminth intensity was clearly higher in adult rodents than juveniles (Fig. 5a). This may be explained by the assumption that older hosts have a longer time to expose and accumulate the parasite infections throughout their life (Rossin et al. 2009, Kataranovski et al. 2011). Besides, adult rodents explore a larger area for foraging and breeding purposes and thus are potentially more in contact with infective stages of parasites.
FIG. 5.
Differences in total zoonotic helminth intensity with rodent maturity (a) and season (b) after comparison by a Mann–Whitney U-test. (*) p<0.05, (**) p<0.01, (***) p<0.001.
Again, seasonal differences were observed for total zoonotic helminth intensity. Rodents captured during the wet season were infected with a higher number of zoonotic helminths than the rodents from the dry season (Fig. 5b). High humidity and moisture in the wet season promote a good rate of development and survival of parasite eggs and larvae in environment (O'Connor et al. 2007), inferring that infective stages of the parasites are likely to be more abundant and highly active than during the dryer period. Accordingly, we conclude that the wet season is potentially the risky period for humans to contract those zoonotic helminthiases.
The major rodent reservoir of the zoonotic helminths
Compared to the others rodent species in the present study, the Asian house rat R. tanezumi harbors the highest zoonotic helminth species richness (six out of eight). This rat is also the most captured by the project, reflecting a high density of this rodent species in this region. Unlike the others, R. tanezumi is the only species that is found ubiquitously in a large habitat range, from urban area to various wild or agricultural landscapes (Bordes et al. 2013). This rodent can act as a potential mechanical vector carrying the parasites across the different habitat types and spreading them into new environment. Due to the reasons given above, R. tanezumi is the main reservoir of rodent-borne zoonotic helminthiases in the Indochinese Peninsula.
Conclusions
A number of rodent-borne helminthiases were found in rodents from the Indochinese Peninsula. Most of these showed significant variation in intensity between different habitats. Rodents captured in the wet season harbored a significantly higher zoonotic helminth intensity than those trapped in the dry season, suggesting that the wet season represents a period of greater zoonotic risk. The Oriental house rat R. tanezumi is the most important reservoir of the zoonotic helminthiases and might carry and spread the parasites between habitats. Humans can be infected accidentally by ingestion of infective stage larvae contaminating vegetables and other foods or the environment. Finally, understanding the route of transmission, potentially riskier habitats, as well as good hygienic awareness will help in prevention of zoonotic helminthiases.
Acknowledgments
This study was supported by the CERoPath project (Community Ecology of Rodents and Their Pathogens in a Changing Environment, the French ANR Biodiversity grant ANR 07 BDIV 012) and the BiodivHealthSEA project (Local impacts and perceptions of global changes: Health, biodiversity and zoonoses in Southeast Asia, the French ANR CP&ES grant ANR 11 CPEL 002). We gratefully acknowledge the student teams from the Faculty of Veterinary Technology, Kasetsart University, and the Faculty of Science, Mahasarakham University, Thailand, for their valuable help on gut dissection and helminth isolation. In addition, we thank Dr. Ben Makepeace (IGH, University of Liverpool) for English editing and valuable comments improving our manuscript. Finally, we sincerely thank all friends, colleagues, as well as local collaborators for their cooperation, resulting in great success in the field.
Author Disclosure Statement
No competing financial interests exist.
References
- Al-Rawas AY, Mirza MY, Shafig A, Al-Kindy L. First finding of Moniliformis moniliformis (Bremser 1811) Travassos 1915 (Acanthocephala: Oligacanthorhynchidae) in Iraq from human child. J Parasitol 1977; 63:396–397 [PubMed] [Google Scholar]
- Alvarez-Fernandez BE, Rodrfguez-Bataz E, Dfaz-Chiguer DL, Marquez-Navarro A., et al. . Mixed Hymenolepis species infection in two family members: A case report from an urban area of Chilpancingo, Guerrero, México. Trop Gastroenterol 2012; 33:83–84 [PubMed] [Google Scholar]
- Anderson RC. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed. New York; CABI Publishing, 2000 [Google Scholar]
- Andreassen J, Bennet-Jenkins EM, Bryant C. Immunology and biochemistry of Hymenolepis diminuta. Adv Parasitol 1999; 42:223–275 [DOI] [PubMed] [Google Scholar]
- Aplin KP, Brown PR, Jacob J, Krebs C, et al. . Field Methods for Rodent Studies in Asia and the Indo-Pacific. Canberra, Australia; ACIAR Monograph No. 100, 2003 [Google Scholar]
- Areekul S, Radomyos P. Preliminary report of Raillietina sp. infection in man and rats in Thailand. Southeast Asian J Trop Med Public Health 1970; 1:559 [Google Scholar]
- Asada J. Studies on the Hymenolepis diminuta Rudolph, which finds the host in a dog. Okayama Igakki Zasshi 1923; 407:876–880 [Google Scholar]
- Asada J, Otagaki H, Morita M, Takeuchi T, Sakai Y, Konoshi T, Okahashi K. A case report on the human infection with Plagiorchis muris Tanabe, 1922 in Japan. Jpn J Parasitol 1962; 11:512–516 [Google Scholar]
- Beaver PC, Jung RC, Cupp EW. Clinical Parasitology, 9th ed. Philadelphia, PA: Lea and Febiger, 1984 [Google Scholar]
- Behnke JM, Barnard CJ, Bajer A, Bray D, et al. . Variation in the helminth community structure in bank voles (Clethrionomys glareolus) from three comparable localities in the Mazury Lake District region of Poland. Parasitol 2001; 123:401–414 [DOI] [PubMed] [Google Scholar]
- Behnke JM, Bajer A, Harris PD, Newington L, et al. . Temporal and between-site variation in helminth communities of bank voles (Myodes glareolus) from N.E. Poland 2 The infracommunity level. Parasitology 2008; 135:999–1018 [DOI] [PubMed] [Google Scholar]
- Belizario V, Geronilla G, Marilyn-Benedith AM, Winifreda U, et al. . Echinostoma malayanum infection, the Philippines. Emerg Infect Dis 2007; 13:1130–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Musa NA. Intestinal parasites in school aged children and the first case report on amoebiasis in urinary bladder in Tripoli, Libya. J Egypt Soc Parasitol 2007; 37:775–784 [PubMed] [Google Scholar]
- Berenji F, Fata A, Hosseininejad ZA. Case of Moniliformis Moniliformis (Acanthocephala) infection in Iran. Korean J Parasitol 2007; 45:145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betterton C. The intestinal helminths of small mammals in the Malaysian tropical rain forest: Patterns of parasitism with respect to host ecology. Int J Parasitol 1979; 9:313–320 [Google Scholar]
- Betterton C, Lim BL. Digenetic trematodes from rats, squirrels and tree shrews in Malaysia. Southeast Asian J Trop Med Public Health 1975; 3:343–358 [PubMed] [Google Scholar]
- Bettiol S, Goldsmid JM. A case of probable imported Moniliformis moniliformis infection in Tasmania. J Travel Med 2000; 7:336–337 [DOI] [PubMed] [Google Scholar]
- Bhaibulaya M, Indra-ngarm S. Man, an accidental host of Cyclodontostomum purvisi (Adam, 1933) and the occurrence in rat in Thailand. Southeast Asian J Trop Med Public Health 1975; 6:391–393 [PubMed] [Google Scholar]
- Bhaibulaya M, Charoenlarp P, Harinasuta C. Report of cases of Echinostoma malayanum and Hypoderaeum conoideum in Thailand. J Med Assoc Thai 1964; 47:720–731 [Google Scholar]
- Bordes F, Herbreteau V, Dupuy S, Chaval Y, et al. . The diversity of microparasites of rodents: A comparative analysis that helps in identifying rodent-borne rich habitats in Southeast Asia. IEE 2013; 3, published online at http://dx.doi.org/10.3402/iee.v3i0.20178 [DOI] [PMC free article] [PubMed]
- Brenes-Madrigal R, Hangen G, Monge E, Mu G, et al. . Primer caso humano de parasitosis por Raillietina sp. en Costa Rica. Revista Medica de Costa Rica 1983; 4:81–87 [Google Scholar]
- Calvo FR. Incidence of Hymenolepis diminuta in human parasitic infections in Cuba. Revista Kuba de Medicina Tropical y Parasitologia 1951; 7:67–68 [PubMed] [Google Scholar]
- Carney WP, Sudomo M, Purnomo Echinostomiasis: A disease that disappeared. Trop Geogr Med 1980; 32:106–111 [PubMed] [Google Scholar]
- Castex MR, Wanke L, Camponovo LE, Rechniewski C. New Argentine case of human infection with Hymenolepis diminuta. La Prensa Medica Argentina 1951; 38:1415–1418 [PubMed] [Google Scholar]
- Chai J, Shin E, Lee S, Rim H. Foodborne intestinal flukes in Southeast Asia. Korean J Parasitol 2009; 47:S69–S102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J, Sohn WM, Yong TS, Eom KS, et al. . Echinostome flukes recovered from human in Khammouane Province, Lao PDR. Korean J Parasitol 2012; 50:269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisiri K, Chaeychomsri W, Siruntawineti J, Ribas A, et al. . Diversity of gastrointestinal helminths among murid rodents from northern and northeastern Thailand. Southeast Asian J Trop Med Public Health 2012; 43:21–28 [PubMed] [Google Scholar]
- Chandler AC, Pradatsundarasar A. Two cases of Raillietina infection in infants in Thailand, with a discussion of the taxonomy of Raillietina (Cestoda) in man, rodents and monkeys. J Parasitol 1957; 43:81–89 [PubMed] [Google Scholar]
- Cheah JS, Kan SP. A case of Hymenolepis nana infestation in Singapore responding to niclosamide. J Trop Med Hygiene 1971; 74:272–273 [PubMed] [Google Scholar]
- Chenchittikul M, Daengpium S, Hasegawa M, Itoh T, et al. . A study of commensal rodents and shrews with reference to the parasites of medical importance in Chanthaburi Province, Thailand. Southeast Asian J Trop Med Public Health 1983; 14:255–259 [PubMed] [Google Scholar]
- Chero JC, Saito M, Bustos JA, Blanco EM, et al. . Hymenolepis nana infection: Symptoms and response to nitazoxanide in field conditions. Trans Royal Soc Trop Med Hygiene 2007; 101:203–205 [DOI] [PubMed] [Google Scholar]
- Chitchang S, Sooksala N, Radomyos P. A case report of Hymenolepis diminuta in Bangkok, Thiland. Southeast Asian J Trop Med Public Health 1978; 9:534–535 [PubMed] [Google Scholar]
- Chhakda T, Muth S, Socheat D, Odermatt P. Intestinal parasites in school-aged children in villages bordering Tonle Sap Lake, Cambodia. Southeast Asian J Trop Med Public Health 2006; 37:859–864 [PubMed] [Google Scholar]
- Chung WC, Fan PC, Chiu HM. Survey of helminthic infections and treatment of Taenia species infection among the aborigines in Chien-Shih District, Hsin-Chu County, northern Taiwan. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 1985;18:96–103 [PubMed] [Google Scholar]
- Cohen IP. A case report of Hymenolepis diminuta in a child in St. James Parish, Jamaica. J La State Med Soc 1989; 141:23–24 [PubMed] [Google Scholar]
- Cordova PSO, Vargas VF, Gonzalez VA, Peréz CG, et al. . Intestinal parasitism in Peruvian children and molecular characterization of Cryptosporidium species. Parasitol Res 2006; 98:576–581 [DOI] [PubMed] [Google Scholar]
- Counselman K, Field C, Lea G, Nickol B, et al. . Moniliformis moniliformis from a child in Florida. Am J Trop Med Hygiene 1989; 41:88–90 [PubMed] [Google Scholar]
- Cross JH, Basaca-Sevilla V. Studies on Echinostoma ilocanum in the Philippines. Southeast Asian J Trop Med Public Health 1986; 17:23–27 [PubMed] [Google Scholar]
- Cross JH, Clarke MD, Cole WC, Lien JC, et al. . Parasitic infections in humans in West Kalimantan (Borneo), Indonesia. Trop Geogr Med 1976; 28:121–130 [PubMed] [Google Scholar]
- Cutting JW. A survey of intestinal parasitism in a community on the Pan American Highway route in eastern Panama. Rev. Panam. Salud Publica 1975; 9:13–18 [PubMed] [Google Scholar]
- Delobel P, Signate A, El-Guedj M, Couppie P, et al. . Unusual form of neurocysticercosis associated with HIV infection. Euro J Neurol 2004; 11:55–58 [DOI] [PubMed] [Google Scholar]
- El-Shazly AM, Awad SE, Sultan DM, Sadek GS, et al. . Intestinal parasites in Dakahlia governorate, with different techniques in diagnosing protozoa. J Egypt Soc Parasitol 2006; 36:1023–1034 [PubMed] [Google Scholar]
- El-Sheikh SM, El-Assouli SM. Prevalence of viral, bacterial and parasitic enteropathogens among young children with acute diarrhoea in Jeddah, Saudi Arabia. J Health Popul Nutr 2001; 19:25–30 [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males and the immunocompetence handicap. Am Naturalist 1992; 139:603–622 [Google Scholar]
- Ghadirian E, Arfaa F. Human infection with Hymenolepis diminuta in villages of Minab, southern Iran. Int J Parasitol 1972; 2:481–482 [DOI] [PubMed] [Google Scholar]
- Goldsmid JM. A note on the occurrence of Hymenolepis diminuta (Rudolphi, 1819) Blanchard, 1891 (Cestoda) in Rhodesia. Cent Afr J Med 1973; 19:51–52 [PubMed] [Google Scholar]
- Graczyk TK, Fried B. Echinostomiasis: A common but forgotten food-borne disease. Am J Trop Med Hygiene 1998; 58:501. [DOI] [PubMed] [Google Scholar]
- Hadidjaja P, Oemijati S. Echinostoma infection in Indonesia with a special study on Echinostoma malayanum. Proceedings of the Fourth Southeast Asian Seminar on Parasitology and Tropical Medicine, Schistosomiasis and other snail-transmitted helminthiasis, Manila, 1969:167–170 [Google Scholar]
- Hasegawa H, Syafruddin Cyclodontostomum purvisi (syn. Ancistronema coronatum) (Nematoda: Strongyloidea: Chabertiidae) from rats of Kalimantan and Sulawesi, Indonesia. J Parasitol 1994; 80:657–660 [PubMed] [Google Scholar]
- Herbreteau V, Jittapalapong S, Rerkamnuaychoke W, Chaval Y, et al. . Protocols for field and laboratory rodent studies. Bangkok, Thailand: Kasetsart University Press, 2011 [Google Scholar]
- Herbreteau V, Bordes F, Jittapalapong S, Supputamongkol Y, et al. . Rodent-borne diseases in Thailand: targeting rodent carriers and risky habitats. IEE 2012; 2, published online at http://dx.doi.org/10.3402/iee.v2i0.18637 [DOI] [PMC free article] [PubMed]
- Heicher DS, Gallati WW. Three new hosts for the cysticercoid of Hymenolepis diminuta. Ohio J Sci 1978; 78:149–151 [Google Scholar]
- Hilarrio JS, Wharton LD. Echinostoma ilocanum (Garrison): A report of five cases and a contribution to the anatomy of the fluke. Phil J Sci 1917; 12:203 [Google Scholar]
- Hong SJ, Woo HC, Chai JY. A human case of Plagiorchis muris (Tanabe, 1922: Digenea) infection in the Republic of Korea: Freshwater fish as a possible source of infection. J Parasitol 1996; 82:647–649 [PubMed] [Google Scholar]
- Ikeh EI, Anosike JC, Okon E. Acanthocephalan infection in man in northern Nigeria. J Helminthol 1992; 66:241–242 [DOI] [PubMed] [Google Scholar]
- Jacobsen KH, Ribeiro PS, Quist BK, Rydbeck BV. Prevalence of intestinal parasites in young Quichua children in the highlands of rural Ecuador. J Health Popul Nutr 2007; 25:399–405 [PMC free article] [PubMed] [Google Scholar]
- Joe LK. Studies on Echinostomatidae in Malaya. IV. The animal hosts of Echinostoma malayanum Leiper, 1911 (Trematoda). Z F Parasitenkunde 1963; 23:136–140 [DOI] [PubMed] [Google Scholar]
- Jongsuksantigul P, Chaeychomsri W, Techamontrikul P, Jeradit P, et al. . Study on prevalence and intensity of intestinal helminthiases and opisthorchiasis. J Trop Med Parasitol 1992;15:80–95 [Google Scholar]
- Jueco NL, Zabala ZR. The cestodes and trematodes of Rattus norvegicus and Rattus rattus mindanensis. Phil J Vet Med 1990; 27:47–51 [Google Scholar]
- Kaminsky RG. Parasitism and diarrhoea in children from two rural communities and marginal barrio in Honduras. Trans Royal Soc Trop Med Hygiene 1991; 85:70–73 [DOI] [PubMed] [Google Scholar]
- Kan SK, Kok RT, Marto S, Thomas I, et al. . The first report of Hymenolepis diminuta infection in Sabah, Malaysia. Trans Royal Soc Trop Med Hygiene 1981; 75:609. [DOI] [PubMed] [Google Scholar]
- Kataranovski M, Mirkov I, Belij S, Popov Z, et al. . Intestinal helminths infection of rats (Ratus norvegicus) in the Belgrade area (Serbia): The effect of sex, age and habitat. Parasite 2011; 18:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Micro Rev 2004; 17:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol 2004; 26:247–264 [DOI] [PubMed] [Google Scholar]
- Krishnasamy M, Singh KI, Ambu S, Ramachandran P. Seasonal prevalence of the helminth fauna of the wood rat Rattus tiomanicus (Miller) in West Malaysia. Folia Parasitologica 1980; 27:231–235 [PubMed] [Google Scholar]
- Kunwar CB, Subba B, Shrestha M, Chapagain RH, et al. . A human case of Hymenolepis diminuta infection in Nepal. JIOM-Nepal 2005; 27:66–67 [Google Scholar]
- Le Hung Q, de Vries PJ, Giao PT, Binh TQ, et al. . Intestinal helminth infection in an ethnic minority commune in southern Vietnam. Southeast Asian J Trop Med Public Health 2005; 36:623–628 [PubMed] [Google Scholar]
- Leong TS, Lim BL, Yap LF, Krishnasamy M. Parasite fauna of the house rat Rattus rattus diardii in Kuala Lumpur and nearby villages. Southeast Asian J Trop Med Public Health 1979; 10:122–126 [PubMed] [Google Scholar]
- Levi MH, Raucher BG, Teicher E, Sheehan DJ, et al. . Hymenolepis diminuta: One of three enteric pathogens isolated from a child. Diag Micr Infec Dis 1987; 7:255–259 [DOI] [PubMed] [Google Scholar]
- Lie KJ, Viric HK. Human infection with Echinostoma malayanum Leiper, 1911, (Trematoda: Echinostomatidae). J Trop Med Hygiene 1963; 66:77–82 [PubMed] [Google Scholar]
- Lie KJ, Basch PF, Umathevy T. Studies on Echinostomatidae (Trematoda) in Malaya. XII. Antagonism between two species of Echinostome trematodes in the same Lymnaeid snail. J Parasitol 1966; 52:454–457 [PubMed] [Google Scholar]
- Maji AK, Bera DK, Manna B, Nandy A, et al. . First record of human infection with Echinostoma malayanum in India. Trans R Soc Trop Med Hygiene 1993; 87:673. [DOI] [PubMed] [Google Scholar]
- Marangi M, Zechini B, Fileti A, Quaranta G, et al. . Hymenolepis diminuta Infection in a child living in the urban area of Rome, Italy. J Clin Microbiol 2003; 41:3994–3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margono S. Cestodes in man in Indonesia. Buletin Penelitian Kesehatan 1989; 17:60–66 [Google Scholar]
- Markell EK, Voge M, John DT. Medical Parasitology, 7th ed. Philadelphia, PA: WB Saunders, 1992 [Google Scholar]
- Marshall JT. Family Muridae: Rats and mice. In: Lekagul B, Mc Neely JA, eds. Mammals of Thailand. Bangkok: Association for the Conservation of Wildlife, 1988:397–487 [Google Scholar]
- McMillan B, Kelly A, Walker JC. Prevalence of Hymenolepis diminuta infection in man in the New Guinea Highlands. Trop Geogr Med 1971; 23:390–392 [PubMed] [Google Scholar]
- McMullen DB. An experimental infection of Plagiorchis muris in man. J Parasitol 1937; 23:113–115 [Google Scholar]
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol 2009; 35:221–270 [DOI] [PubMed] [Google Scholar]
- Min DY. Cestode infections in Korea. Kisaengchunghak Chapchi 1990; 28:123–144 [DOI] [PubMed] [Google Scholar]
- Moayedi B, Izadi M, Maleki M, Ghadirian E. Human infection with Moniliformis moniliformis (Bremser, 1811) Travassos, 1915 (Syn. Moniliformis dubius) report of case in Isfahan, Iran. Am J Trop Med Hygiene 1971; 20:445–448 [DOI] [PubMed] [Google Scholar]
- Mohd Zain SN, Behnke JM, Lewis JW. Helminth communities from two urban rat populations in Kuala Lumpur, Malaysia. Parasites Vectors 2012; 5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzon RB, Kitikoon V. Lymnaea (Bullastra) cumingiana Pfeiffer (Pulmonata: Lymnaeidae): Second intermediate host of Echinostoma malayanum in the Philippines. Southeast Asian J Trop Med Public Health 1989; 20:453–460 [PubMed] [Google Scholar]
- Moore SL, Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science 2002; 297:2015–2018 [DOI] [PubMed] [Google Scholar]
- Namue C, Wongsawad C. Survey of helminth infection in rats (Rattus spp) from Chiang Mai Moat. Southeast Asian J Trop Med Public Health 1997; 28:179–183 [PubMed] [Google Scholar]
- Ngoenklan R, Piangjai S, Somwang P, Moophayak K, et al. . Emerging helminths infections in snails and cyprinoid fish in sewage treatment wetland waters in Cambodia. Asian J Water Environ Pollut 2010; 7:13–21 [Google Scholar]
- Nguyen TL. The trematode of birds and mammals in South Vietnam. Tap Chi Sinh Hoc, Vietnam; 1991:23–26 [Google Scholar]
- O'Connor LJ, Kahn LP, Walkden-Brown SW. Moisture requirements for the free living development of Haemonchus contortus: Quantitative and temporal effects under conditions of low evaporation. Vet Parasitol 2007; 150:128–138 [DOI] [PubMed] [Google Scholar]
- Owen IL. Parasitic zoonoses in Papua New Guinea. J Helminthol 2005; 79:1–14 [DOI] [PubMed] [Google Scholar]
- Pakdeenarong N, Siribat P, Chaisiri K, Douangboupha B, et al. . Helminth communities in murid rodents from southern and northern localities in Lao PDR: The role of habitat and season. J Helminthol 2014; 88:302–309 [DOI] [PubMed] [Google Scholar]
- Park SK, Kim DH, Deung YK, Kim HJ, et al. . Status of intestinal parasite infections among children in Bat Dambang, Cambodia. Korean J Parasitol 2004; 42:201–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patamia I, Cappello E, Castellano-Chiodo D, Greco F, et al. . A human case of Hymenolepis diminuta in a child from eastern Sicily. Korean J Parasitol 2010; 48:167–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul FM, Zaman V. Hymenolepis diminuta infestation in a Chinese baby. J Singapore Paediatr Soc 1969; 11:67–72 [PubMed] [Google Scholar]
- Pham XD, Tran CL, Hasegawa H. Helminths collected from Rattus spp. in Bac Ninh Province, Vietnam. Comp Parasitol 2001; 68:261–264 [Google Scholar]
- Poulin R. Helminth growth in vertebrate hosts: Does host sex matter? Int J Parasitol 1996; 26:1311–1315 [DOI] [PubMed] [Google Scholar]
- Radomyos P, Bunnag D, Harinasuta T. Echinostoma ilocanum (Garrison, 1908) Odhner, 1911, infection in man in Thailand. Southeast Asian J Trop Med Public Health 1982; 13:265–269 [PubMed] [Google Scholar]
- Radomyos P, Wongsaroj T, Wilairatana P, Radomyos P, et al. . Opisthorchiasis and intestinal fluke infections in northern Thailand. Southeast Asian J Trop Med Public Health 1998; 29:123–127 [PubMed] [Google Scholar]
- Reyes H, Inzunza E, Doren G. Incidence of human infection by Hymenolepis diminuta in Santiago de Chile, 1957–1971. Boletin chileno de parasitologia 1972; 27:29–33 [PubMed] [Google Scholar]
- Rim HJ. Echinostomiasis. CRC Handbook Series in Zoonoses. Section C: Parasitic Zoonoses (Trematode Zoonoses), vol. III Boca Raton, FL: CRC Press, 1982 [Google Scholar]
- Roberts M. The parasites of the Polynesian rat within and beyond New Zealand. Int J Parasitol 1991; 21:777–783 [DOI] [PubMed] [Google Scholar]
- Rohela M, Ngui R, Lim YAL, Kalaichelvan B, et al. . A case report of Hymenolepis diminuta infection in a Malaysian child. Trop Biomed 2012; 29:224–230 [PubMed] [Google Scholar]
- Rossin MA, Malizia AI, Timi JT, Poulin R. Parasitism underground: Determinants of helminth infections in two species of subterranean rodents (Octodontidae). Parasitology 2010; 137:1569–1575 [DOI] [PubMed] [Google Scholar]
- Rougier Y, Legros F, Durand JP, Cordoliani Y. Four cases of parasitic infection by Raillietina (R.) celebensis (Kanicki, 1902) in French Polynesia. Trans Royal Soc Trop Med Hygiene 1981; 75:121. [DOI] [PubMed] [Google Scholar]
- Rozsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. J Parasitol 2000; 86:228–232 [DOI] [PubMed] [Google Scholar]
- Salcedo LE, Glenn QL. Some zoonotic trematodes from the Philippine field rat, Rattus mindanensis mindanensis (Mearns, 1905) (Mammalia: Rodentia) in Bay, Laguna, Philippines with redescription and new records of species. Phil J Vet Med 2006; 43:33–45 [Google Scholar]
- Salehabadi A, Mowlavi G, Sadjjadi SM. Human infection with Moniliformis moniliformis (Bremser 1811) (Travassos 1915) in Iran: Another case report after three decades. Vector Borne Zoonotic Dis 2008; 8:101–103 [DOI] [PubMed] [Google Scholar]
- Schmidt GD. Handbook of Tapeworm Identification. Boca Raton, FL: CRC Press Inc., 1986 [Google Scholar]
- Sedaf HS, Khan SS, Kanwal N, Tasawer BM, et al. . A review on diarrhoea causing Hymenolepis nana, Dwarf tapeworm. Int Res J Pharmacy 2013; 4:32–35 [Google Scholar]
- Sherchand JB, Larsson S, Shrestha MP. Intestinal parasites in children and adults with and without abdominal discomfort from the Kathmandu area of Nepal. Trop Gastroenterol 1996; 17:15–22 [PubMed] [Google Scholar]
- Singh M, Chee-Hock C. On a collection of nematode parasites from Malayan rats. Southeast Asian J Trop Med Public Health 1971; 2:516–522 [PubMed] [Google Scholar]
- Sinniah B. Hymenolepis diminuta infection in a Malaysian oil palm estate worker first case from Malaysia. Southeast Asian J Trop Med Public Health 1978; 9:453–454 [PubMed] [Google Scholar]
- Sinniah B. Parasites of Some Rodents in Malaysia. Southeast Asian J Trop Med Public Health 1979; 10:115–121 [PubMed] [Google Scholar]
- Sirivichayakul C, Radomyos P, Praevanit R, Pojaroen AC, et al. . Hymenolepis nana infection in Thai children. J Med Assoc Thai 2000; 83:1035–1038 [PubMed] [Google Scholar]
- Sirivichayakul C, Looareesuwan S, Radomyos P. Textbook of Clinical Parasitology, 2nd ed. Bangkok: Medical Media Press, 2006 [Google Scholar]
- Sithithaworn P, Sukavat K, Vannachone B, Sophonphong K, et al. . Epidemiology of food-borne trematodes and other parasite infections in a fishing community on the Nam Ngum reservoir, Lao PDR. Southeast Asian J Trop Med Public Health 2006; 37:1083–1090 [PubMed] [Google Scholar]
- Sohn WM, Kim HJ, Yong TS, Eom KS, et al. . Echinostoma ilocanum infection in Oddar Meanchey Province, Cambodia. Korean J Parasitol 2011; 49:187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornmani S. Echinostomiasis: A review. Proceedings of the Fourth Southeast Asian Seminar on Parasitology and Tropical Medicine, Schistosomiasis and Other Snail-Transmitted Helminthiases, Manila, 1969:171–175 [Google Scholar]
- Spratt DM. Australian ecosystems, capricious food chains and parasitic consequences for people. Int J Parasitol 2005; 35:717. [DOI] [PubMed] [Google Scholar]
- Stafford EE, Sudomo M, Masri S, Brown RJ. Human parasitoses in Bali, Indonesia. Southeast Asian J Trop Med Public Health 1980; 11:319–323 [PubMed] [Google Scholar]
- Syafinaz AN, Hamat RA, Malina O, Siti Norbaya M, et al. . Hymenolepis nana in a renal transplant recipient: To treat or not to treat? Med J Malaysia 2011; 66:259–260 [PubMed] [Google Scholar]
- Syed Arnez ASK, Mohd Zain SN. A Study on wild rats and their endoparasite fauna from the Endau Rompin National Park, Johor. Malaysian J Sci 2006; 25:19–39 [Google Scholar]
- Team R Core. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. Available at http://www.R-project.org [Google Scholar]
- Tena D, Pérez SM, Gimeno C, Pérez PMT, et al. . Human infection with Hymenolepis diminuta: Case report from Spain. J Clin Microbiol 1998; 36:2375–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley RC. The effects of host sex on transmission success. Parasitol Today 1989; 5:190–195 [DOI] [PubMed] [Google Scholar]
- Toma A, Miyagi I, Kamimura K, Tokuyama Y, et al. . Questionnaire survey and prevalence of intestinal helminthic infections in Barru, Sulawesi, Indonesia. Southeast Asian J Trop Med Public Health 1999; 30:68–77 [PubMed] [Google Scholar]
- Varughese G. Studies on the lifecycle and developmental morphology of Cyclodontostomum purvisi (Adam, 1933), a hookworm parasite of Malayan giant rats. Southeast Asian J Trop Med Public Health 1973; 4:78–95 [PubMed] [Google Scholar]
- Voge M, Heyneman D. Development of Hymenolepis nana and Hymenolepis diminuta (Cestoda: Hymenolepididae) in the Intermediate Host Tribolium confusum. UC Publications in Zoology 1957; 59:549–580 [Google Scholar]
- Waikagul J, Thairungroj M. Human Worms in Southeast Asia. Bangkok; Department of Helminthology, Tropical Medicine, Mahidol University, 1997 [Google Scholar]
- Waloch M. Cestode infections in Poland in 2001. Przegl Epidemiol 2003; 57:159–163 [PubMed] [Google Scholar]
- Watwe S, Dardi CK. Hymenolepis diminuta in a child from rural area. Indian J Pathol Microbiol 2008; 51:149–150 [DOI] [PubMed] [Google Scholar]
- Wiroreno W. Nematode parasites of rats in West Java, Indonesia. Southeast Asian J Trop Med Public Health 1978; 9: 520–525 [PubMed] [Google Scholar]
- Wiwanitkit V. Overview of Hymenolepis diminuta infection among Thai patients. Med Gen Med 2004; 6:7. [PMC free article] [PubMed] [Google Scholar]
- Wolday D, Mayaan S, Mariam ZG, Berhe N, et al. . Treatment of intestinal worms is associated with decreased HIV plasma viral load. J Acquir Immune Defic Syndr 2002; 31:56–62 [DOI] [PubMed] [Google Scholar]
- World Health Organization. Prevention and Control of Intestinal Parasitic Infections. Report of WHO Expert Committee, Technical Report Series, 1987:749. [PubMed]
- Yamaguti S. The Digenetic Trematodes of Vertebrates Part I: Volume I. In: Yamaguti S, ed. Systema Helminthum. New York: Interscience Publishers, Inc., 1958:800–972 [Google Scholar]
- Zuk M, McKean KA. Sex differences in parasite infections: Patterns and processes. Int J Parasitol 1996; 26:1009–1024 [PubMed] [Google Scholar]