Abstract
Background
Cancer immunotherapy attempts to stimulate the immune system to reject and destroy tumors and is one of the cancer treatment strategies. Recently, interluekin36 (IL36) has been used as immunotherapeutic agents in cancer gene therapy. Present study investigated that the IL36 gene therapy effects on the regression of tumor masses in mouse model. Aim of this study is determination of the gene therapy effects by IL36 in the regression of tumor masses in mouse model.
Methods
To study the therapeutic efficacy of this cytokine, WEHI-164 tumor cells were transected with mIL36 plasmids. ELISA test was used to check cytokine production by transected cells. To establish fibro sarcoma mouse model, Tumoral transfected cells were injected subcutaneously to inoculate tumor in BALB/C mice. Tumor volumes were measured by caliper. Mice were sacrificed and tumors were extracted. The expression of IL36 and IFN-γ was studied with Real-time PCR and immunoblotting. The expression of Ki-67 (a tumor proliferation marker) in tumor masses was studied by immunohistochemistry staining. In this study we had 2 groups which are treated with IL-36 and Untreated with IL-36 as a blank.
Results
The group treated with IL36 indicated decrease of tumor mass volume (p<0.001). The results of western blotting and real-time PCR showed the IL36 expression increased in the group treated with IL36 (with relative expression of 1.9).
Conclusion
Immunohistochemistry staining indicated that the Ki-67expression has been reduced in the group interfered with IL36. IL36 gene therapy has therapeutic effects on the regression of tumor masses in fibro sarcoma mouse model.
Keywords: IL-36, Gene therapy, tumor Mass, ELISA, Immunobloting
Introduction
Immunotherapy is a new class of cancer treatment that works to harness the innate powers of the immune system to fight cancer. Major conceptual and technical advances in immunology have led to a new understanding of cellular and molecular interplays between the immune system and a tumor over the past decades [1-3]. Because of the immune system's unique properties, these therapies may hold greater potential than current treatment approaches to fight cancer more powerfully, to offer longer-term protection against the disease, to come with fewer side effects, and to benefit more patients with more cancer types. Since recent therapies for cancer are based on drugs or radiotherapy which can kill proliferating cells or suppress cell proliferation, these treatments have several side effects on normal dividing cells. The potential roles of immunologic approaches for treating of cancer patients are specific for tumors [1, 2]. Targeting neoplastic cells can improve the therapeutic effects of gene delivery by preventing healthy tissues injury and reduce the risk of germ line transduction [3, 4]. Immunotherapy strategies consists of cancer vaccines, antitumor monoclonal antibodies, adoptive transfer of ex vivo activated T and NK cells, and administration of recombinant proteins and antibodies or that either co-stimulate immune cells or suppress immune inhibitory pathways (immune checkpoints). Also the immune memory can rebalance the equivalence between tumor and host cells. The aim of cancer immunotherapy is to boost the weak host immune response to develop tumors. One strategy is to use cytokines such as IL36 [5-7].
Gene therapy prepared a novel strategy of therapeutic intervention for lots of genetic and non-genetic disorders. With recent progress in gene therapy field, a wide variety of viral and non-viral vectors have emerged that can transfer genetic materials to target cells. Non-targeted delivery of transgenes often causes undesirable effects, low tumor transduction, and decreased therapeutic effects [8, 9].
Cancer-specific cytokine cascade is one of the manifestations of the underlying paraneoplastic systemic disease, and this hypothesis links the stage of cancer with both the functional status of the immune system and the patient's prognosis [10].
Cancer gene immunotherapy is a delivery of cytokine genes to tumor cells to modify the local tumor environment in order to induce anti-tumor immune responses [11]. In comparison to the therapeutic protein therapy, delivery of cytokine genes avoids the necessity of production and purification of large quantities of recombinant proteins. Moreover, gene immunotherapy is capable of delivering cytokines in a more efficient and safe manner [12].
Interluekin36 (IL36) is a 74 Kda heterodimeric cytokine that consists of 35 Kda (p35) and 40 Kda (p40) subunits [13-15]. It was characterized as a Natural Killer-Stimulating Factor (NKSF) and Cytotoxic Lymphocyte Maturation Factor (CLMF) by Trinchieri’s group in 1989 and Gately’s group in 1990. It is produced by macrophages, monocytes, and dendritic cells [14, 16]. Investigations have shown that IL36 posse’s superior antitumor effect compared with different cytokines. Also the researches demonstrated that IL36 can be efficacious in prevention of primary tumor growth [17]. IL36 is a multifunctional cytokine which can develops the multi-functional effects such as rising the proliferation and cytotoxic activity of T cells and NK cells, regulating the production of IFN-γ, inducing cytokine production, progressing the CD4+ Th1 cells development and also promoting the activity and generation of CTLs, via activation of STAT4. IFN-γ up regulates the expression of MHC class I and II molecules, adhesion molecules such as Intracellular Adhesion Molecules (ICAM)-1 and transcription factors such as T-box expressed in T cells (T-bet) with inducing by IL36 [15,18-19].Also, IL36 possesses potent anti-angiogenic activity produced by neuotrophils, macrophages and dendritic cells [20].
The endogenous production of IFN-γ is needed for the antitumor effect of IL36 in most cases. IL36 is one of the potent cytokines for cancer immunotherapy [18-19]. The aim of this study was to investigate the effects of gene therapy with IL36 in the regression of tumor masses in fibro sarcoma mouse model.
Materials and Methods
Plasmid amplification and isolation
An IL36 expression vector, pUMVC3-mIL36, was purchased from (Aldevron company, UK.LONDON). The plasmid DNA (6247 bp) is in size and contains CMV IE promoter.
PUMVC1-IL36 was amplified in Escherichia coli DH5α strain which was obtained from Drug Applied Research Center (Tabriz, Iran). The purified plasmid was detected by agarose gel electrophoresis. The DNA concentration was quantified by measuring the UV absorbance at 260 nm using UV spectrophotometer (Shimadzu, Japan, Tokyo).
Transformation of E. coli
pUMVC3-m IL36 plasmid was transformed into host strain E. coli, DH5α, BL21, respectively which was obtained, amplified and extracted according to TENS protocol. The purified plasmid was detected by agarose gel electrophoresis. The DNA concentration was quantified by measuring the UV absorbance (Biorad company product, USA, Illinois).
Determination of transfection efficiency
The transfection efficiency of mIL36 was measured by enzyme-linked immunosorbent assay (ELISA) kit (Koma Biotech Company, China, Zhenjiang) according to the manufacturer’s instructions.
Cell culture
BALB/C mouse fibro-sarcoma cells (WEHI-164) were bought from Pasteur Institute, Tehran. The WEHI-164 cells were cultured in RPMI1640 medium (Sigma, Germany, Frankfort) supplemented with 10% fetal bovine serum (Sigma, Germany, Frankfort), in presence of penicillin (100 U/ml), streptomycin (100 μg/ml), (Sigma, Germany, Frankfort), and incubated in humidified incubator with 5% CO2 at 37 °C.
In vitro transfection studies
The cells were trypsinized and seeded into 6-well plate at a density of 4× 105 cells/well; the cells were carried out before 2 days of transfection procedure. The cells were washed with Phosphate-Buffer Solution (PBS) twice prior to the addition of 2 mL RPMI1640 without FBS and antibiotic. Six μg of pUMVC1-m IL36 plasmid was diluted in 250 λ OptiMem in one micro-centrifuge tube. Ten λ Lipofectamine 2000 was diluted in 250 λ OptiMem in separate micro-centrifuge tube and incubated for 5 minutes. The contents of micro-centrifuge tubes were mixed together gently and incubated at room temperature for 20 minutes. The cells were in-cubated at 37°C in 5% CO2. After 6 hours, the complexes were aspirated and replaced with culture medium. After 48 hours of transfection, supernatants were harvested and released IL36 was confirmed with ELISA, by Mouse IL36 ELISA kit (Koma Biotech, China, Zhenjiang), following manufacturer's instructions.
Tumor implantation
Female BALB/C mice (6 to 8 weeks old) were purchased from the Pasteur Institute, Tehran, Iran. One hundred and six of WEHI-164 cells were inoculated into the right flank of the BALB/C mice subcutaneously to establish a tumor model. The viability of the cells used for inoculation was over 95% as determined by the Trypan blue dye exclusion test. Palpable tumors developed after 10 days. Tumor growth was monitored three times a week with calipers after tumor challenge until the experiment was completed. Tumor volume (mm3) was calculated by the formula: 1/2× (length×width2).
RNA extraction and Real time PCR
Fallowing tumor mass extraction, total RNA was extracted by AccuZolTM reagent (Bioneer, Daedeok-gu, Daejeon, Korea) as described by the manufacturer. Complementary DNA (cDNA) was generated from 1 µg of total RNA by using Oligo-DT primer and MMLV reverse transcriptase (Promega, Madison, WI, USA, Illinoise) according to the manufacturer’s recommendations. QRT-PCR was performed with SYBR Premix Ex Taq (Takara Bio, Otsu, and Shiga, Japan) in the Rotor-GeneTM 6000 system (Corbett Life Science, Mortlake, NSW, Australia). The PCR was done in a 20 µl reaction system containing 12 µl of SYBR green reagent, 0.2 µM for each primer, 1 µl of cDNA template and 6 µl of nuclease-free distilled water. The primer sequences were as followed: MouseIL36p40:
5’- GAGCACTCCCCATTCCTACT -3’ (as a Forward primer) and 5’-GCATTGGACTTCGGTAGATG-3’ (as a Reverse primer), Mouse IFN-γ:
5’-TCAGCAACAGCAAGGCGAAAAAG-3’ (as a Forward primer) and 5’-ACCCCGAATCAGCAGCGACTC-3’ (as a Reverse primer) and GAPDH was used as an internal expression control:
5’-CCTCGTCCCGTAGACAAAA-3’ (as a Forward primer) and 5’-AATCTCCACTTTGCCACTG-3’ (as a Reverse primer). GAPDH cDNA was served as an internal standard. The initial denaturation step was at 95°C for 10 min and was followed by 45 cycles at 95°C for 20 sec and 60°C for 1 min. Relative GM-CSF mRNA expression was calculated with the 2- (∆∆CT) 17, using GAPDH as a reference gene. The primer designs were done in Immunology center of Tabriz Medical University Laboratory.
Immunohistochemistry
Immunohistochemical assays were performed to detect Ki67 protein expression. Ki67 is a nuclear protein and is considered as a tumor proliferation biomarker in tumor masses. Four micrometer frozen sections were cut, air-dried, fixed in acetone, and rehydrated in PBS con-taining 0.05% Tween-20. Non-specific binding sites were blocked by Blocking buffer which is preformulated with Tween-20 for 30 minutes. Slides were incubated by primary antibody (Purified anti-mouse Ki67), (Biolegend, UK, London), for 60 minutes. Subsequently, slides were washed in PBS containing 0.05% Tween 20 and then slides were incubated with HRP labeled secondary antibody [Rabbit Polycolonal secondary antibody to Rat IgG (HRP-conjugated)], (abcam), for 30 minutes. H2O2 was added to DAB solution (Substrate solution) and DAB and H2O2 were added to the slides for 5 minutes. The slides were consequently washed with PBS containing 0.05% Tween-20 and studied by invert microscopy.
Western blotting
The purified anti-mouse IL36 (1:1000) (Biolegend, UK, London), and anti-β-actin (l: 1000) (Sigma) were used as primary antibodies. Cell extracts were prepared in RIPA-B buffer (0.5% Non-idetP40, 20 mM Tris, (pH 8.0), 50 mM NaCl, 50 mM NaF, 100 μM Na3VO4, 1 mM dithio-threitol, and 50 μg/mL phenyl-methylsulfonyl fluorides.
The protein concentration was quantified using a UV spectrophotometer. Protein samples were fractionated on a 12% polyacrylamide gel and transferred to the nitrocellulose membrane. The membrane was blocked with 3% skim milk for 1 hour at room temperature and incubated with primary antibody at 4°C overnight. After extensively washing, the membrane was incubated with [Rabbit Polyclonal secondary antibody to Rat IgG (HRP-conjugated)], (abcam). The results were visualized using the enhanced chemiluminescence (ECL) system (Amersham Biosciences, Piscataway, NJ, USA) and exposured to autoradiography film (Ko-dak XAR film).
Statistical analysis
Statistical analysis was measured by using the Student’s t test. p<0.05 was considered to be statistically significant.
Results
Confirmation of IL36 production by tumor transected cells
Enzyme-Linked Immunosorbent Assay (ELISA) test was used for confirmation of IL36 production by tumor transected cell. The concentration of IL36 in supernatant of tumor transected cell culture was assessed by spectrophotometer in 540 nm λ. The IL36 concentration in the supernatant of the tumor transected cell culture was 1000 pg/m9l (figure1).
Figure 1.
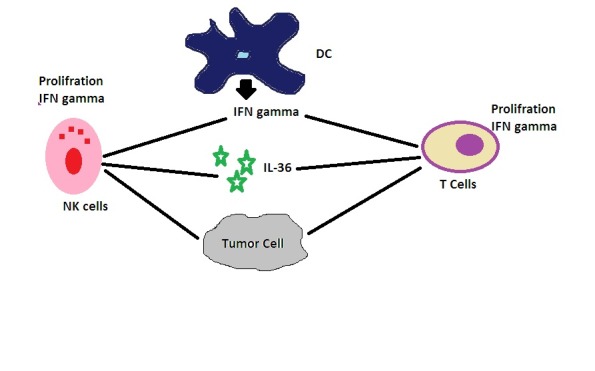
IL36 induces proliferation, production of IFN-γ and enhanced cytotoxic activity of these cells and induces the polarization of CD4+ T cells to the Th1 phenotype that mediates immunity against intracellular pathogens. IL36, especially in combination with IL-18, also acts on macrophages and dendritic cells to induce IFN-γ production even in antigen presenting cells.
Antitumor effects of IL36 in vivo
To test the antitumor response induced by IL36 secreted in site, BALB/C mice (n=7) were injected subcutaneously with fibro sarcoma/IL36 cells (1×106), and monitored for tumor growth 3 times a week. After 21 days of tumor inoculation the volume of the tumor masses was less than control group. So gene therapy with IL36 has effect in the regression of tumor masses (Figure 2).
Figure 2.
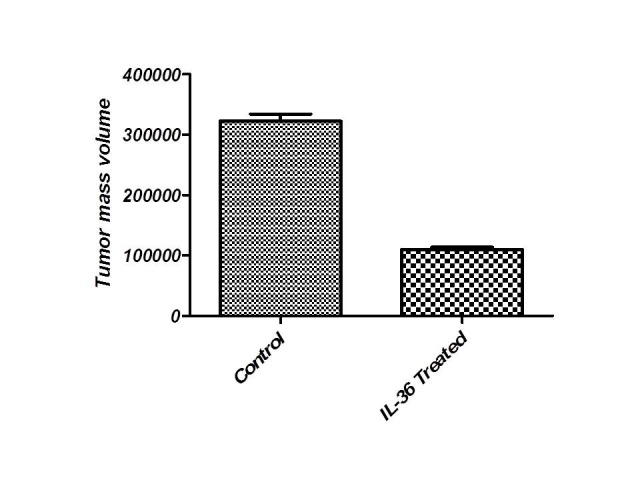
Tumor mass regression in group treated with IL36. The results showed that the tumor mass volume was reduced in group treated with IL36 (Sig: 0.00).
Expression of IL36 & IFN-γ mRNA and protein in tumor tissue
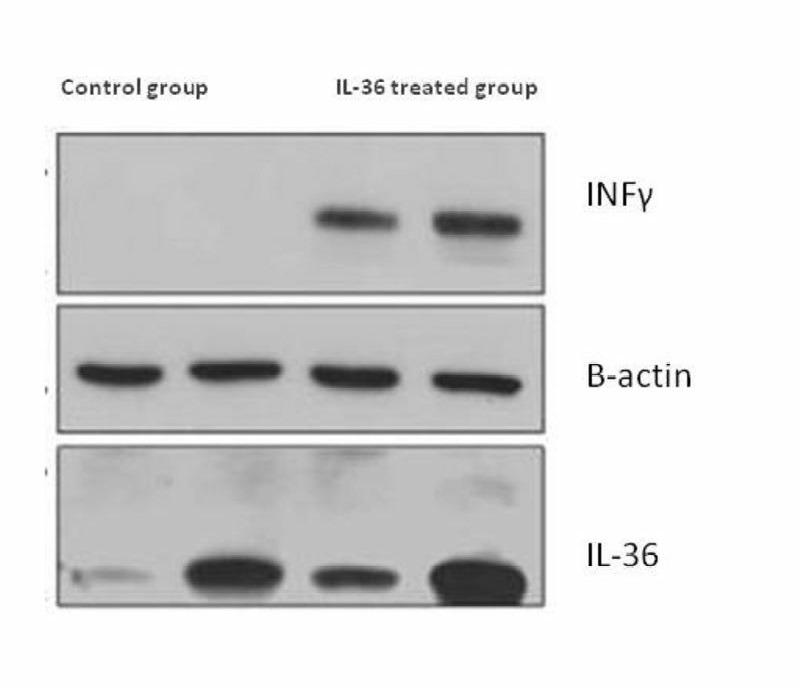
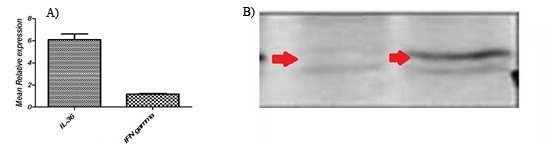
The extracted RNA with 28S/18S ratios between 1.5 and 1.9 gp/ml as determined by agarose gel electrophoresis analysis, using ethidium bromide staining. The median total RNA recovery from tissue was 98% (range, 90% to 105%). To investigate whether fibro sarcoma/IL36 cells can express IL36 in vivo, tumor mass was harvested from mice on day 21 after inoculation with fibro sarcoma/IL36 cells, respectively, total RNA was extracted from tumor masses and cell lyses were prepared. The results of real-time PCR indicated that the expression of IL36 and IFN-γ was enhanced in group treated with IL36 in comparison to the control group (Figure 3). The results of immunoblotting showed that the expression of IL36 and IFN-γ was enhanced in group treated with IL36 in comparison to the control group (Figure 4).
Figure 3.
Relative expression of IL36 and IFN-γ.
Figure 4.
The cytokine expression results of western blot: A) IL36 and INF-γ expression has been proved by western blotting analysis. B) Two samples of each group have been shown.
The Group treated with IL36 were UP-regulated (compared to the control group) by a mean factor of 1.90 (S.E. range is 1.900 - 1.900).
Discussion
Despite advances in our understanding of the processes involved in the development and progression of cancer, treatment options for many patients are limited and prognosis still remains poor. Gene therapy is the insertion of a functional gene into the cells of a patient to correct an inborn error of metabolism, to alter or repair an acquired genetic abnormality, and to provide a new function to a cell [2, 5]. Cancer gene therapy by delivery of cytokine gene is an appealing strategy which can be efficiently transducer into the tumor cells and extracts potential immune responses. Cancer gene therapy consists of 2 main therapeutic strategies: One approach is to cause a direct effect on cancer cells by transferring suicide genes siRNA for ontogenesis and proteins related to the cell cycle and apoptosis [21-24]. In this method, transfer of the therapeutic gene into the cancer cells is necessary to induce cytotoxicity. The second approach is indirect and activates antitumor immunity mediated by introducing a cytokine gene such as IL36 [25-29]. In such cytokine gene therapies, the therapeutic gene does not need to be delivered into all the cancer cells, because the cytokine is secreted from the cells.. Many anti-tumor effects are related to this method for IL36 gene delivery [30, 31]. The high IL36-producing cells show a NK- or T cell-independent antiangiogenic effect induced by IP-10, although the final rejection in immunocompetent mice is totally dependent on T cells [32, 33]. In prostate cancer, adenoviral-mediated IL36 gene therapy had an antimetastatic effect in partial dependency on NK cells [34]. In bladder carcinoma and colon cancer, adenoviral-mediated IL36 gene therapy is depend on T cell- local antitumor effects .In this study, we investigated that the effect of non-viral gene therapy by using plasmid DNA expressing IL36, is a potent primer of anti-tumor immunity [35-37]. Ki-67 expression has been reduced in the treated group. In addition, a significant level of intratumoral IFN-γ was also observed as an inducer of 2 important antitumor chemokines, IP-10 and MIG 37. No one denies that gene therapy for cancer holds extraordinary promise or that it will eventually yield results. But critics have grown increasingly concerned that the initial excitement led to a premature rush to get unproved gene therapies for cancer out of the laboratory and into human patients. Researchers are still not sure which are the best methods to transport genes into cancer cells, nor have figured out how to stop a person's own immune systems from rejecting what are, in effect, microscopic transplants of foreign materials. Even more troubling is the signs that indicate financial considerations may have replaced scientific rigor in determining how and when to use gene therapy for cancer.
Conclusion
The possibility of gene therapy opens a new area of therapeutics and hope for individuals afflicted with several types of cancers. We demonstrated that the local IL36 gene delivery into tumor tissue is an immune response to the tumor cells. Therefore, gene delivery by Lipofectamine could be a useful non-viral vector system in cancer gene therapy.
Acknowledgments
This article was done with Tabriz medical university, faculty of dentistry department.
Footnotes
Conflicts of Interest
The authors declare that they have no competing interests.
Authors’ Contribution
Shiva Kahnamouii and Pouralibaba designed the project, also Shiva Kahnamouii did the project, Farrokh Farhadi did the practical work of dentistry section. Farzaneh Pakdel designed the project and also guided the immunology sections.
REFERENCES
- 1.Helmy KY, Patel SA, Nahas GR, Rameshwar P. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 2013;4(10):1307–20. doi: 10.4155/tde.13.88. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimoto T, Morishima N, Okumura M, Chiba Y, Xu M, Mizuquchi J. Interleukins and cancer immunotherapy. Immunotherapy. 2009;1(5):825–44. doi: 10.2217/imt.09.46. [DOI] [PubMed] [Google Scholar]
- 3.Wang JH, Liu XY. Targeting strategies in cancer gene therapy. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao. 2003;35(4):311–6. [PubMed] [Google Scholar]
- 4.Hughes RM. Strategies for cancer gene therapy. J Surg Oncol. 2004;85(1):28–35. doi: 10.1002/jso.20001. [DOI] [PubMed] [Google Scholar]
- 5.Gajewski TF. Cancer immunotherapy. Mol Oncol. 2012;6(2):242–50. doi: 10.1016/j.molonc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowalczyk DW, Wysocki PJ, Mackiewicz A. Cancer immunotherapy using cells modified with cytokine genes. Acta Biochim Pol. 2003;50(3):613–24. [PubMed] [Google Scholar]
- 7.Zarour HM, Ferrone S. Cancer immunotherapy: Progress and challenges in the clinical setting. Eur J Immunol. 2011;41(6):1510–5. doi: 10.1002/eji.201190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. 2013;13(6):421–33. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia S, Menezes ME, Das SK, Emdad L, Dasgupta S, Wang XY, et al. Innovative approaches for enhancing cancer gene therapy. Discov Med. 2013;15(84):309–17. [PubMed] [Google Scholar]
- 10.Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005;10(3):450–4. doi: 10.1002/14651858.CD002225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich D, Gabay C. Inflammation: IL-36 has proinflammatory effects in skin but not in joints. Nat Rev Rheumatol. 2014;9:156. doi: 10.1038/nrrheum.2014.156. [DOI] [PubMed] [Google Scholar]
- 12.Nezhadi SH, Valizadeh H, Dastmalchi S, Baradaran B, Lotfipour F. Preparation of Chitosan-Plasmid DNA Nanoparticles Encoding IL-12 and their Expression in CT-26 Colon Carcinoma Cells. J Pharm Pharmaceut Sci. 2011;14(2):181–95. doi: 10.18433/j3tp4t. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez-Ortega A, Sandoval-Montes C, de Olivera-Flores TJ, Santos-Argumedo L, Gómez-Lim MA. Expression of functional IL-12 from mouse in transgenic tomato plants. Transgenic Res. 2005;14(6):877–85. doi: 10.1007/s11248-005-1464-8. [DOI] [PubMed] [Google Scholar]
- 14.Xu D, Gu P, Pan PY, Li Q, Sato Al, Chen SH. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer. 2004;109(4):499–506. doi: 10.1002/ijc.11696. [DOI] [PubMed] [Google Scholar]
- 15.Toda M, Martuza RL, Rabkin SD. Combination suicide/cytokine gene therapy as adjuvants to a defective herpes simplex virus-based cancer vaccine. Gene Ther, 2001;8(4):332–9. doi: 10.1038/sj.gt.3301392. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Mizoguchi I, Morishima N, Chiba Y. Regulation of antitumor immune responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27. Clin Dev Immunol. . 2010;20(10):832–44. doi: 10.1155/2010/832454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR. Antitumor and antimetastatic activity of interlukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara I, Nagai H, Miyake H, Yamanaka K, Hara S, Micallef MJ, et al. Effectiveness of cancer vaccine therapy using cells transduced with the IL-12 gene combined with systemic interleukin-18 administration. Cancer Gene Therapy. 2000;7(1):83–90. doi: 10.1038/sj.cgt.7700083. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki R, Namai E, Oda Y, Nishiie N. Cancer gene therapy by IL12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J Control Release. 2010;142(2):245–50. doi: 10.1016/j.jconrel.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interlukin 12. J Natl Cancer Inst. 1995;87(8):581–6. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 21.Shi XH, Liang ZY, Ren XY, Liu TH. Combined silencing of K-ras and Akt2 oncogenes achieves synergistic effects in inhibiting pancreatic cancer cell growth in vitro and in vivo. Cancer Gene Ther. 2009;16(3):227–36. doi: 10.1038/cgt.2008.82. [DOI] [PubMed] [Google Scholar]
- 22.Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115(4):978–85. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beh CW, Seow WY, Wang Y, Zhang Y, Ong ZY, Ee PL, et al. Efficient delivery of Bcl-2-targeted siRNA using cationic polymer nanoparticles: downregulating mRNA expression level and sensitizing cancer cells to anticancer drug. Biomacromolecules. 2009;10(1):41–8. doi: 10.1021/bm801109g. [DOI] [PubMed] [Google Scholar]
- 24.Folini M, Pennati M, Zaffaroni N. RNA interference-mediated validation of genes involved in telomere maintenance and evasion of apoptosis as cancer therapeutic targets. Methods Mol Biol. 2009;48(7):303–30. doi: 10.1007/978-1-60327-547-7_15. [DOI] [PubMed] [Google Scholar]
- 25.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401 (6753):517. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 26.Conwell CC, Huang L. Recent advances in non-viral gene delivery. Adv Genet. 2005;53(2):1–18. doi: 10.1016/S0065-2660(05)53001-3. [DOI] [PubMed] [Google Scholar]
- 27.Newman CM, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14(6):465–75. doi: 10.1038/sj.gt.3302925. [DOI] [PubMed] [Google Scholar]
- 28.Shen ZP, Brayman AA, Chen L, Miao CH. Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene Ther. 2008;15(16):1147–55. doi: 10.1038/gt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105(10):1233–9. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 30.Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. J Immunother. 2007;30(5):490–8. doi: 10.1097/CJI.0b013e318031b551. [DOI] [PubMed] [Google Scholar]
- 31.Pertl U, Luster AD, Varki NM. IFN-gamma-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J Immunol. 2001;166(11):6944–51. doi: 10.4049/jimmunol.166.11.6944. [DOI] [PubMed] [Google Scholar]
- 32.Pertl U, Luster AC, Varki NM, Lode N, Homann D, Gaedicke G, et al. IFN-γ-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain 1L-12 gene therapy. J Immunol. 2001;16(6):6944. doi: 10.4049/jimmunol.166.11.6944. [DOI] [PubMed] [Google Scholar]
- 33.Lode HN, Dreier T, Xiang R, Varki NM, Kang AS, Reisfeld RA. Gene therapy with a single chain interleukin 12 fusion protein induces T cell-dependent protective immunity in a syngeneic model of murine neuroblastoma. Proc Natl Acad Sci USA. 1998;95(5):2475–80. doi: 10.1073/pnas.95.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasu Y, Bangma CH, Hull GW, Lee HM, Hu J, Wang J, et al. Adenovirus-mediated interluekin36gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 1999;6(3):338–49. doi: 10.1038/sj.gt.3300834. [DOI] [PubMed] [Google Scholar]
- 35.Mazzolini G, Qian C, Xie X, Sun Y, Lasarte JJ, Drozdzik M, et al. Regression of colon cancer and induction of antitumor immunity by intratumoral injection of adenovirus expressing interleukin-12. Cancer Gene Ther. 1999;6(7):514–22. doi: 10.1038/sj.cgt.7700072. [DOI] [PubMed] [Google Scholar]
- 36.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63(14):4095–100. [PubMed] [Google Scholar]
- 37.Li S, Xia X, Mellieon FM, Liu J, Steele S. Candidate genes associated with tumor regression mediated by intratumoral IL-12 electroporation gene therapy. Mol Ther. 2004;9(3):347–54. doi: 10.1016/j.ymthe.2003.11.022. [DOI] [PubMed] [Google Scholar]


