Abstract
Background
Although the incidence of cervical cancer has reduced during last years, but it causes mortality among women. Many efforts have performed to develop new drugs and strategy for treatment of cervical cancer. Adipose Tissue-Derived mouse Mesenchymal Stem Cells (MSCs) has many advantages which make them a suitable choice as a cell therapeutic agent in cancer treatment. In this study, we aimed to develop an improved protocol for Mouse MSCs transduction as well as assess the homing capacity and incorporation of MSCs in cervical cancer model.
Methods
MScs were isolated from the mouse adipose tissue and characterized by differentiation and flow cytometry. In our study, lentiviral vector transductions of MSCs performed. Their penetrations were detected in tissue sections after injection of transduced MSCs to female C57BL/6 mice as a cervical cancer model.
Results
Results showed that MSCs were efficiently transduced with lentiviral vector resulting in efficient tumor penetration.
Conclusion
The results provide evidence that MSCs were able to penetrate into the tumor mass of cervical tumor model and are good vehicles for gene transfer to cervical cancer.
Keywords: Lentivector, Adipose Tissue-Derived mouse Mesenchymal Stem Cells, cervical cancer, Transduction
Introduction
Incidence of cervical cancer and the death rate of it have declined recently, but it is still the third most common cancer worldwide and the second leading cause of mortality among women. Approximately, 500,000 new cases of cervical cancer with 280,000 deaths are reported each year. Human Papilloma Virus (HPV) infection is the major factor related to development of cervical cancer in developing world [1, 2]. Surgery, chemotherapy and radiotherapy can cure 80%-95% of patients with early diagnosed cancer; although the recurrent or metastatic disease remains a leading factor of cancer mortality. Development of new diagnosis, prognostic, and treatment strategies is a major interest for public health [3]. Mesenchymal Stem Cells (MSCs) are non-haematopoietic stem cells that have attracted interest as potential platforms for transgene delivery and cell-based therapy in diseases such as viral infections [4]. MSCs have the capability to self-renew and to differentiate into the three lineages, including osteoblasts, chondrocytes and adipocytes [5]. Minimal criteria for defining MSC are as follow: First, MSC must be plastic-adherent in culture conditions. Second, MSC must express CD105 CD73 and CD90, and absence of CD14, CD34, CD19, HLA-DR and CD45. Third, MSC must be able to differentiate to osteoblasts, adipocytes and chondroblasts [6]. Mesenchymal Stem Cells (MSCs) represent a new tool for delivery of therapeutic genes to cells. MSCs have been modified by multiple methods including viral transduction and lipid transfection. Lipid transfection of MSCs results (30-40%) in relatively low numbers of transducing cells. Viral transduction generally results in higher efficiency. A method is to use lenti viruses to deliver the DNA construct in to MSCs [7]. Recent studies have indicated that lentiviral vectors are efficient to deliver and express transgenes in MSCs. An advantage of these vectors over retrovectors is that they can transduce non-dividing cells. This is important given the fact that a large subset (population) (20%) of Mesenchymal Progenitor Cells (MPCs) has been reported to be quiescent [8]. Moreover, the capacity of lentiviral vectors to transfer relatively large transgenes is an advantage over Adeno-Associated Viral Vectors (AAVs), which have a packaging limit of ~5 kb [9]. McGinley and colleagues group have reported a study comparing various modified methods for rat MSCs. They reported that lentiviral vectors showed efficient transduction of rat MSCs in contrast to adenovirus, AAV, lipid transfection and electroporation [10].
Mouse MSCs have demonstrated more difficult to transduce with lentiviral vectors than their human counterparts, and because of many studies use mouse models of human disease, an optimized method of transduction is required to facilitate studies applying mouse MSCs [4, 2]. The main problem for the development of effective cancer therapy is believed to be the lack of sufficient specificity. In recent years, cell-based cancer therapy strategies have proposed. An example of this is the use of Mesenchymal stem cells as gene delivery vehicles.
The MSC-based multiple-targeted anticancer strategy is based on MSCs’ capacity of tumor-directed migration and incorporation and in situ expression of tumor-specific anticancer genes. They can migrate to tumor and incorporate into the tumor architecture. Since the introduction of tumor-oriented homing ability of MSCs, the application of specific anticancer gene-engineered MSCs has demonstrated the great potential for cancer therapies [11-15].
The homing ability of MSCs has been shown in many tested cancer cell lines, as Kaposi sarcoma, breast cancer, lung cancer, malignant glioma, pancreatic cancer, colon carcinoma, melanoma and ovarian cancer [13].
In this study, we aimed to develop the efficient lentiviral transduction of Adipose Tissue-Derived mouse Mesenchymal Stem Cells and assessment of their penetration in female mice cervical tumor model and assess the homing capacity and incorporation of MSCs in cervical cancer model.
Materials and Methods
Isolation and seeding of MSC
Murine MSCs were isolated from the inguinal adipose tissue of C57/BL6 mice, as previously described [16]. The plastic-adherent population of cells was grown in complete culture medium, consisting of Dulbecco's Modified Eagle's Medium (DMEM; GIBCO-BRL, Tokyo, Japan), 10% fetal bovine serum (FBS; GIBCO-BRL, Tokyo, Japan) and passaged in 75 cm flask. We perfoemed all the experiments under biosafety class II and sterility condition.
Characterization of Mesenchymal stem cells using flow cytometry
Adipo-derived MSCs are phenotypically defined by the presence or absence of defined cell surface markers. The phenotype of MSCs from mouse adipose was determined by flow cytometry. At the third passage, the cells were used for phenotype analysis. Cells (105 cells) were resuspended in 200 µL of PBS and incubated for 30 min at 4°C with fluorescence-labeled antibodies against mouse CD73-Fluorescein Isothiocyanate (FITC), CD90- and CDSca1, CD45, CD11b or the appropriate isotype controls (all antibodies were purchased from eBioscience). The cells were analyzed using flow cytometry (Attune acoustic focusing cytometer, Applied biocystems, USA) by flowJo software (Tree Star, Inc., Ashland, OR, USA).
Evaluation of MSC differentiation
For osteogenic differentiation, the cells at the third passage were incubated in Dulbecco's Modified Eagle Media (DMEM) containing 10% FBS, dexamethasone 10-7 M, β-Glycerol-Phosphate 10 mM and ascorbic acid 2-phosphate 50 μg/ml. Control MSCs were incubated only in DMEM containing 10% FBS and after 21 days of culture in 6-wells plate, the osteogenic differentiation of stem cells was confirmed by positive alizarin red staining of the Mineralized matrix.
For confirmation of adipogenic differentiation, cells were incubated with DMEM medium containing 10% FBS Isobutylmethylxanthine (IBMX) 0.5 mM, dexamethasone 10-7 M, Insulin 66 nM, Indomethacin 0.2 mM. At 30 days of culture, the adipocytic phenotype is characterized by intracellular accumulation of lipid droplets that visualized with 0il-Red O staining.
Production and concentration of the Lentiviral vector
Lentiviral vectors were produced based on Prof. Trono lab protocols with some modifications [17]. Replication-incompetent lentiviral particles were generated by transient co-transfection of HEK293T cells, human embryonic kidney cells, that have been transformed by adenovirus type 5 DNA [18], with the three plasmids (psPAX2, PMD2G, pCDH-CMV-MCS-EF1-GFP-T2A-Puro), using a CaPO4 precipitation method. Briefly, 293T cells were plated on 10 cm plates to 80-90% confluence and co-transfected with 10.5 μg PMD2G, 21 μg psPAX2 and 21 μg pCDH-CMV-MCS-EF1-GFP-T2A-Puro. The cells were rinsed with PBS and given fresh media within 2 h before initiating transfection.
The plasmid DNA was diluted into Ca3(PO4)2 buffer that contains 21 μg pCDH-CMV-MCS-EF1-GFP-T2A-Puro, 21 μg Pspax2, 10.5 μg pMD2, 33 μl TE 1X, 105 μl CaCl2 2.5 M, and 1050 μl 2x HBSS was used for one of 10 cm plate. The 2X HBSS was added during solution vortexing. The DNA/ CaCl2/HBS mixture was then added drop wise to the cells.
The medium was removed 16 h later and replaced with 10 ml of fresh DMEM with 10% fetal bovine serum (FBS; GIBCO-BRL, Tokyo, Japan). Culture supernatants were collected every 24 h for three days, centrifuged at low speed to remove cell debris and filtered through a 0.45 μm filter. The viral supernatants were concentrated by precipitation with polyethylene glycol (PEG)-NACL, resuspended in sterile phosphate-buffered saline (PBS), and then stored at −80°C.
Vector titration using Fluorescence Activated Cell Sorting (FACS)
Vector titration was performed based on Prof. Trono lab protocols with some modification [19]. To determine vector titers (TU), 293T cells were grown in 12-well plate at a density 105 cells per well in one ml DMEM supplemented with 10% FBS. The medium was removed and cells transduced in 500 μl of fresh DMEM-10 with serial dilutions of the vector that correspond to final amount of: 1 μl, 10-1, 10-2, 10-3, and 10-4μl of vector. After 24 h, the medium was removed and one ml of fresh medium was added to each well. 72 h after transduction, cells were processed for Fluorescence Activated Cell Sorting (FACS) analysis.
Transduction of MSCs with Lentiviral Vectors
Cells were plated 24 h before transduction at a density of 105 cells per well in 6-well plates in DMEM supplemented with 10% FBS in the presence of 8 μg/mL polybrene. 6-well plates were centrifuged in 2000 rpm for 1 hour at 25ºC and incubated overnight without centrifugation. Transductions were performed three times with interval of eight hours. After 24 h, the medium was replaced with two ml fresh culture medium. 48 h after transduction, Green Fluorescent Protein (GFP) gene expression was examined by Fluorescent microscopy. Transduced cells were passaged, and selected with puromycin (1.5 mg/ml) for 5 days.
Mice
Six to seven week-old female C57BL/6 mices were purchased from the Pasteur Institute of Iran. All experiments and manipulation were performed in accordance with the Animal Care and Use Protocol of Tarbiat Modares University.
Tumor induction and MSCs administration
TC-1 cell line were purchased from a cell bank (Pasteur Institute of Iran). This cell line was derived from primary lung epithelial cells of C57BL/6 mice. The cells were immortalized with HPV-16 E6 and E7 and c-Ha-ras oncogenes [20]. Total number of 106 TC-1 cells were suspended in 100 μl Phosphate Buffered Saline (PBS) and subcutaneously injected into the left flanks of the female C57BL/6 mice. Tumor formation was visualized, one week after inoculation.
At day 7, rapid tumor formation appeared in C57BL/6 mice inoculated with 106 TC-1 cells. After tumor formation, mice were intratumorally injected with 2.5*105 MSCs transduced with lentiviral vector.
Histological study
Seven days following administration of MSCs to tumor, the mice were euthanized and histological studies were conducted. Autopsied tissues from animals were fixed in 10% phosphate-buffered formalin. They were embedded in paraffin and sectioned at 4-6 μm; the slides were mounted and evaluated by fluorescent microscopy (×40).
Results
The FACS phenotype analysis showed that the MSCs were positive for CD73, CD90 and Sca-1, but negative for CD45 and CD11b (Figure1).
Figure 1.
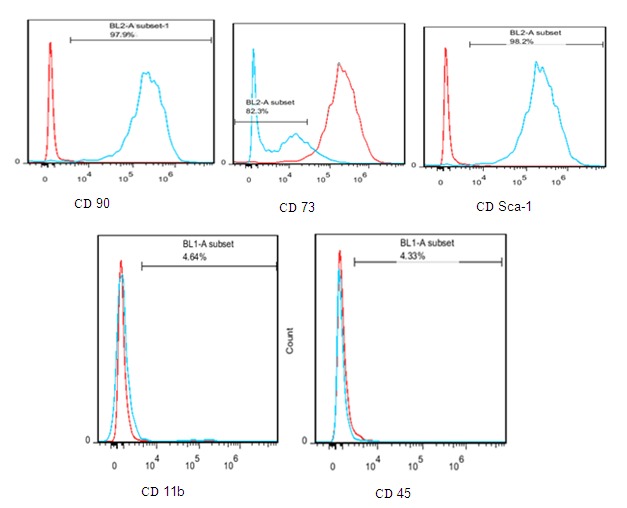
FACS analysis for isolated MSCs. The results revealed that the isolated cells were positive for CD73, CD90 and Sca-1, but negative for CD45 and CD11b and confirmed to be MSCs.
21 days after culture the MSCs with osteogenic-inducing media at three passages, osteogenic differentiation was observed with alizarin red staining (Figure 2A).
Figure 2.

Isolation of MSCs from mouse adipose tissue and characterized by differentiation A: Adipo MSCs were cultured in osteogenic differentiation medium and after 21 days of culture stained with Alizarin red. Alizarin red stained the calcium deposits. Formation calcium deposits indicate that osteogenic differentiation process performed. B: Figure B shows MSCs were differentiated to adipocyte. Lipid Vesicle seen in cells with Oil Red staining and indicated successful adipogenic differentiation. C: Figure c shows the MSCs at 3rd passage.
MScs were incubated with adipogenic media at three passages for 30 days. Polybrene and centrifugation Oil droplets were confirmed in the cytoplasm using Oil Red-O staining (Figure 2B).
After Transient co-transfection of HEK293T cells containing the three plasmids, GFP expression was visualized using a fluorescence microscope (Figure 3). The virus titer was determined by flow cytometry, the final virus titer found to be TU/mL after concentration.
Figure 3.

Transfection of HEK-293T by Ca3(PO4)2 method for virus production. HEK-293T cells co-transfected with PMD2G, psPAX2 and pCDH-CMV-MCS-EF1-GFP-T2A-Puro (A): Figure A shows the culture of HEK-293Tby light microscopy and (B) HEK-293T cells 24 h after transfection visualized by fluorescent microscopy High expression of GFP in HEK-293T demonstrated the high level of transfection.
MSCs were plated 24 h before transduction at a density of 50,000 cells per well of 6-well plates. Transduction was performed three times with 8 h intervals. In first time, cells were infected with lentiviral vector as well as 8 μg/mL polybrene and the plates were centrifuged in 2000 rpm for 1 h (Figure 4). In other twice, same MSCs were infected only with lentiviral vector.
Figure 4.

A: Transductions were carried out in the presence of 8 μg of Polybrene (Sigma) per ml. After incubation at 37°C for 48 h, transduced MSCs seen by Fluorescence microscopy. High expression of GFP in MSCs shows the high rate of transduction. B: shows transduced MScs by light microscopy after incubation at 37°C for 48 h.
After seven days following intratumoral injection of MSCs, the mice were sacrificed and tumors were removed for histological examination. Tumor tissues from mice were fixed in 10% phosphate-buffered formalin. They were embedded in paraffin and were sectioned at 4-6 μm; the slides were mounted and visualized by fluorescent microscopy (×40). MSCs with high frequency in tissues sections were seen (Figure 5).
Figure 5.

Tumor tissue was removed for histological examination after the mice were sacrificed at 14days. Sections prepared from central region of tumor. Figure shows, transduced MSCs (green cells) have good penetration in cervical tumor tissue.
Discussion
Whereas bone marrow (BM) MSC has been the first identified source of MSC [21], adipose tissue recognized a valid reservoir of Mesenchymal stem cells [22]. Adipose tissue can be isolated and easily processed to release large numbers of adipose-derived MSC. AD-MSCs are particularly suitable for cell and gene therapy methods because they can be expanded and then transduced by several vectors [21]. The main benefit of MSCs in this field is that they are considered as immunoprivileged, because they expresslow level of Ag (HLA) MHC class 1, but not CD40, CD80 and CD86 [23]. In addition, they can secret prostaglandin, transforming growth factor beta and hepatocyte growth factor, which regulate the T-cell immune response, leading to reduce the possibility of a cytotoxic T-cell response to transduced cells [14]. MSCs have gained interest as promising tools for cell-based gene therapy approaches for various diseases. An important advantage of MSCs as cellular vehicles is their accessibility for genetic modification. MSCs have some advantages as cellular vehicles: they are approximately easy to isolate and expand, specifically migrate to tumors following systemic delivery, can be transduced with a range of viral vectors. Current studies have reported the use of lentivirus-mediated Transduction for MSCs [15]. Generally, HIV-1 replication is not supported by mouse cells because a number of barriers at various steps including virus entry, nuclear import, RNA splicing, polyprotein processing, assembly, and release [4, 24].
In this study, we isolate adipo MScs form C57BL/6 mouse tissue. All these MSCs showed plastic-adherent and fibroblastic like morphologic characteristics. The property of plastic adherent itself is not sufficient for identification of MScs as well as further characterization of these cells that is performed by differentiation potential and cell surface marker expression (three criteria required for MSCs confirmation: plastic-adherent, differentiation potential and surface markers expression). Adipogenic inducing media Cultured MSCs differentiate to adipocyte cells with formation of lipid droplets. These droplets were stained by Oil Red. The differentiation of MSC to osteoblasts carried out in vitro using appropriate media. Mesenchymal Stem Cells have no extracellular calcium deposit, whereas osteoblasts feature vast extracellular calcium deposits. Therefore, Calcium deposits are indication of successful differentiation that stained by Alizarin Red. In addition to differentiation potential, MSCs surface markers expression evaluated by Flow cytometry. Result showed that MSCs were positive for CD73, CD90 and Sca-1 and these markers are expressed in 97.9%, 82.3%, and 98.2% of MSCs, respectively. MSCs were negative for CD45 (4.33%) and CD11b (4.64%).
To improve lenti viral vectors for efficient transduction of MSCs, we have tested various transduction protocols. The current protocol for efficient lentiviral transduction of MSCs involves the addition of polybrene during transduction and the achieved results showed that a three round of transduction using concentrated lentiviral vectors can lead to the efficient transduction of mouse MSCs.
In recent years, combinations of cell and gene therapy have been used for cancer therapy. Cell therapies are based on biological agents contain cells to be used to patients [11]. One of the cells with high attraction in cell-based strategy is Mesenchymal stem cell as a gene delivery vehicle.
MSCs are of interest as a vehicle for the expression of therapeutic genes, because they are easy isolated, expanded and transduced with viral vectors, as well as MSCs have the ability to home to the tumor tissue, they may thus be promising tools for the specific delivery of antitumor agents to tumors [25].
Conclusion
Our results demonstrate that lentiviral vectors can efficiently transduce mouse MSCs in vitro with high efficiency and lentiviral vectors are good choice for stable gene delivery to these stem cells. These findings confirm other studies [7, 26, 27]. After intratumoral injection of MSCs, histological examination of cervical tumor tissue showed that MSCs have good penetration in cervical tumor tissue, as well as these cells seen with high frequency in microscopic sections.[28] It seems that these cells are the potent vehicles for transfer and expression of therapeutic genes to cervical cancer microenvironment.
Acknowledgments
This work was performed as part of the requirement for the fulfillment of the degree of PhD in Medical Virology of Azra Kenarkoohi at Tarbiat Modares University. We would like to thank the office of applied research of Tarbiat Modares University for its support of the project.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this study.
Authors’ Contribution
Azra Kenarkoohi: PhD student who has performed as her PhD research project, Masoud Soleimani and Taravat Bamdad: Design and Management of project, and all the other authors have been involved in experiments, technical support of project.
REFERENCES
- 1.Tomar A, Kushwah A. Advances in human papilloma virus vaccines: a review. International Journal of Basic & Clinical Pharmacology. 2014;3(1):37–43. [Google Scholar]
- 2.Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, et al. Isolation and characterization of mouse Mesenchymal stem cells. Transplant Proc. 2008;40(8):2649–54. doi: 10.1016/j.transproceed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Ricks Dm, Kutner R, Zhang X-Y, Welsh DA, Reiser J. Optimized Lentiviral Transduction of Mouse Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells and Development. 2008;17(3):441–50. doi: 10.1089/scd.2007.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of Adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Dominici M, Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 7.Lin P, Lin Y, Lennon Dp, Correa D, Schluchter M, Caplan Ai. Efficient Lentiviral Transduction of Human Mesenchymal Stem Cells That Preserves Proliferation and Differentiation Capabilities. stem cells translational medicine. 2012;1(12):886–97. doi: 10.5966/sctm.2012-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5(12):1571–84. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matrai J, Chuah M, Vanden-Driessche T. Recent Advances in Lentiviral Vector Development and Applications. Molecular Therapy. 2010;18(3):477–90. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O’Toole D, et al. Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Research & Therapy. 2011;2(2):12. doi: 10.1186/scrt53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peralta-Zaragoza O, Hugo Bermúdez-Morales V, Pérez-Plasencia C, Salazar-León J, Gómez-Cerón C, Madrid-Marina V. Targeted treatments for cervical cancer: a review. Onco Targets Ther. 2012;5:315–28. doi: 10.2147/OTT.S25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Castro J, Rubio D, de la Fuente R, Bernad A. Antitumoral cell-based therapies. Cancer Therapy. 2003;1:163–71. [Google Scholar]
- 13.Fritz V, Jorgensen CH. Mesenchymal Stem Cells: An Emerging Tool for Cancer Targeting and Therapy. Current Stem Cell Research & Therapy. 2008;3(1):32–42. doi: 10.2174/157488808783489462. [DOI] [PubMed] [Google Scholar]
- 14.Mohr A, Lyons M, Deedigan L, Harte T, Shaw G, Howard L, et al. Mesenchymal stem cells expressing TRAIL lead to tumor growth inhibition in an experimental lung cancer model. J Cell Mol Med. 2008;12(6B):2628–43. doi: 10.1111/j.1582-4934.2008.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwyer RM, Khan S, Barry FP, O’Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Research & Therapy. 2010;1(3):25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai LJ, Moniri MR, Zeng ZR, Zhou JX, Rayat J. Potential implications of mesenchymal stem cells in cancer therapy. Cancer letters. 2011;305(1):8–20. doi: 10.1016/j.canlet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Klages N, Zufferey R, Trono D. A Stable System for the High-Titer Production of Multiply Attenuated Lentiviral Vectors. Mol Ther. 2000;2(2):170–6. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- 18.Graham F. L, Smiley J. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J Gen Virol. 1997;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 19.Barde I, Salmon P, Trono D. Production and Titration of Lentiviral Vectors. Current Protocols in Neuroscience. 2010;4:4. doi: 10.1002/0471142301.ns0421s53. [DOI] [PubMed] [Google Scholar]
- 20.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21–6. [PubMed] [Google Scholar]
- 21.Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, et al. Adipose-Derived Mesenchymal Stem Cells as Stable Source of Tumor Necrosis Factor -Related Apoptosis-Inducing Ligand Delivery for Cancer Therapy. Cancer Res. 2010;70(9):3718–29. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- 22.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 23.Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10(7):657–67. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- 24.Tsurutani N, J Yasuda, N Yamamoto, BI Choi, M Kadoki, Y Iwakura. Nuclear import of the preintegration complex is blocked upon infection by human immunodeficiency virus type 1 in mouse cells. J Virol. 2007;81(2):677–88. doi: 10.1128/JVI.00870-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galderisi U, Giordano A, Paggi MG. The bad and the good of mesenchymal stem cells in cancer: Boosters of tumor growth and vehicles for targeted delivery of anticancer agents. World J Stem Cell. . 2010;2(1):5–12. doi: 10.4252/wjsc.v2.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truong Nguyen K. Mesenchymal Stem Cells as Targeted Cell Vehicles to Deliver Drug-loaded Nanoparticles for Cancer Therapy. J Nanomed Nanotecho. 2013;4(1) [Google Scholar]
- 27.Azadmanesh K, Gheysari Y, Negahdari B. Gene Delivery to Mesenchymal Stem Cells. International Journal of Pediatrics. 2014;2((2-3)):12. [Google Scholar]
- 28.Hajizadeh-Sikaroodi SH, Hosseini A, Fallah A, Estiri H, Noormohammadi Z, Salehi M, et al. Lentiviral Mediating Genetic Engineered Mesenchymal Stem Cell for Releasing IL-27 as a Gene Therapy Approach for Autoimmune Diseases. Cell Journal (Yakhteh). 2013;16(3):2–18. [PMC free article] [PubMed] [Google Scholar]


