Abstract
Background
Although feeding behavior and food habit are ecologically and economically important properties, little is known about formation and evolution of herbivory. Grass carp (Ctenopharyngodon idella) is an ecologically appealing model of vertebrate herbivore, widely cultivated in the world as edible fish or as biological control agents for aquatic weeds. Grass carp exhibits food habit transition from carnivory to herbivory during development. However, currently little is known about the genes regulating the unique food habit transition and the formation of herbivory, and how they could achieve higher growth rates on plant materials, which have a relatively poor nutritional quality.
Results
We showed that grass carp fed with duckweed (modeling fish after food habit transition) had significantly higher relative length of gut than fish before food habit transition or those fed with chironomid larvae (fish without transition). Using transcriptome sequencing, we identified 10,184 differentially expressed genes between grass carp before and after transition in brain, liver and gut. By eliminating genes potentially involved in development (via comparing fish with or without food habit transition), we identified changes in expression of genes involved in cell proliferation and differentiation, appetite control, circadian rhythm, and digestion and metabolism between fish before and after food habit transition. Up-regulation of GHRb, Egfr, Fgf, Fgfbp1, Insra, Irs2, Jak, STAT, PKC, PI3K expression in fish fed with duckweed, consistent with faster gut growth, could promote the food habit transition. Grass carp after food habit transition had increased appetite signal in brain. Altered expressions of Per, Cry, Clock, Bmal2, Pdp, Dec and Fbxl3 might reset circadian phase of fish after food habit transition. Expression of genes involved in digestion and metabolism were significantly different between fish before and after the transition.
Conclusions
We suggest that the food habit transition from carnivory to herbivory in grass carp might be due to enhanced gut growth, increased appetite, resetting of circadian phase and enhanced digestion and metabolism. We also found extensive alternative splicing and novel transcript accompanying food habit transition. These differences together might account for the food habit transition and the formation of herbivory in grass carp.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-015-1217-x) contains supplementary material, which is available to authorized users.
Keywords: Food habit transition, Carnivory, Herbivory, Grass carp, Transcriptome sequencing
Background
Although there are intensive research efforts on feeding behavior and food habit, little is known about the formation and evolution of herbivory. Previous studies have reported that the gut microbiota in herbivores play an important role in nutrient digestion and assimilation [1-3]. Sullam et al. suggest that herbivorous fish and mammals share the process of gut fermentation to obtain nutrients from plants [4]. However, many herbivorous fishes display low levels of gastrointestinal fermentation [5]. Several freshwater herbivorous fishes such as grass carp do not rely on microbial cellulolysis, but rather pass large quantities of plant material rapidly through the gut [6-9]. Little is known about the molecular mechanism of the formation of herbivory. Grass carp (Ctenopharyngodon idella) is an ecologically appealing model of vertebrate herbivore, widely cultivated in China as well as in many other countries as edible fish or as biological control agents for aquatic weeds. Grass carp goes through a transition from carnivory to herbivory during its life cycle. Grass carp smaller than 3 cm (total length) is carnivorous, fish of 3-5.5 cm (total length) is at the food transition stage from zooplankton or benthos to aquatic macrophytes, whereas fish lager than 5.5 cm (total length) is herbivorous [10]. However, little is currently known about genes determining the food habit transition, and how they could achieve higher growth rates on plant materials, which have a relatively poor nutritional quality. Therefore, grass carp is an excellent model for studying the formation mechanism of herbivory as it shows the food habit transition from carnivory to herbivory. It could facilitate the comparison analysis between carnivory and herbivory in one species, eliminating the differences result from different species.
Grass carp is not able to synthesize cellulase enzyme, and its intestinal microbiota harbors many cellulose-decomposing bacteria [11]. The cellulase enzymes produced by cellulolytic bacteria and fungi are active in a wide range of invertebrate taxa [12,13]. However, relatively few higher vertebrates are able to utilize this resource efficiently [14]. Moreover, our previous study suggests that the cellulase enzyme synthesized by certain microorganism is too limited to digest and absorb crude fiber sufficiently in grass carp, and exogenous cellulase needs to be added to the artificial diets, especially when using plant ingredients [15]. This agrees well with the results that digestion of fiber in grass carp is incomplete, with about half the food material ingested excreted as feces [16]. Therefore, the food habit transition of grass carp might be not attributed to intestinal cellulose-decomposing bacteria, but rather due to high feeding rates. It has been reported that the daily ration (the relation of the total weight of feed taken in a day to the weight of the fish) of grass carp may reach 49.9% when feeding on aquatic plants [17]. Cui et al. [18] also found that grass carp fed with plant diet spend longer time on feeding, have higher feeding intensities, and consume less dry matter per bite than those fed with animal diet. Grass carp fed with plant diet feed almost continuously for most of the diet cycle. In addition, our previous study reported that the gut length relative to body length in grass carp fed with duckweed is higher than those fed with chironomid larvae [19], suggesting that gut growth could be also involved in the food habit transition.
To elucidate the relationship between gene expression and the formation of herbivory, we performed transcriptome sequencing of grass carp before and after the food habit transition from carnivory to herbivory. We showed that expression of genes in several pathways, including cell proliferation and differentiation, appetite control, circadian rhythm, and digestion and metabolism, were significantly different in fish before and after the food transition. These potential determinants provide a glimpse of genetic architecture of the formation of herbivory. Elucidating the genes regulating the unique food habit transition from carnivory to herbivory in grass carp could lead to a better understanding of the mechanism of higher intake and utilization of plant feedstuff in grass carp or other herbivores and formation of herbivore during speciation.
Results
Determination of morphological characteristics
Grass carp fed with duckweed (Group C) had significantly higher relative length of gut (gut length/body length) than fish before food habit transition (Group A) or those without transition (fed with chironomid larvae) (Group B) (Figure 1). Moreover, fish fed with duckweed (Group C) had significantly higher growth than those fed with chironomid larvae (Groups B) (P < 0.05) in terms of total length, body length, gut length, body weight, gut weight (Figure 1).
Figure 1.
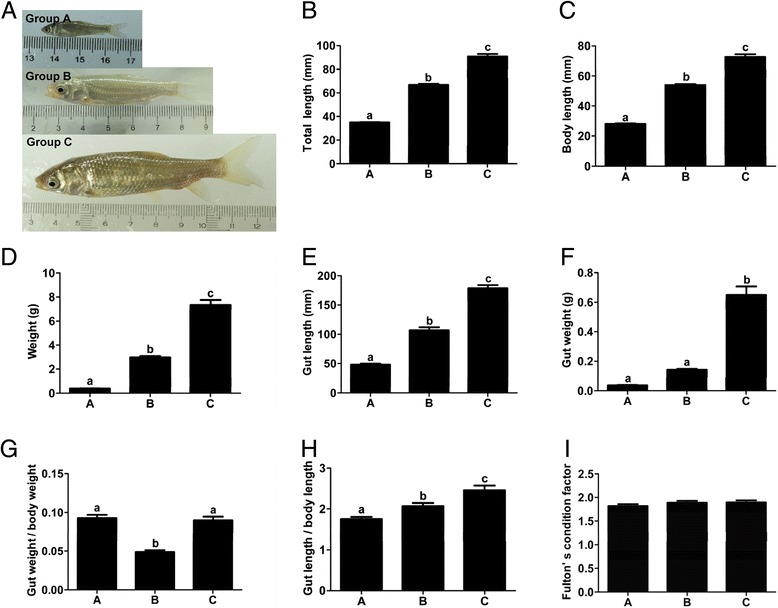
The morphological index of grass carp with differential feeding patterns, including photos of fish (A), total length (B), body length (C), weight (D), gut length (E), gut weight (F), gut weight/body weight (G), gut length/body length (H), Fulton's condition factor (I). Group A: fish fed with chironomid larvae before food habit transition; Group B: fish fed with chironomid larvae without transition; Group C: fish fed with duckweed after food habit transition to herbivory. Total length means the length from the rostral tip of jaw to the caudal tip of the expanded tail, and body length refers to the length from the rostral tip of jaw to the caudal end of last lateral line scale. Data are means ± S.E.M. (n = 6). A value followed by a superscript differs significantly (P < 0.05) from all other values not followed by the same superscript.
High-throughput sequencing and mapping
To obtain an overview of gene expression profile in grass carp before and after food habit transition, cDNA libraries were constructed from brain (AB), liver (AL), gut (AG) of grass carp before the food transition (before the feeding trial (Group A)); brain (BB), liver (BL), gut (BG) of fish without transition (fed with chironomid larvae (Group B)); brain (CB), liver (CL), gut (CG) of fish after food transition (fed with duckweed (Group C)), and sequenced using the Illumina HiSeq2000 system. After removing the low-quality reads, we obtained 64,914,000 (AB), 62,801,686 (AL), 62,679,236 (AG), 66,466,322 (BB), 65,442,204 (BL), 63,096,538 (BG), 62,196,672 (CB), 62,079,494 (CL), 66,338,198 (CG) clean reads, respectively (Table 1). About 66.32-76.59% of clean reads could be mapped to grass carp genome and 30.60-55.24% mapped to gene (Table 1, Additional file 1). Most of the mapped reads were perfect match (46.72-58.74% and 24.07-42.81% of clean reads to genome and genes, respectively). The vast majority of all reads were mapped to unique positions except a very small percentage (1.69-3.09%). To evaluate the quality of the transcriptomes, sequencing randomness assessment was performed to detect the random distribution of reads in reference genes. The distributions of reads from nine samples were homogeneous in grass carp genes, suggesting that the quality of our sequencing data was good. The distributions of genes’ coverage were shown by pie charts in the electronic supplementary material, Additional file 2. A large percentage of genes (26-50%) showed perfect coverage (90-100%). Most of genes’ coverage (65-83%) was higher than 50%. Overall, high quality sequencing and mapping results were obtained. The sequencing data in this study have been deposited in the Short Read Archive (SRA) at the National Center for Biotechnology Information (NCBI) (accession number: SRX532425, SRX532426, SRX532427, SRX532428, SRX532455, SRX532456, SRX532457, SRX532459 and SRX532460).
Table 1.
Summary of data generated from grass carp transcriptome
| Summary | A | B | C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain | Gut | Liver | Brain | Gut | Liver | Brain | Gut | Liver | ||
| Map to genome | Total Reads | 64914000 (100.00%) | 62679236 (100.00%) | 62801686 (100.00%) | 66466322 (100.00%) | 63096538 (100.00%) | 65442204 (100.00%) | 62196672 (100.00%) | 66338198 (100.00%) | 62079494 (100.00%) |
| Total BasePairs | 5842260000 (100.00%) | 5641131240 (100.00%) | 5652151740 (100.00%) | 5981968980 (100.00%) | 5678688420 (100.00%) | 5889798360 (100.00%) | 5597700480 (100.00%) | 5970437820 (100.00%) | 5587154460 (100.00%) | |
| Total Mapped Reads | 49715390 (76.59%) | 42833150 (68.34%) | 42985997 (68.45%) | 50210581 (75.54%) | 43776373 (69.38%) | 43400816 (66.32%) | 47148316 (75.81%) | 45696711 (68.88%) | 43017860 (69.29%) | |
| Perfect match | 37846054 (58.30%) | 31528381 (50.30%) | 30706529 (48.89%) | 37491544 (56.41%) | 31800339 (50.40%) | 30572108 (46.72%) | 36533605 (58.74%) | 33008461 (49.76%) | 31336236 (50.48%) | |
| ≤5 bp mismatch | 11869336 (18.28%) | 11304769 (18.04%) | 12279468 (19.55%) | 12719037 (19.14%) | 11976034 (18.98%) | 12828708 (19.60%) | 10614711 (17.07%) | 12688310 (19.13%) | 11681624 (18.82%) | |
| Unique match | 48342500 (74.47%) | 40897483 (65.25%) | 41471932 (66.04%) | 48691487 (73.26%) | 41959590 (66.50%) | 42291590 (64.62%) | 45938250 (73.86%) | 43731437 (65.92%) | 41795927 (67.33%) | |
| Multi-position match | 1372890 (2.11%) | 1935667 (3.09%) | 1514065 (2.41%) | 1519094 (2.29%) | 1816783 (2.88%) | 1109226 (1.69%) | 1210066 (1.95%) | 1965334 (2.96%) | 1221933 (1.97%) | |
| Total Unmapped Reads | 15198610 (23.41%) | 19846086 (31.66%) | 19815689 (31.55%) | 16255741 (24.46%) | 19320165 (30.62%) | 22041388 (33.68%) | 15048356 (24.19%) | 20641427 (31.12%) | 19061634 (30.71%) | |
| Map to gene | Total Reads | 64914000 (100.00%) | 62679236 (100.00%) | 62801686 (100.00%) | 66466322 (100.00%) | 63096538 (100.00%) | 65442204 (100.00%) | 62196672 (100.00%) | 66338198 (100.00%) | 62079494 (100.00%) |
| Total BasePairs | 5842260000 (100.00%) | 5641131240 (100.00%) | 5652151740 (100.00%) | 5981968980 (100.00%) | 5678688420 (100.00%) | 5889798360 (100.00%) | 5597700480 (100.00%) | 5970437820 (100.00%) | 5587154460 (100.00%) | |
| Total Mapped Reads | 19866103 (30.60%) | 29656785 (47.32%) | 31817288 (50.66%) | 27628167 (41.57%) | 26090271 (41.35%) | 35023773 (53.52%) | 26422927 (42.48%) | 30446461 (45.90%) | 34294871 (55.24%) | |
| Perfect match | 15622780 (24.07%) | 22768765 (36.33%) | 24089872 (38.36%) | 21432825 (32.25%) | 19713788 (31.24%) | 26347072 (40.26%) | 21345319 (34.32%) | 22979918 (34.64%) | 26576843 (42.81%) | |
| ≤4 bp mismatch | 4243323 (6.54%) | 6888020 (10.99%) | 7727416 (12.30%) | 6195342 (9.32%) | 6376483 (10.11%) | 8676701 (13.26%) | 5077608 (8.16%) | 7466543 (11.26%) | 7718028 (12.43%) | |
| Unique match | 19376349 (29.85%) | 28694650 (45.78%) | 30598309 (48.72%) | 26605613 (40.03%) | 25430799 (40.30%) | 34464752 (52.66%) | 25908582 (41.66%) | 29607406 (44.63%) | 33598282 (54.12%) | |
| Multi-position match | 489754 (0.75%) | 962135 (1.54%) | 1218979 (1.94%) | 1022554 (1.54%) | 659472 (1.05%) | 559021 (0.85%) | 514345 (0.83%) | 839055 (1.26%) | 696589 (1.12%) | |
| Total Unmapped Reads | 45047897 (69.40%) | 33022451 (52.68%) | 30984398 (49.34%) | 38838155 (58.43%) | 37006267 (58.65%) | 30418431 (46.48%) | 35773745 (57.52%) | 35891737 (54.10%) | 27784623 (44.76%) | |
A: fish fed with chironomid larvae before food habit transition; B: fish fed with chironomid larvae without transition; C: fish fed with duckweed after food habit transition to herbivory.
Alternative splicing and novel transcript predication
Alternative splicing is essential for protein diversity and functional complexity [20,21]. We examined four major alternative splicing events of each group, including exon skipping, intron retention, alternative 5’ splicing and alternative 3’ splicing. 15,739, 16,380 and 16,826 alternative splicing events were identified in Groups A, B and C, respectively (Additional file 3a). As in other vertebrates, 5’- and 3’- alternative splicing is the major class accounting for about 79.3%, 84.2% and 85.9% (12,480 in Group A, 13,790 in Group B and 14,450 in Group C) of all alternative splicing events in grass carp. Because some genes produced two or more alternative splicing events, a total of 8,859 genes (6,246 in Group A, 6,251 in Group B and 6,327 in Group C) were estimated to undergo alternative splicing (Additional file 3a). We found the number of exon skipping, alternative 5’ splicing and alternative 3’ splicing in Group C was higher than those in Groups A and B, whereas the number of intron retention in Group C was lower than those in Groups A and B (Additional file 3b). We also found 115 food habit transition-specific alternative splicing genes, involved in cell proliferation and differentiation, appetite control, circadian rhythm, mitogen-activated protein kinases (MAPK) signaling, adipocytokine, glutamatergic synapase, calcium signaling, GABAergic synapase, insulin signaling, peroxisome proliferator activated receptors (PPAR) signaling, pancreatic secretion, protein digestion and absorption, bile secretion and gastric acid secretion pathways. We suggested that these genes with alternative splicing might play important roles in the food habit transition of grass carp through regulating diverse pathways (Figure 2).
Figure 2.
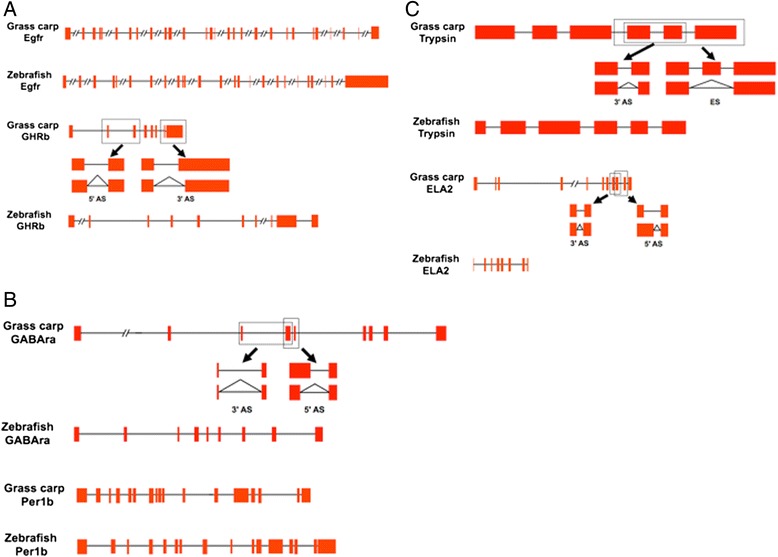
Gene structure and alternative splicing of the most representative differentially expressed genes in cell proliferation and differentiation pathway (A), in appetite control and circadian rhythm pathway (B), in digestion and metabolism pathway (C). The red blocks show the exons. Egfr means epidermal growth factor receptor, GHRb means growth hormone receptor b, ELA2 means elastase 2, GABAra means GABA A receptor, and Per1b means period 1b. ES, 5’AS and 3’AS mean exon skipping, alternative 5’ splicing, and alternative 3’ splicing, respectively.
In addition, novel transcript could be determined by highthroughput sequencing to enrich the present genome database. We predicted 69,520, 43,953, 26,592, 49,109, 41,754, 16,897, 46,457, 36,675 and 18,331 novel transcripts in AB, AG, AL, BB, BG, BL, CB, CG and CL, respectively. Of these about 14.5, 15.6, 11.1, 16.6, 15.4, 13.7, 17.5, 15.1 and 14.4% (10,078 in AB, 6,870 in AG, 2,942 in AL, 8,176 in BB, 6,417 in BG, 2,313 in BL, 8,109 in CB, 5,540 in CG and 2,641 in CL) were longer than 500 bp. In all three tissues, more novel transcripts were identified in Group A than in Groups B and C, suggesting that novel transcripts were developmentally regulated.
Identification of differentially expressed genes
We found 10,184 genes to be differentially expressed between Groups A and C, 8,711 genes between Groups A and B, 4,435 genes between Groups B and C; and 40,149 genes to be differentially expressed between brain and gut, 47,849 genes between brain and liver, 35,434 genes between liver and gut (False Discovery Rate (FDR) ≤ 0.001, fold-change ≥ 2, Additional file 4). Genes differentially expressed between Groups A and C, but not differentially expressed between Groups A and B were potentially involved in the food habit transition of grass carp. We mapped the differentially expressed genes to the reference canonical pathways in Kyoto Encyclopedia of Genes and Genomes (KEGG) to identify the biological pathways. The representative pathways with the differentially expressed genes were MAPK signaling, adipocytokine, glutamatergic synapase, calcium signaling, GABAergic synapase, insulin signaling, PPAR signaling, pancreatic secretion, protein digestion and absorption, bile secretion and gastric acid secretion and mammalian circadian rhythm pathways. Analysis of these genes, which were differentially expressed between Groups A and C, but not differentially expressed between Groups A and B, revealed the signaling pathways involved, including cell proliferation and differentiation (growth hormone receptor b (GHRb), epidermal growth factor receptor (Egfr), fibroblast growth factor (Fgf), FGF-binding protein 1 (Fgfbp1), insulin receptor a (Insra), insulin receptor substrate 2 (Irs2), Janus kinase (Jak), signal transducer and activator of transcription 1 (STAT), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), protein kinase C (PKC), suppressor of cytokine signaling 1 (SOCS1)) (Figure 3); appetite control (agouti gene-related protein 2 (Agrp2), neuropeptide Y receptor 2 (Npy y2), dopamine receptor D1 (Drd1), GABA A receptor (GABAra), leptin (Leptin), cholecystokinin (Cck), insulin receptor a (Insra), insulin receptor substrate 2 (Irs2), thyrotropin-releasing hormone receptor 1 (Trhr1)) (Figure 4); circadian rhythm (period 1 (Per1), Per3, cryptochrome 5 (Cry5), Cry2, clock protein (Clock), Bmal2, hepatic leukemia factor (Pdp), class B basic helix-loop-helix protein (Dec), F-box and leucine-rich repeat protein 3 (Fbxl3), nocturnin) (Figure 5).
Figure 3.
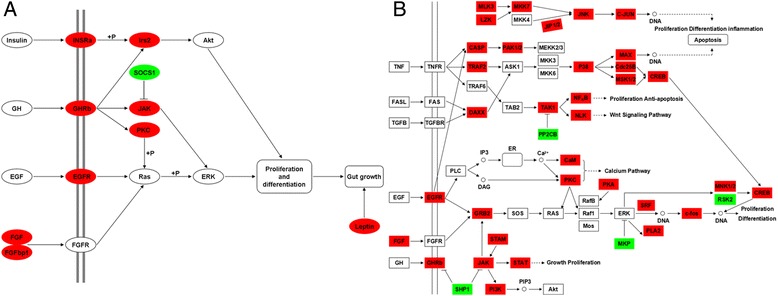
Differentially expressed genes in cell proliferation and differentiation pathway between grass carp before and after food habit transition from transcriptome analysis. The most important pathways are cell proliferation and differentiation (A), MAPK signaling (B). The colors of ellipses or rectangles were shaded according to the different expression (red: the mRNA expression levels of fish in Group C were significantly higher than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1); green: the mRNA expression levels of fish in Group C were significantly lower than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1)). All of these genes were not differentially expressed between Groups A and B.
Figure 4.
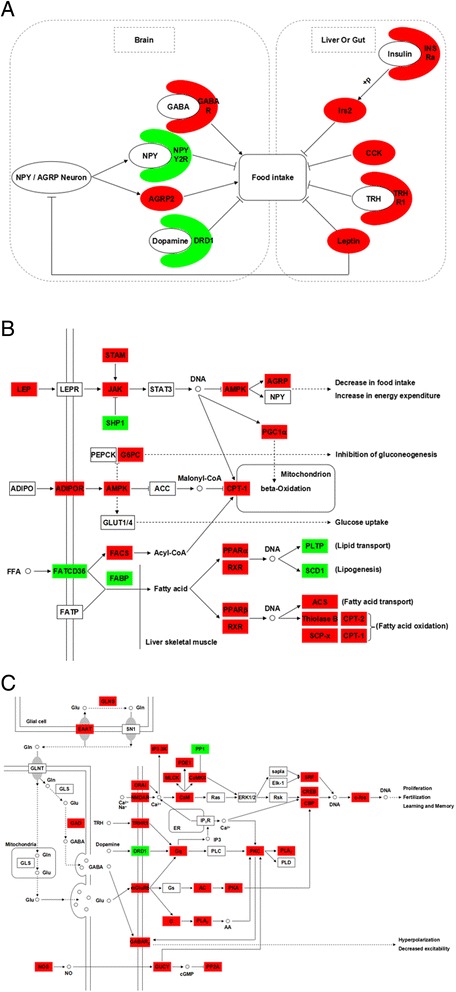
Differentially expressed genes in appetite control pathway between grass carp before and after food habit transition from transcriptome analysis. The most important pathways are appetite control (A), adipocytokine signaling (B), glutamatergic synapase, calcium signaling and GABAergic synapase (C). The colors of ellipses or rectangles were shaded according to the different expression (red: the mRNA expression levels of fish in Group C were significantly higher than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1); green: the mRNA expression levels of fish in Group C were significantly lower than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1)). All of these genes were not differentially expressed between Groups A and B.
Figure 5.
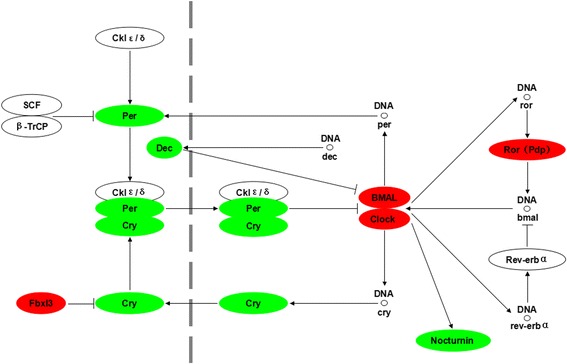
Differentially expressed genes in circadian rhythm pathway between grass carp before and after food habit transition from transcriptome analysis. The colors of ellipses were shaded according to the different expression (red: the mRNA expression levels of fish in Group C were significantly higher than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1); green: the mRNA expression levels of fish in Group C were significantly lower than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1)). All of these genes were not differentially expressed between Groups A and B.
In addition, expression of genes involved in digestion and metabolism were significantly different between fish before and after food habit transition, including increase of hexokinase (GK) and glucose-6-phosphatase (G6PC) involved in glycolysis (Figure 6A); trypsin (PRSS), pancreatic elastase (CELA), carboxypeptidase A (CPA), carboxypeptidase B (CPB), bile salt-stimulated lipase (CEL), secretory phospholipase A2 (PLA2) involved in protein digestion (Figure 6B); solute carrier family 1 member 1 (EAAT3), solute carrier family 1 member 5 (ASCT2), solute carrier family 6 member 19 (B0AT1), solute carrier family 7 member 9 (B0,+AT1), solute carrier family 16 member 10 (TAT1), solute carrier family 7 member 7 (y+LAT1) involved in protein metabolism and absorption (Figure 6C); microsomal epoxide hydrolase (mEH), solute carrier family 22 member 7 (OATs), ATP-binding cassette subfamily B (MDR/TAP) member 11 (BSEP) involved in bile secretion (Figure 6D); solute carrier family 26 member 7 (AE) and H+/K+-exchanging ATPase alpha polypeptide (H/K-ATPase) involved in gastric acid secretion (Figure 6E); carnitine O-palmitoyltransferase 1 (CPT-1), carnitine O-palmitoyltransferase 2 (CPT-2), sterol carrier protein 2 (SCP-x), acetyl-CoA acyltransferase 1 (Thiolase B) and long-chain acyl-CoA synthetase (ACS) involved in fatty acid oxidation and transport (Figure 4B).
Figure 6.
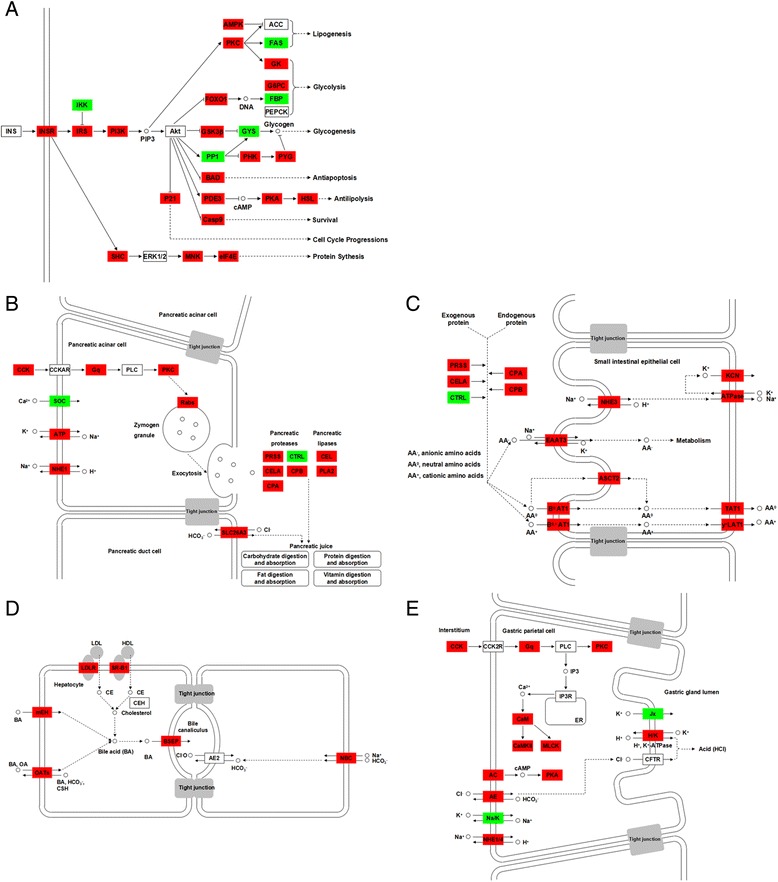
Differentially expressed genes in digestion and metabolism pathway between grass carp before and after food habit transition from transcriptome analysis. The most important pathways are insulin signaling and PPAR signaling (A), pancreatic secretion (B), protein digestion and absorption (C), bile secretion (D), gastric acid secretion (E). The colors of rectangles were shaded according to the different expression (red: the mRNA expression levels of fish in Group C were significantly higher than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1); green: the mRNA expression levels of fish in Group C were significantly lower than those in Group A (FDR ≤ 0.001, the absolute value of log2[Ratio] ≥ 1)). All of these genes were not differentially expressed between Groups A and B.
Furthermore, the importance of the differentially expressed genes indicated above was further supported by the identification of significant alternative splicing in these genes. 56% of these differentially expressed genes had alternative splicing events. We also found that gene structure of these differentially expressed genes in grass carp were different from those in zebrafish, suggesting that the unique gene structure of grass carp might contribute to its unique food habit transition (Figure 2). We used Real-time RT-PCR to confirm the important differential expression genes related to the food habit transition in grass carp. The data obtained were consistent with those obtained from the transcriptome sequencing and DGE analysis (Figure 7).
Figure 7.
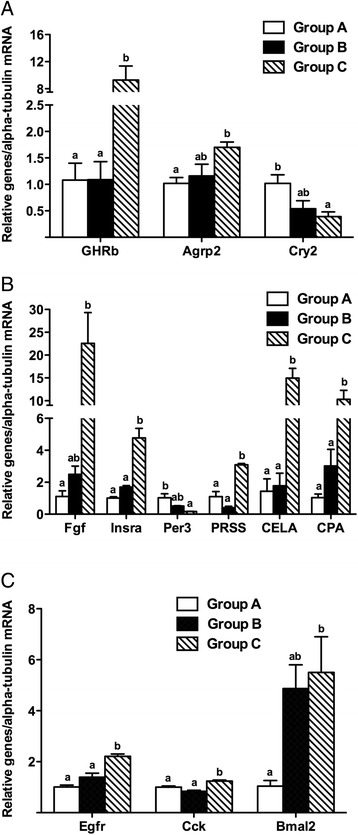
Validation of differentially expressed genes with Real-time RT-PCR. The relative mRNA abundance of differentially expressed genes in brain (A), liver (B) and gut (C) were determined by Real-time RT-PCR. Data are presented as mean ± standard error (n = 4). A value followed by a superscript differs significantly from all other values not followed by the same superscript in the same kind of columns based on one-way analysis of variance (ANOVA) followed by the post hoc test (P < 0.05).
Discussion
Although grass carp is an ecologically appealing model of vertebrate herbivore, little is known about the genes and biological mechanisms of herbivory formation and food habit transition in grass carp. In this study, by profiling the transcriptomes of grass carp before and after food habit transition, we identified differentially expressed genes potentially influencing the food habit transition from carnivory to herbivory, including those affecting cell proliferation and differentiation, appetite control, circadian rhythm, and digestion and metabolism. Real-time RT-PCR confirmed the differential expression in selected genes. Gene structures of several differentially expressed genes in grass carp were different from those in zebrafish. We also found numerous alternative splicing and novel transcript related to the food habit transition of grass carp. These differences together might account for the formation of herbivore, and the higher intake and utilization of plant feedstuff in grass carp.
Gut growth and differentially expressed genes involved in cell proliferation and differentiation, and digestion and metabolism
Fish after food habit transition to herbivory (Group C) had significantly higher relative length of gut than fish before food habit transition (Group A) or those without food habit transition fed with chironomid larvae (Group B). It is suggested that longer gut could enable fish to achieve higher growth rates on plant materials, which have a relatively poor nutritional quality [18]. The digestive system plays an essential role in vertebrate physiology as the site of nutrient digestion and absorption [22]. Previous studies demonstrated that the time of exposure of ingested food to proteolytic enzymes rises with increasing gut length in herbivorous fish, therefore many herbivorous fish have long coiled digestive tract [23]. Furthermore, we observed higher expression of several growth factors, their receptors and downstream signaling molecular involved in cell proliferation and differentiation in Group C, including GHRb, Egfr, Fgf, Fgfbp1, Insra, Irs2, Jak, STAT, PI3K, PKC [24-31]. Previous study has reported that exogenous growth hormone stimulates structural and functional intestinal adaptation in rats [32]. GH receptors are present throughout the human gastrointestinal tract [33] and transgenic mice that overexpressed GH have higher total body weights and heavier small intestines than the control (nontransgenic) mice [34]. The intestinal EGF and EGFR are involved in the processes of gastrointestinal cell proliferation, differentiation, and migration [35]. Fibroblast growth factors have also been implicated in proliferation regulation in the gut [36]. Our results suggested that the up-regulation of these genes in grass carp after food transition might lead to increased cell proliferation and differentiation, contributing to the gut growth, food habit transition from carnivory to herbivory, and increase of intake and utilization of plant feedstuff in grass carp (Figure 3).
In addition, several genes involved in digestion and metabolism were significantly increased in grass carp after food habit transition, including PRSS, CELA, CPA, CPB, CEL, PLA2 involved in protein digestion [37-39]; EAAT3, ASCT2, B0AT1, B0,+AT1, TAT1, y+LAT1 involved in protein metabolism and absorption [40]; mEH, OATs, BSEP involved in bile secretion [41]; AE and H/K-ATPase involved in gastric acid secretion [42]; GK and G6PC involved in glycolysis; CPT-1, CPT-2, SCP-x, Thiolase B and ACS involved in fatty acid oxidation and transport [43] (Figure 6). It is suggested that longer gut could enable fish after the food transition to achieve higher growth rates on plant materials through increased digestion and metabolism, such as better protein digestion with increased PRSS, CELA and CPA expressions, better food digestion with enhanced bile and gastric acid secretion, and better protein absorption with improved amino acid transportation.
Differentially expressed genes involved in appetite control
In the present study, grass carp had free access to food 24 h a day. Fish fed with low nutritional plant diets (Group C) had higher growth than those fed with high nutritional animal diets (Group B), therefore grass carp after food habit transition to herbivory could consume more food per day. Previous studies provide a framework for understanding the regulation of food intake in mammals and fish. Peripheral signals such as leptin from adipocytes, insulin from endocrine pancreas, cholecystokinin and peptide YY from gastrointestinal tract are incorporated in the hypothalamus to generate orexigenic (such as NPY and ghrelin) or anorexigenic (such as α-melanocyte stimulating hormone derived from proopiomelanocortin) signals [44]. We observed higher expression of orexigenic genes (Agrp2, GABAra), and lower expression of anorexigenic genes (Npy y2, Drd1) in brain of grass carp in Group C than those in Group A. Moreover, the expression of anorexigenic genes (Leptin, Cck, Insra, Irs2, Trhr1) were increased in liver or gut of grass carp in Group C compared to those in Group A (Figure 4). These genes are well-established regulators of energy homeostasis and play important roles in determination of food intake [45-49]. The changes in gene expression suggested that grass carp after food habit transition to herbivory had increased appetite signal in brain. This agrees well with the results obtained in grass carp fed with plant food that appears to feed throughout the diet cycle [18]. This foraging strategy may represent an adaptation to herbivory and enable the grass carp to achieve high growth rates on plant materials.
CCK is released from the duodenum in response to the presence of digested food [50], potentially explaining the increased expression in fish after food habit transition. In addition, leptin appears to be a growth factor for normal small intestine [51], and the increased expression of Leptin in liver might stimulate the gut growth of grass carp after food transition. Herbivorous fish consume more food per day and have much longer gut than carnivorous and omnivorous fish [52,53]. Our previous study on food preference of mandarin fish, a piscivore, showed that dead prey fish feeders have decreased appetite [54], suggesting that the appetite control pathway plays an important role in food habit formation of fish. In addition, several genes involved in glutamategic synapase, calcium signaling and GABAergic synapase pathway, such as EAAT, GAD, NMDAR, PKC, PLA2, mGluR5, PKA and NOS [55,56], were increased in grass carp after food habit transition, which might contribute to its higher appetite.
Differentially expressed genes involved in circadian rhythm
Previous studies demonstrated that the molecular mechanisms of circadian rhythm generation in zebrafish appear to be generally consistent with the mammalian model [57]. We identified homologs of the mammalian clock genes in grass carp. We found differential expression in several clock genes, including Per1, Per3, Cry, Clock, Bmal2, Pdp, Dec, Fbxl3, nocturnin between fish before and after food transition (Figure 5). These genes are known to be critical regulators of circadian rhythm [58-63], with the heterodimerization of CLOCK and BMAL1 proteins activating transcription of Period and Cryptochrome genes. The PER and CRY proteins form complexes that enter the nucleus, bind to the CLOCK:BMAL1 complex and inhibit transcription. The disruption of these genes could cause the reset of behavioral rhythmicity. Grass carp fed with plant diet spent longer time on feeding, and feed almost continuously for most of the diet cycle [18]. Taken together, changes in expression levels of these clock genes in grass carp might reset circadian phase of feeding to accommodate the food habit transition from carnivory to herbivory, because fish fed with plant diet consumes less dry matter per bite than those fed with animal diet. This result agrees with our previous research in mandarin fish, with the acquisition of novel food preference (dead prey fish) partly due to resetting of circadian phase [54].
Conclusions
In summary, our results showed that grass carp after food habit transition from carnivory to herbivory had higher relative length of gut than those before transition and without transition. The food habit transition in grass carp might be due to enhanced gut growth, increased appetite, resetting of circadian phase and enhanced digestion and metabolism. Interaction of expression and alternative splicing in genes related to cell proliferation and differentiation, appetite control, circadian rhythm outputs, and digestion and metabolism might drive the formation of herbivory in grass carp.
Methods
Fish and sample preparation
The embryos of grass carp were obtained from Wuhan Academy of Agricultural Science and Technology (Wuhan, Hubei Province, China). Larvae were raised in tanks at 25 ± 2°C and fed with chironomid larvae Chironomus tentans. At 46 days post-hatch (dph) (body weight 0.39 ± 0.05 g, body length 28.05 ± 0.99 mm), 30 fish were randomly selected for sample collection as fish before food habit transition (Group A). And then the rest of the fish were randomly divided into two groups (n = 1000 for each group) fed with either chironomid larvae Chironomus tentans as fish without transition (Group B) or duckweed Lemna minor as fish after food habit transition to herbivory (Group C). Fish had free access to food 24 h a day and fed for 70 days. At 116 dph (body weight and body length for Group B were 2.97 ± 0.3 g and 53.96 ± 1.80 mm, respectively; those for Group C were 7.34 ± 1.43 g and 72.78 ± 6.15 mm, respectively), 30 fish were randomly selected from each group for sample collection. Total RNA was isolated from brain, liver and gut tissues using SV TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer's protocol. Equal amount of total RNA of each group were pooled for each tissue and used to construct the libraries for transcriptome analysis. The following formula were used to calculate three ratios: the ratio of gut length to body length (gut length/body length), the ratio of gut weight to body weight (gut weight/body weight) and the ratio of hepatopancreas weight to body weight (hepatopancreas weight/body weight) [23,64]. The animal protocol was approved by the Ethical Committee of Huazhong Agricultural University.
Transcriptome library preparation and Illumina sequencing
The samples for transcriptome analysis were prepared using Illumina's kit following manufacturer's instructions (San Diego, CA, USA). Poly(A) mRNA was purified from total RNA using oligo-dT-attached magnetic beads. Paired-end cDNA libraries were sequenced using Illumina HiSeq2000 system. Image deconvolution and base calling were performed with the Illumina CASAVA v1.7. The reliability of the reads was 89.1% with average length of the reads at 90 bp. Clean reads were obtained by removing adaptor reads and low quality reads (Q ≤ 5), on which all following analysis are based. The library construction and sequencing were performed by Beijing Genomics Institute at Shenzhen (Shenzhen, China). To estimate expression levels and discover novel genes and transcripts, the RNA-Seq reads generated were mapped to the grass carp genome using the Short Oligonucleotide Analysis Package SOAPaligner/soap2 [65], up to five base mismatches were allowed in the genome alignment while up to two base mismatches were allowed in gene alignment. The reference genome and annotation data of grass carp were obtained from the State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, China.
Alternative splicing and novel transcript predication
To identify all potential splice sites, we searched for three types of splice of site (Class I: GT-AG/CT-AC; Class II: GC-AG/CT-GC; and Class III: AT-AC/GT-AT) in the intronic regions. Alternative splicing events were classified into four basic types: exon skipping, intron retention, alternative 5’ splice site, alternative 3’ splice site. SOAPsplice [66] (with all default parameters) was used to detect the splice junction sites which give information about boundaries and combinations of different exons in a transcript. Then all splice junction sites of the same gene are used to distinguish type of its alternative splicing event. To detect novel genes in the putative intergenic region, we compared the reference gene models and the transcriptome, the potential gene models found in intergenic regions (200 bp away from upstream or downstream of genes) with lengths > 150 bp and average coverage > 2 were considered to be candidate of novel transcript.
Identification of differentially expressed genes
Gene expression levels were measured through short reads mapping in Reads Per Kb per Million reads (RPKM) [67]. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were then carried out in differentially expressed genes. To annotate the differentially expressed genes, we performed the BLASTx alignment (e-value < 0.00001) against protein databases such as NCBI, Swiss-Prot, KEGG and COG. SYBR Green Real-time RT-PCR was performed to validate the transcriptome data (Additional file 5). Alpha-tubulin was amplified in parallel as an internal control. There were four biological and three technical replicates respectively.
Statistical analysis
We used FDR ≤ 0.001 and the absolute value of log2[Ratio] ≥ 1 as the threshold to judge the significance of gene expression difference. Statistical analysis was performed with SPSS13.0 software. Data normality and homogeneity of variances were analyzed. Results were presented as the means ± S.E. for each group. One-way analysis of variance (ANOVA) followed by the post hoc test were carried out to determine whether the differences between groups were significant (P < 0.05).
Availability of supporting data
All the supporting data are included as additional files.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program, No. 2014CB138601), the National Natural Science Foundation of China (No. 31272641, 31172420), the National High Technology Research and Development Program (863 Program, No. 2011AA100403), and the Fundamental Research Funds for the Central Universities (No. 2010PY010, 2011PY030).
Additional files
Distribution statistics of reads mapped to reference genes. AB, AL and AG indicate brain, liver and gut in Group A, respectively; BB, BL and BG indicate brain, liver and gut in Group B, respectively; CB, CL and CG indicate brain, liver and gut in Group C, respectively.
Distribution statistics of genes’ coverage. AB, AL and AG indicate brain, liver and gut in Group A, respectively; BB, BL and BG indicate brain, liver and gut in Group B, respectively; CB, CL and CG indicate brain, liver and gut in Group C, respectively. Gene coverage is the percentage of a gene covered by reads. The value equals to ratio of the number of bases in a gene covered by unique mapping reads to number of total bases in that gene.
Alternative splicing prediction. (A) Numbers of alternative splicing events and involved genes in the three groups. (B) Numbers of four major alternative splicing events and involved genes in the three groups. The x-axis represents types of alternative splicing events (AS Event). A: fish fed with chironomid larvae before food habit transition; B: fish fed with chironomid larvae without transition; C: fish fed with duckweed after food habit transition to herbivory.
Differentially expressed genes (DEG) analyzed by transcriptome sequencing. AB, AL and AG indicate brain, liver and gut in Group A, respectively; BB, BL and BG indicate brain, liver and gut in Group B, respectively; CB, CL and CG indicate brain, liver and gut in Group C, respectively. The superscripts of each column represent the number of differentially expressed genes between groups.
Primer sequences for Real-time RT-PCR.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SH, LL, JS and ZYW contributed to the fish and sample preparation. SH, LL, XYC, AXL, YHH, WJC and XCY performed the assembly, bioinformatical analysis and the RT-PCR experiments. XFL and YPW gave technical advice and contributed to the study design. SH, XFL and YXT co-wrote the paper. All authors read and approved the final manuscript.
Contributor Information
Shan He, Email: laile1985@163.com.
Xu-Fang Liang, Email: xufang_liang@hotmail.com.
Ling Li, Email: 20584917@qq.com.
Jian Sun, Email: 378340487@qq.com.
Zheng-Yong Wen, Email: 761612083@qq.com.
Xiao-Yan Cheng, Email: 916005218@qq.com.
Ai-Xuan Li, Email: 147309817@qq.com.
Wen-Jing Cai, Email: 332756006@qq.com.
Yu-Hui He, Email: 328449359@qq.com.
Ya-Ping Wang, Email: wangyp@ihb.ac.cn.
Ya-Xiong Tao, Email: taoyaxi@auburn.edu.
Xiao-Chen Yuan, Email: 554962964@qq.com.
References
- 1.Van Soest PJ. Nutritional Ecology of the Ruminant. 2. Ithaca, NY: Cornell University Press; 1994. [Google Scholar]
- 2.Bryant MP. Introduction to gastrointestinal microbial ecology. In: Mackie RI, White BA, editors. Gastrointestinal Microbiology. Vol. 1: Gastrointestinal Ecosystems and Fermentations. New York: Chapman and Hall; 1997. pp. 3–12. [Google Scholar]
- 3.Mackie RI. Gut environment and evolution of mutualistic fermentative digestion. In: Mackie RI, White BA, editors. Gastrointestinal Microbiology. Vol. 1: Gastrointestinal Ecosystems and Fermentations. New York: Chapman and Hall; 1997. pp. 156–98. [Google Scholar]
- 4.Sullam KE, Essinger SD, Lozupone CA, O’connor MP, Rosen GL, Knight R. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol Ecol. 2012;21:3363–78. doi: 10.1111/j.1365-294X.2012.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choat JH, Clements KD. Vertebrate herbivores in marine and terrestrial environments: a nutritional ecology perspective. Annu Rev Ecol System. 1998;29:375–403. doi: 10.1146/annurev.ecolsys.29.1.375. [DOI] [Google Scholar]
- 6.Hickling CF. On the feeding process in the White Amur, Ctenopharyngodon idella. J Zool. 1966;148:408–19. doi: 10.1111/j.1469-7998.1966.tb02960.x. [DOI] [Google Scholar]
- 7.Van Dyke JM, Sutton DL. Digestion of duckweed (Lemna spp.) by the grass carp (Ctenopharyngodon idella) J Fish Biol. 1977;11:273–8. doi: 10.1111/j.1095-8649.1977.tb04120.x. [DOI] [Google Scholar]
- 8.Lindsay GJH, Harris JE. Carboxymethylcellulase activity and the digestive tracts of fish. J Fish Biol. 1980;16:219–33. doi: 10.1111/j.1095-8649.1980.tb03700.x. [DOI] [Google Scholar]
- 9.Lesel R, Fromageot C, Lesel M. Cellulose digestibility in grass carp, Ctenopharyngodon idella and in goldfish, Carassius auratus. Aquaculture. 1986;54:11–7. doi: 10.1016/0044-8486(86)90249-8. [DOI] [Google Scholar]
- 10.Watkins CE, Shireman JV, Rottmann RW, Colle DE. Food habits of fingerling grass carp. Prog Fish-Culturist. 1981;43:95–7. doi: 10.1577/1548-8659(1981)43[95:FHOFGC]2.0.CO;2. [DOI] [Google Scholar]
- 11.Wu S, Wang G, Angert ER, Wang W, Li W, Zou H. Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS ONE. 2012;7:e30440. doi: 10.1371/journal.pone.0030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin MM. Cellulose digestion in insects. Comp Biochem Physiol. 1983;75:313–24. doi: 10.1016/0300-9629(83)90088-9. [DOI] [Google Scholar]
- 13.Zinkler D, Gotze M. Cellulose digestion by the firebrat Thermobia domestica. Comp Biochem Physiol B. 1987;88:661–6. [Google Scholar]
- 14.Goodenough S, Goodenough P. Who needs cellulase? J Biol Educ. 1993;27:97–102. doi: 10.1080/00219266.1993.9655313. [DOI] [Google Scholar]
- 15.Zhou Y, Yuan X, Liang XF, Fang L, Li J, Guo X, Bai X, He S. Enhancement of growth and intestinal flora in grass carp: the effect of exogenous cellulase. Aquaculture. 2013;416–417:1–7. doi: 10.1016/j.aquaculture.2013.08.023. [DOI] [Google Scholar]
- 16.Ni DS, Wang JG. Biology and Diseases of Grass Carp. Beijing, China: Science Press; 1999. [Google Scholar]
- 17.Li SF, Yang HQ, Lu WM. Preliminary research on diurnal feeding rhythm and the daily ration for silver carp, bighead carp and grass carp. J Fish China. 1980;4:275–83. [Google Scholar]
- 18.Cui YB, Chen SL, Wang SM, Liu XF. Laboratory observations on the circadian feeding patterns in the grass carp (Ctenopharyngodon idella Val.) fed three different diets. Aquaculture. 1993;113:57–64. doi: 10.1016/0044-8486(93)90340-5. [DOI] [Google Scholar]
- 19.He S, Liang XF, Li L, Sun J, Shen D. Differential gut growth, gene expression and digestive enzyme activities in young grass carp (Ctenopharyngodon idella) fed with plant and animal diets. Aquaculture. 2013;410:18–24. doi: 10.1016/j.aquaculture.2013.06.015. [DOI] [Google Scholar]
- 20.Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002;30:13–9. doi: 10.1038/ng0102-13. [DOI] [PubMed] [Google Scholar]
- 21.McGuire AM, Pearson MD, Neafsey DE, Galagan JE. Cross-kingdom patterns of alternative splicing and splice recognition. Genome Biol. 2008;9:R50. doi: 10.1186/gb-2008-9-3-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson LR. Physiology of the Gastrointestinal Tract. New York: Raven; 1994. [Google Scholar]
- 23.Hofer R, Schiemer F. Proteolytic activity in the digestive tract of several species of fish with different feeding habits. Oecologia. 1981;48:342–5. doi: 10.1007/BF00346492. [DOI] [PubMed] [Google Scholar]
- 24.Sotiropoulos A, Perrot-Applanat M, Dinerstein H, Pallier A, Postel-Vinay MC, Finidori J, Kelly PA. Distinct cytoplasmic regions of the growth hormone receptor are required for activation of JAK2, mitogen-activated protein kinase, and transcription. Endocrinology. 1994;135:1292–8. doi: 10.1210/endo.135.4.7925092. [DOI] [PubMed] [Google Scholar]
- 25.Argetsinger LS, Hsu GW, Myers MG, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem. 1995;270:14685–92. doi: 10.1074/jbc.270.24.14685. [DOI] [PubMed] [Google Scholar]
- 26.Ridderstrale M, Degerman E, Tornqvist H. Growth hormone stimulates the tyrosine phosphorylation of the insulin receptor substrate-1 and its association with phosphatidylinositol 3-kinase in primary adipocytes. J Biol Chem. 1995;270:3471–4. doi: 10.1074/jbc.270.8.3471. [DOI] [PubMed] [Google Scholar]
- 27.Argetsinger LS, Carter-Su C. Mechanism of signaling by growth hormone receptor. Physiol Rev. 1996;76:1089–107. doi: 10.1152/physrev.1996.76.4.1089. [DOI] [PubMed] [Google Scholar]
- 28.Playford RJ, Marchbank T, Mandir N, Higham A, Meeran K, Ghatei MA, Bloom SR, Goodlad RA. Effects of keratinocyte growth factor (KGF) on gut growth and repair. J Pathol. 1998;184:316–22. doi: 10.1002/(SICI)1096-9896(199803)184:3<316::AID-PATH3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, Wellstein A. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem. 2001;276:40247–53. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]
- 30.Brubaker PL, Drucker DJ. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–9. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 31.Washizawa N, Gu LH, Gu L, Openo KP, Jones DP, Ziegler TR. Comparative effects of glucagon-like peptide-2 (GLP-2), growth hormone (GH), and keratinocyte growth factor (KGF) on markers of gut adaptation after massive small bowel resection in rats. JPEN-Parenter Enter. 2004;28:399–409. doi: 10.1177/0148607104028006399. [DOI] [PubMed] [Google Scholar]
- 32.Shulman DI, Hu CS, Duckett G, Lavallee-Grey M. Effects of short-term growth hormone therapy in rats undergoing 75% small intestinal resection. J Pediatr Gastroenterol Nutr. 1992;14:3–11. doi: 10.1097/00005176-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Delehaye-Zervas MC, Mertani H, Martini JF, Nihoul-Fekete C, Morel G, Postel-Vinay MC. Expression of the growth hormone receptor gene in human digestive tissues. J Clin Endocrinol Meta. 1994;78:1473–80. doi: 10.1210/jcem.78.6.8200952. [DOI] [PubMed] [Google Scholar]
- 34.Ulshen MH, Dowling RH, Fuller CR, Zimmermann EM, Lund PK. Enhanced growth of small bowel in transgenic mice overexpressing bovine growth hormone. Gastroenterology. 1993;104:973–80. doi: 10.1016/0016-5085(93)90263-c. [DOI] [PubMed] [Google Scholar]
- 35.Pillai SB, Hinman CE, Luquette MH, Nowicki PT, Besner GE. Heparin-binding epidermal growth factor-like growth factor protects rat intestine from ischemia/reperfusion injury. J Surg Res. 1999;87:225–31. doi: 10.1006/jsre.1999.5764. [DOI] [PubMed] [Google Scholar]
- 36.Cove FL, Evans GS. The use of an intestinal epithelial cellline as a biological assay system for growth factor activity. Biochem Soc Trans. 1992;20:175S. doi: 10.1042/bst020175s. [DOI] [PubMed] [Google Scholar]
- 37.Hernell O, Olivecrona T. Human milk lipases. II. Bile salt-stimulated lipase. Biochim Biophys Acta. 1974;369:234–44. doi: 10.1016/0005-2760(74)90254-9. [DOI] [PubMed] [Google Scholar]
- 38.Hamosh M, Hamosh P. Development of digestive enzyme secretion. Development of the gastrointestinal tract. Sanderson IR, Walker WA, Decker Hamilton, Ontario, Chap. 16. 1999. p. 261-277.
- 39.Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Digest Dis Sci. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 40.Verrey F, Singer D, Ramadan T, Vuille-dit-Bille RN, Mariotta L, Camargo SMR. Kidney amino acid transport. Pflugers Arch-Eur J Physiol. 2009;458:53–60. doi: 10.1007/s00424-009-0638-2. [DOI] [PubMed] [Google Scholar]
- 41.Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med. 2003;9:558–64. doi: 10.1016/j.molmed.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Hersey SJ, Sachs G. Gastric acid secretion. Physiol Rev. 1995;75:155–89. doi: 10.1152/physrev.1995.75.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Reddy JK, Hashimoto T. Peroxisomal-oxidation and peroxisome proliferator-activated receptor: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 44.Tao YX, Yuan ZH, Xie J. G protein-coupled receptors as regulators of energy homeostasis. Prog Mol Biol Transl Sci. 2013;114:1–43. doi: 10.1016/B978-0-12-386933-3.00001-7. [DOI] [PubMed] [Google Scholar]
- 45.Morley JE. Appetite regulation by gut peptides. Annu Rev Nutr. 1990;10:383–95. doi: 10.1146/annurev.nu.10.070190.002123. [DOI] [PubMed] [Google Scholar]
- 46.Terry P, Gilbert DB, Cooper SJ. Dopamine receptor subtype agonists and feeding behavior. Obesity Res. 1995;3:515S–23. doi: 10.1002/j.1550-8528.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 47.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–16. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos T Roy Soc B. 2006;361:1187–209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilding JP. Neuropeptides and appetite control. Diabetic Med. 2002;19:619–27. doi: 10.1046/j.1464-5491.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 51.Alavi K, Schwartz MZ, Prasad R, O'connor D, Funanage V. Leptin: a new growth factor for the small intestine. J Pediatr Surg. 2002;37:327–30. doi: 10.1053/jpsu.2002.30805. [DOI] [PubMed] [Google Scholar]
- 52.Al-Hussaini AH. On the functional morphology of the alimentary tract of some fish in relation to differences in their feeding habits: cytology and physiology. Q J Microsco Sci. 1949;s3-90:323–54. [PubMed] [Google Scholar]
- 53.Kapoor BG, Smit H, Verighina IA. The alimentary canal and digestion in teleosts. Adv Mar Biol. 1976;13:109–239. doi: 10.1016/S0065-2881(08)60281-3. [DOI] [Google Scholar]
- 54.He S, Liang XF, Sun J, Li L, Yu Y, Huang W, Qu CM, Cao L, Bai XL, Tao YX. Insights into food preference in hybrid F1 of Siniperca chuatsi (♀) × Siniperca scherzeri (♂) mandarin fish through transcriptome analysis. BMC Genomics. 2013;14:601. doi: 10.1186/1471-2164-14-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Newton DC, Marsden PA. Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol. 1999;13:21–43. doi: 10.1615/critrevneurobiol.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- 56.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Rev. 2004;45:250–65. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Cahill GM. Clock mechanisms in zebrafish. Cell Tissue Res. 2002;309:27–34. doi: 10.1007/s00441-002-0570-7. [DOI] [PubMed] [Google Scholar]
- 58.Falvey E, Fleury-Olela F, Schibler U. The rat hepatic leukemia factor (HLF) gene encodes two transcriptional activators with distinct circadian rhythms, tissue distributions and target preferences. EMBO J. 1995;14:4307–17. doi: 10.1002/j.1460-2075.1995.tb00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–10. doi: 10.1016/S0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 60.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 61.Green CB, Douris N, Kojima S, Strayer CA, Fogerty J, Lourim D, Keller SR, Besharse JC. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc Natl Acad Sci U S A. 2007;104:9888–93. doi: 10.1073/pnas.0702448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–4. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 63.Shi J, Wittke-Thompson JK, Badner JA, Hattori E, Potash JB, Willour VL, McMahon FJ, Gershon ES, Liu C. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1047–55. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hidalgo MC, Urea E, Sanz A. Comparative study of digestive enzymes in fish with different nutritional habits: proteolytic and amylase activities. Aquaculture. 1999;170:267–83. doi: 10.1016/S0044-8486(98)00413-X. [DOI] [Google Scholar]
- 65.Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25:1966–7. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- 66.Huang S, Zhang J, Li R, Zhang W, He Z, Lam TW, Peng Z, Yiu SM. SOAPsplice: genome-wide ab initio detection of splice junctions from RNA-Seq data. Front Genet. 2011;2:46. doi: 10.3389/fgene.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]


