Abstract
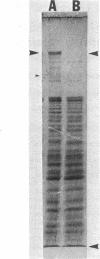
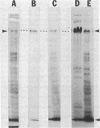
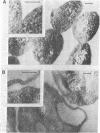
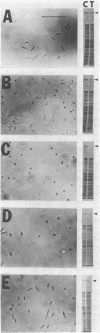
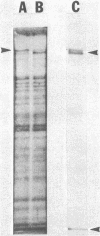
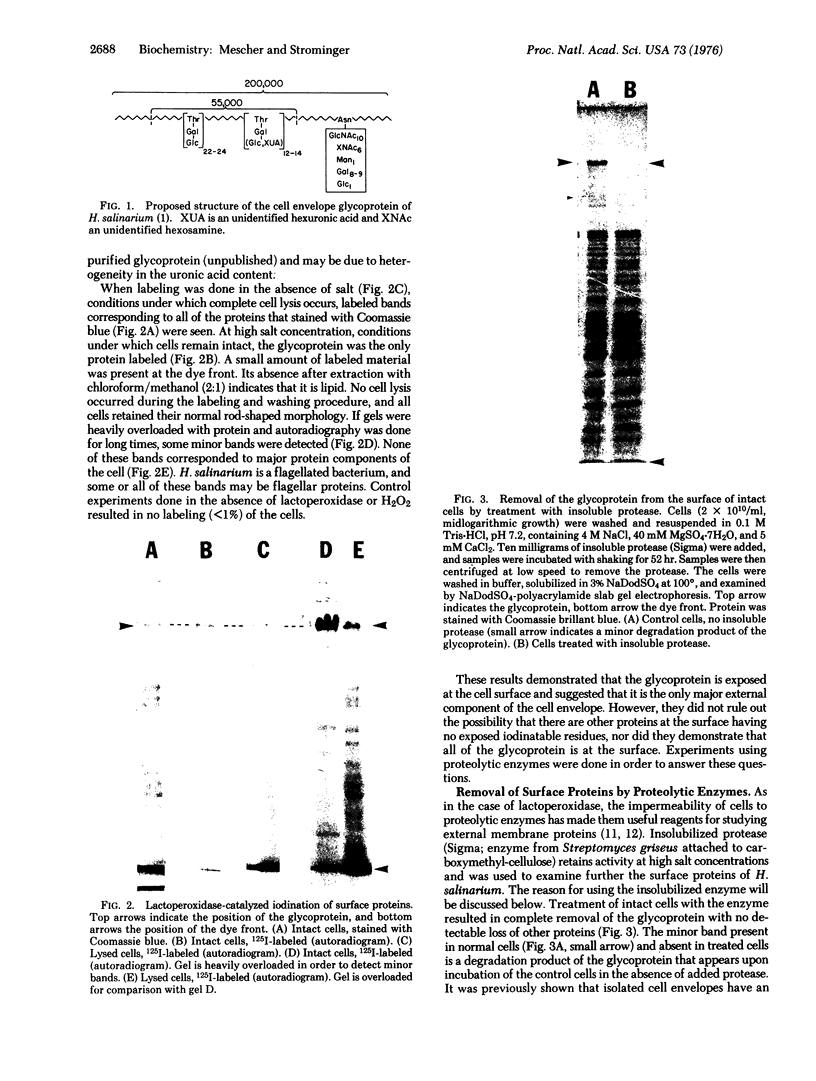
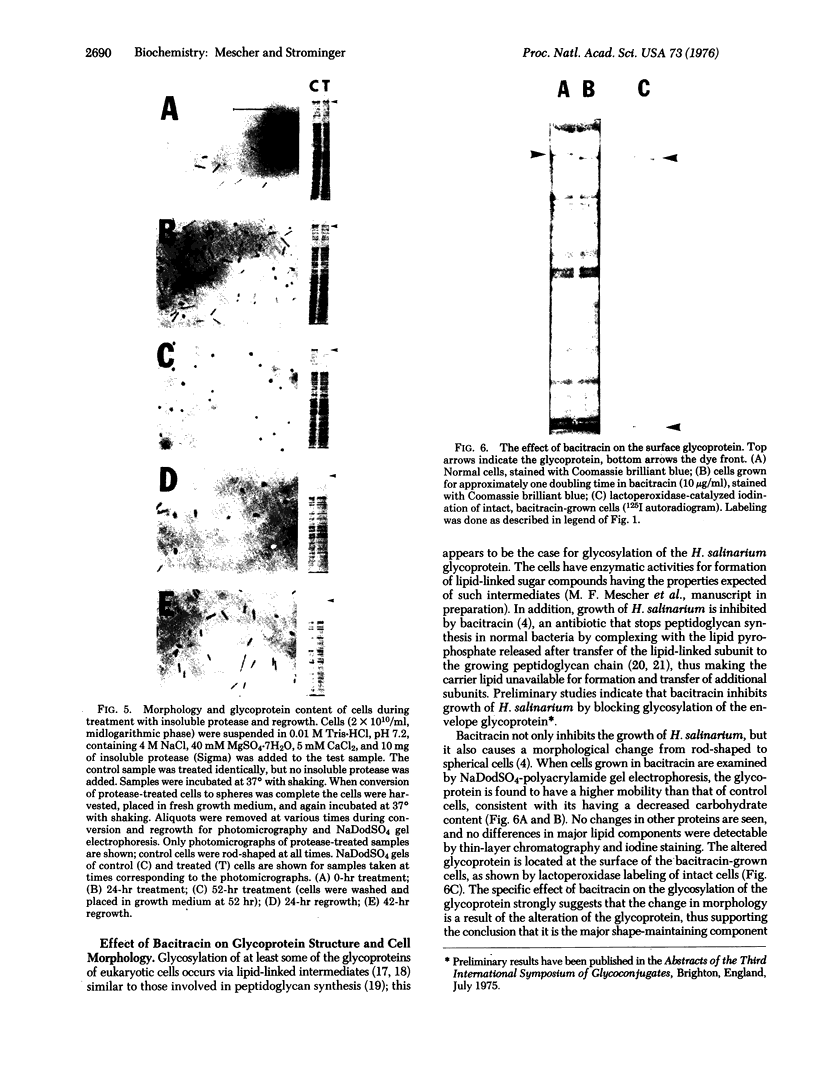
The obligate halophile, Halobacterium salinarium, maintains a rod-shaped morphology under normal growth conditions. Lactoperoxidase(EC 1.11.1.7;donor:hydrogen-peroxide oxidoreductase)-catalyzed iodination and treatment with proteolytic enzymes were used to demonstrate that the recently described envelope glycoprotein (Mescher, M.F. & Strominger, J.L. (1976) J. Biol. Chem. 251, 2005-2014) is the only major cell surface component of this organism. The morphological changes that accompany alteration of the structure of the glycoprotein by growth in the presence of bacitracin or its removal with proteolytic enzymes strongly suggest that it forms a rigid matrix at the cell surface and is responsible for maintenance of the characteristic rod shape.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN A. D. THE PERIPHERAL STRUCTURES OF GRAM-NEGATIVE BACTERIA.IV. THE CATION-SENSITIVE DISSOLUTION OF THE CELL MEMBRANE OF THE HALOPHILIC BACTERIUM, HALOBACTERIUM HALOBIUM. Biochim Biophys Acta. 1963 Nov 29;75:425–435. doi: 10.1016/0006-3002(63)90630-9. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Cho K. Y., Doy C. H., Mercer E. H. Ultrastructure of the obligate halophilic bacterium Halobacterium halobium. J Bacteriol. 1967 Jul;94(1):196–201. doi: 10.1128/jb.94.1.196-201.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Henning U. L. Determination of cell shape in bacteria. Annu Rev Microbiol. 1975;29:45–60. doi: 10.1146/annurev.mi.29.100175.000401. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J. Lipid linked sugars in glycoprotein synthesis. Science. 1975 Jun 6;188(4192):986–991. doi: 10.1126/science.167438. [DOI] [PubMed] [Google Scholar]
- Marshall C. L., Wicken A. J., Brown A. D. The outer layer of the cell envelope of Halobacterium halobium. Can J Biochem. 1969 Jan;47(1):71–74. doi: 10.1139/o69-013. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Bacitracin induces sphere formation in Halobacterium species which lack a wall peptidoglycan. J Gen Microbiol. 1975 Aug;89(2):375–378. doi: 10.1099/00221287-89-2-375. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L., Watson S. W. Protein and carbohydrate composition of the cell envelope of Halobacterium salinarium. J Bacteriol. 1974 Nov;120(2):945–954. doi: 10.1128/jb.120.2.945-954.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland H., Larsen H. A study of the cell envelope of the halobacteria. J Gen Microbiol. 1969 Mar;55(3):325–336. doi: 10.1099/00221287-55-3-325. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Rowen R. A morphological study of Halobacterium halobium and its lysis in media of low salt concentration. J Cell Biol. 1967 Jul;34(1):365–393. doi: 10.1083/jcb.34.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R., Strominger J. L. Complex formation between bacitracin peptides and isoprenyl pyrophosphates. The specificity of lipid-peptide interactions. J Biol Chem. 1973 Jun 10;248(11):3940–3945. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Staining of protein zones after isoelectric focusing in polyacrylamide gels. Biochim Biophys Acta. 1971 Aug 27;243(2):345–348. doi: 10.1016/0005-2795(71)90094-8. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Colley C. M. Localization of red cell membrane constituents. Biochim Biophys Acta. 1973 Sep 10;300(2):159–182. doi: 10.1016/0304-4157(73)90003-8. [DOI] [PubMed] [Google Scholar]