Abstract
Significance: Hydrogen sulfide (H2S), once associated with rotten eggs and sewers, is now recognized as a gasotransmitter that is synthesized in vivo in a regulated fashion. This ancient gaseous molecule has been retained throughout evolution to perform various roles in different life forms. H2S modulates important signaling functions in diverse cellular processes ranging from regulation of blood pressure to redox homeostasis. Recent Advances: One of the modes by which H2S signals is by post-translational modification of reactive cysteine residues in a process designated as sulfhydration, resulting in conversion of the -SH groups of target cysteine residues to -SSH. Using the modified biotin-switch assay and a fluorescent maleimide-based analysis, sulfhydration of several proteins has been detected in various cell types. Aberrant sulfhydration patterns occur in neurodegenerative conditions such as Parkinson's disease. Critical Issues: The exact concentration, source of H2S, and conditions under which various stores of H2S are utilized have not been fully elucidated. Currently, available inhibitors of the biosynthetic enzymes of H2S lack sufficient specificity to shed light on detailed mechanisms of H2S action. Probes with a higher sensitivity that can reliably detect cellular and tissue H2S levels are yet to be developed. Future Directions: Availability of advanced probes and biosynthesis inhibitors would help in the measurement of real-time changes of endogenous H2S levels in an in vivo context. The study of the dynamics of sulfhydration and nitrosylation of critical cysteine residues of regulatory proteins involved in physiology and pathophysiology is an area of interest for the future. Antioxid. Redox Signal. 22, 411–423.
Introduction
Hydrogen sulfide (H2S), once infamous for its toxic effects, is now recognized as a physiological messenger molecule akin to the gasotransmitters, nitric oxide (NO) and carbon monoxide (CO). Although H2S was known to be present in living tissues, it was only recently that the biosynthetic pathways leading to its production were identified. Three enzymes, cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST), synthesize H2S from cysteine in vivo (69, 91, 94). CBS is the major generator of H2S in the brain, whereas CSE generates H2S in peripheral tissues, although both enzymes are present in the central nervous system as well as in peripheral tissues. 3-MST, the third enzyme involved in H2S production, also functions in the brain (82). Recently, a fourth pathway has been reported to generate H2S employing D-cysteine as a substrate along with the enzymes D-amino-acid oxidase and 3-MST (80, 81). Intestinal flora also form H2S, which influences the physiology of both the host and resident microbes (12, 74). H2S can also be released from stores in vivo. Two major stores, capable of releasing H2S, include the acid labile pool, which consists of proteins with iron-sulfur clusters, and the sulfane sulfur pool, which operates in the presence of reductants (40). One of the modes by which H2S functions as a messenger molecule is by sulfhydration of reactive cysteine residues of target proteins in a manner analogous to nitrosylation by proteins (60, 61, 69). In this article, we discuss the modes of signaling by H2S with a focus on sulfhydration and its effects on diverse signaling pathways.
H2S in the Cardiovascular System
New insights were brought to the nascent field of H2S biology by the generation of CSE knockout mice, which provided definitive evidence that H2S physiologically dilates blood vessels and plays a central role in the regulation of blood pressure (101). Mice with CSE deletion exhibit hypertension and impaired vasorelaxation in resistance vessels, leading to the notion that H2S could be an endothelium-derived relaxation factor (EDRF) similar to NO (62, 93). In CSE-deleted mice, vasorelaxation in mesenteric arteries is diminished by about 75%. Hypertension caused due to deletion of CSE was not observed in an independent study, which could reflect effects of strain background and methodological differences (34). Unlike NO and CO, which stimulate guanylyl cyclase to elevate cyclic guanosine monophosphate (cGMP) and promote vasorelaxation, H2S enhances the activity of ATP-dependent K+ channel (KATP) channels that mediate vasodilation (62), and it also stimulates cGMP-dependent protein kinase G (PKG) (9). In addition, H2S inhibits phosphodiesterases (8), which act on cyclic nucleotides such as cGMP, to elevate cGMP levels, thereby promoting vasorelaxation. A role for H2S in the regulation of blood pressure is also supported by studies revealing that pharmacologic inhibition of H2S biosynthetic enzymes elicits hypertension (99, 106). An intraperitoneal injection of D,L-propargylglycine (PAG) decreases H2S production in the liver, aorta, and ileum of rats and elevates systolic blood pressure. Dysregulated H2S biosynthesis occurs in maternal hypertension and pre-eclampsia, where a decrease in CSE expression correlates with reduced H2S levels (92). Pregnant mice injected with PAG exhibit elevated blood pressure and decreased fetal growth. Administration of the H2S donor GYY4137 restores fetal growth. H2S can also induce proliferation and migration of vascular endothelial cells in an Akt-dependent manner, as observed in human umbilical vascular endothelial cells (HUVECs) (10, 67) and bEnd3 microendothelial cells (84). H2S is also cytoprotective for the vasculature. During hyperglycemic stress, viability of endothelial cells decreases due to increased oxidative stress and DNA damage, leading to impaired vasorelaxation. Overexpression of CSE in cultured endothelial cells improves viability, whereas knocking down CSE by siRNA exacerbates hyperglycemia-induced damage (84). In addition to vasorelaxation, H2S is a potent stimulator of angiogenesis (67). Exposure of endothelial cells or mice to the angiogenesis inducer, vascular endothelial growth factor stimulates the production of H2S and induces angiogenesis, actions that are not evident in mice deleted for CSE (67).
An interesting feature of H2S is its intersection with oxygen, the gas indispensable for life. Molecular oxygen is the terminal electron acceptor in the mitochondrial electron transport chain and is, therefore, vital for generation of adenosine triphosphate (ATP) during oxidative phosphorylation. Any change in O2 levels is reflected in the partial pressure of oxygen, Po2. One of the tissues that senses the changes in Po2 is the carotid body (also known as the glomus carotidum or carotid glomus), composed of chemoreceptors and associated cells, present at the fork of the carotid artery. The glomus cells of the carotid body sense the Po2 and release oxygen-sensing signals that modulate compensatory responses to oxygen availability. The chromaffin cells in the fish gills are another cell type specializing in O2 sensing (65). H2S participates in oxygen sensing and regulation of respiratory responses during hypoxia. Both CSE and CBS mRNA are present in the carotid body, and immunohistochemistry has revealed the presence of CSE in the glomus cells. Inhibition of H2S production in the carotid body abolishes hypoxia-induced hyperventilation (45). CSE knockout mice are impaired in their cardiovascular and respiratory responses to hypoxia (72).
In several instances, H2S and NO act coordinately. The synergy between the two gases was first observed in the smooth muscle constituting the rat thoracic aorta, where low concentrations of H2S greatly augmented NO-induced smooth muscle relaxation (29). For instance, the two gasotransmitters cooperate to mediate vasorelaxation and maintain vascular homeostasis. Thus, inhibition of the activity of CSE reduces the response to NO donors and vice versa (15). Treatment of endothelial cells with H2S donors elevates cGMP levels in an NO-dependent manner and activates PKG. H2S also promotes angiogenesis in an NO-dependent manner. The proangiogenic and vasodilatory effects of H2S are absent in mice deleted for endothelial NO synthase (eNOS). Silencing CSE impairs NO-induced cGMP accumulation and angiogenesis as well as cholinergic vasorelaxation, suggesting a requirement for H2S in NO activity in the vasculature (15). The protective effects of H2S on intestinal injury and cardiac arrest are abolished in mice deleted for eNOS (55, 103). Administration of the H2S donor, Na2S significantly ameliorates cardiac arrest and cardiopulmonary resuscitation induced oxidative stress and ventricular as well as neurological dysfunction in mice. These effects are reduced in eNOS knockout mice, suggesting a requirement for NO in the effects of Na2S. In addition, the H2S donor sodium hydrosulfide (NaHS) can directly scavenge superoxide anions O2−• and inhibit vascular NADPH oxidase-derived superoxide production in vitro (2). These activities protect the endothelium and bioavailability of NO under conditions of oxidative stress. H2S also intersects with CO-mediated signaling and regulates microvascular function in the brain (59). Heme oxygenase-2 (HO-2), present in the brain, synthesizes CO in the micromolar range and accounts for about 80% of CO produced. Under normal conditions, CO produced by HO-2 in neurons binds to CBS and inhibits its activity. During hypoxia, the activity of HO-2, an oxygen-dependent enzyme, decreases, thereby relieving the tonic inhibition of CBS. The resulting increase in the production of H2S facilitates vasodilation and maintains ATP levels in the brain. The existence of tonic inhibition of CBS by CO is further supported by the observation that HO inhibitors elicit increased vasodilation and that HO-2 knockout mice have elevated ATP levels. In addition, hypoxia fails to increase vasodilation in HO-2 null mice (59). The localizations of CBS and HO-2 facilitate the interplay between the two gases. CBS localizes exclusively to the astrocytes, whose end feet are in close proximity to the microvasculature and, hence, can promote vasodilation. HO-2 is localized to both the neurons and the endothelium of blood vessels. H2S produced by CBS in the astrocytes can diffuse into the endothelium to mediate its effects. The source of CO, which regulates CBS during hypoxia, whether neuronal or endothelial, is currently unclear. Hypoxia also increases CBS expression in glioblastoma and pheochromocytoma (PC12) cell lines, as well as in the rat cerebral cortex and cerebellum, a phenomenon not observed in vascular endothelial or smooth muscle cells (87). The elevated CBS levels induced by hypoxia reflect transcriptional activation of the cbs gene by the hypoxia-inducible factors, HIF1α and β (87). Responses to hypoxia vary significantly among individuals and occur due to differences in O2 sensing by the carotid body. The differential response is caused by variations in CO-mediated H2S production. Thus, individuals with a hypersensitive response to hypoxia have lower CO production and higher H2S production. Conversely, hyposensitivity to hypoxia correlates with higher CO production and, therefore, lower H2S production (71). In addition to CO, NO has also been shown to modulate CBS activity by binding to its heme center (88). During renal ischemia-reperfusion, elevated NO levels inhibit CBS, resulting in an increase in homocysteine levels and renal damage (75).
H2S and the Gastrointestinal Tract
H2S has long been known to be produced by resident microbes of the colon. In addition, CSE, CBS, and 3-MST of the host contribute to production of the gasotransmitter. H2S is produced throughout the gastrointestinal tract. One of the actions of H2S in the gastrointestinal tract is relaxation of smooth muscle, first demonstrated in a guinea-pig model (44) and subsequently observed in other systems, such as human, mouse and rat colon, and jejunum (24). H2S exerts pronociceptive effects on the transient receptor potential vanilloid (TRPV1) and transient receptor potential subfamily A1 TRPA1 receptors (3, 64, 77). An intraplantar injection of the H2S donor, NaHS and L-cysteine induces mechanical as well as cold hypersensitivity in wild-type mice but not in mice deleted for TrpA1. NaHS stimulates TRPA1 channels expressed in CHO cells and in activated dorsal root ganglion neurons from wild-type mice but not mice deleted for TrpA1. Mechanical hypersensitivity induced by lipopolysaccharides (LPS) is abrogated by PAG, the CSE inhibitor, as well as by TRPA1 antagonists (3). H2S stimulates the T-type voltage-gated calcium channels in the colon to modulate visceromotor responses (51). Intracolonic administration of NaHS elicits visceral nociceptive behavior in mice, effects diminished in mice treated with T-type channel blockers. H2S in the digestive tract has been reported to exert both pro- and anti-inflammatory effects (46). These conflicting reports may reflect variable dose response relationships and cell-type specificities. Recently, it was shown that in healthy mouse colon, CSE is the predominant enzyme responsible for the generation of H2S; whereas in the inflamed colon, 3-MST accounts for the bulk of H2S synthesis, although both CSE and 3-MST are up-regulated in the inflamed colon (21). Increased production and decreased degradation of H2S occur at sites of mucosal ulcers. H2S mediates anti-inflammatory roles in the digestive tract and is protective against ischemic damage (47, 50, 107) and ethanol-induced gastritis (52). The gasotransmitter stimulates bicarbonate secretion, which aids in the defense of the mucosa of the stomach and small intestine against gastric acid (32). Accordingly, H2S-releasing nonsteroidal anti-inflammatory drugs have been developed (13).
H2S in Mitochondrial Function
Toxicity of H2S is attributable, in large part, to its effect on mitochondrial function. Mice treated with 80 ppm of H2S undergo a state of hypometabolism with decreases in CO2 production, O2 consumption, and body temperature within 5 min of exposure (7). H2S inhibits complex IV (cytochrome c oxidase) of the electron transport chain in the mitochondria, leading to inhibition of ATP production (28, 73). By contrast, lower doses of H2S stimulate mitochondrial energetics (56, 86). This stimulation reflects inhibition of mitochondrial phosphodiesterase 2A (PDE2A), leading to elevation of cAMP and activation of protein kinase A (57). H2S production in mitochondria can also occur when CSE translocates to the mitochondria in response to elevated calcium levels. The H2S produced can enhance ATP production (22). H2S generated by CBS can also support mitochondrial function. In cultured hepatocytes and liver tissue, CBS translocates to the mitochondria under ischemic conditions (89). The increased H2S production prevents Ca2+-induced cytochrome c release and increases in reactive oxygen species (ROS). When oxygen levels normalize, the mitochondrial lon protease degrades CBS via the oxygenated heme group of CBS. A similar situation involving oxygen levels and lon protease-mediated degradation has been observed in the regulation of the HO-1. In colon cancer cells, H2S generated by CBS supports cell bioenergetics and proliferation (85). CBS expression is up-regulated in human colon cancer samples with a corresponding increase in H2S synthesis. Colon cancer cell lines such as HCT116 display higher expression of CBS than nonmalignant mucosal cells. CBS in HCT116 cells localizes to the outer mitochondrial membrane in addition to the cystosol. Depleting CBS in these cells by siRNA or reducing its activity by pharmacologic inhibitors leads to reduced mitochondrial function as reflected by diminished oxygen consumption, ATP synthesis, and respiratory reserve capacity. Reducing CBS activity results in lower cell proliferation and reduced endothelial cell migration in tumor/endothelial cell cocultures. The proliferative effects of CBS and its role in bioenergetics were also observed in ovarian carcinoma (6). Primary epithelial ovarian cancer tissues and ovarian cancer cell lines exhibited increased expression of CBS. Silencing CBS resulted in decreased proliferation and impaired mitochondrial function in these cell lines. Further support for a role of H2S in regulating mitochondrial function comes from the observation that silencing of the cbs homologue in Caenorhabditis elegans impairs oxygen consumption (58). Thus, H2S supports mitochondrial function with the enzymatic source of the gas being cell type or condition specific.
Crosstalk Between H2S and NO: Nitrosothiols
The overlap between functions of H2S and NO led to a search for direct interactions between the two gasotransmitters. The identification of the nitrosothiol HSNO provided a molecular basis for the functional similarity in the actions of NO and H2S (97). Biochemical evidence for the formation of nitrosothiol was determined using nitrite assays, amperometric determination of .NO release, and EPR measurements. The physiological presence of this molecule was demonstrated in LPS-treated rats. HSNO can also be formed by the interaction of H2S with GSNO (20). HSNO has unique properties that make it a redox sensor and regulator of metabolic activities. HSNO can give rise to nitroxyl (HNO), which has vasodilatory and cardioprotective functions (23, 66). HSNO can freely cross membranes to elicit its effects such as transnitrosation of molecules such as hemoglobin (20). When HUVECs are incubated with a CSE inhibitor, intracellular SNO levels are markedly reduced, implicating a role for H2S in nitrosation reactions.
H2S Signals by Sulfhydration
One of the modes by which H2S signals is by the attachment of a persulfide group to reactive cysteines in target proteins in a process designated as sulfhydration (60, 61, 69), which is analogous to nitrosylation by NO (36, 83). Sulfhydration typically occurs at cysteine residues with a low pKa, which, in turn, is dependent on the local architecture of the protein side chains and its environment. Recently, it was shown that H2S is also capable of modifying cysteine residues with a high pKa (26). Sulfhydration results in increased reactivity of the modified cysteine by virtue of enhanced nucleophilicity. Nitrosylation, on the other hand, caps the cysteine residue and blunts its reactivity. Sulfhydration, similar to nitrosylation, is reversible by enzymatic systems such as thioredoxin/thioredoxin reductase, thereby providing scope for regulation in vivo (5, 43).
Sulfhydration modulates diverse cellular pathways, including metabolic pathways, protein degradative processes, and stress response pathways. Using the modified biotin switch, sulfhydration was demonstrated in mouse liver samples and individual proteins were identified using mass spectrometry. Sulfhydration was eliminated in livers of CSE knockout mice or in HEK293 cells where CSE was silenced using siRNA, establishing the physiologic relevance of the modification. One of the first proteins shown to be sulfhydrated was glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Sulfhydration of C150 of GAPDH increases its catalytic activity sevenfold (60). Nitrosylation of this residue, on the other hand, abolishes catalytic activity and promotes its interaction with the E3 ubiquitin ligase, Siah1, which transports it to the nucleus to trigger cell death pathways (27). Treatment of GAPDH with the reducing agent dithiothreitol (DTT) decreases its catalytic activity, which is consistent with a role for sulfhydration in modulation of the activity. Catalytic activity of GAPDH is reduced by 35% in the livers of CSE knockout mice, indicating that, under basal conditions, a major proportion of GAPDH is sulfhydrated. Another process that is regulated by sulfhydration is actin polymerization, which is enhanced by sulfhydration of β-actin, an effect reversed by treatment with DTT (60). NaHS enhances actin polymerization by 30%, while having no effect on depolymerization. The EC50 of H2S for sulfhydration of target proteins is within the range of physiologic concentrations of H2S in most tissues. It was recently shown that solutions of H2S or the slow releasing H2S donor GYY4137 form polysulfides in solution, which mediate thiol oxidation and sulfhydration (26). Thus, sulfhydration of target cysteines in proteins adds another layer of complexity to redox homeostasis in vivo.
Detection of Sulfhydration
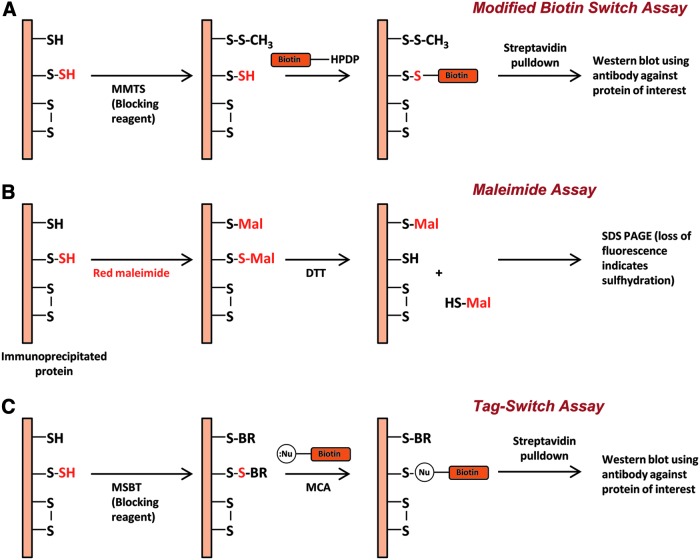
Using the modified biotin switch assay previously developed to detect nitrosylation (37), sulfhydration has been shown to be a widely prevalent post-translational modification (60). In the assay for sulfhydration, unmodified cysteines on proteins are blocked using methyl methanethiosulfonate (Fig. 1A). The persulfide modified cysteine is then exposed by reduction using DTT and labeled with biotin, which can then be captured using streptavidin-conjugated sepharose and analyzed by immunoblotting. Using this technique, 25%–50% of proteins were found to be basally sulfhydrated in vivo. More recently, a new method has been developed that employs fluorescent maleimide (78) to selectively label sulfhydryl groups of cysteines (Fig. 1B). The reagent labels both modified and unmodified cysteine residues, but treatment with DTT releases the maleimide only from the sulfhydrated cysteine moieties, resulting in the loss of fluorescent signal. A critical prerequisite for the use of this assay is labeling of the proteins in their properly folded native conformation, which ensures labeling of only the surface-exposed accessible cysteines. Another method developed to selectively detect sulfhydration is the tag-switch technique (104), where the reagent methylsulfonyl benzothiazole (MSBT) is used as the thiol blocking agent. After blocking by MSBT, the sample is treated with a reagent comprising a nucleophilic component as well as a biotin reporter moiety, which selectively labels persulfide groups. The modified protein can then be captured using streptavidin and detected by Western blotting (Fig. 1C). In addition to these methods, definitive evidence for sulfhydration can be obtained using mass spectroscopy.
FIG. 1.
Methods to detect sulfhydration. (A) The modified biotin switch assay. Shown in the figure is a protein that has unmodified cysteines (-SH), sulfhydrated cysteines (-S-SH), and disulfide bonded cysteines (S-S). The protein (a constituent of the total cell lysate) is treated with methyl methanethiosulfonate (MMTS), which methylthiolates unmodified cysteines. Excess MMTS is removed by acetone precipitation or passage through a gel filtration column and then treated with biotin HPDP, which reacts with thiols. The biotinylated protein is then enriched by affinity capture using streptavidin and detected by Western blotting under nonreducing conditions. (B) The maleimide assay. The protein of interest is first immunoprecipitated and labeled with a fluorescent thiol-reactive maleimide derivative under conditions that retain the protein in its native conformation. One portion of the reaction mixture is treated with the reductant dithiothreitol, resulting in the release of the maleimide moiety at the site of sulfhydration, which is detected by SDS PAGE as a decrease in fluorescent intensity that is directly proportional to the degree of sulfhydration. (C) The tag-switch assay. The sample under study is treated with the thiol blocking agent, methylsulfonyl benzothiazole (MSBT), followed by treatment with a reagent, methyl cyanoacetate (MCA) comprising both a nucleophilic component and a reporter such as biotin. The biotinylated proteins are then captured using streptavidin and analyzed using Western blotting.
Sulfhydration in Vasorelaxation
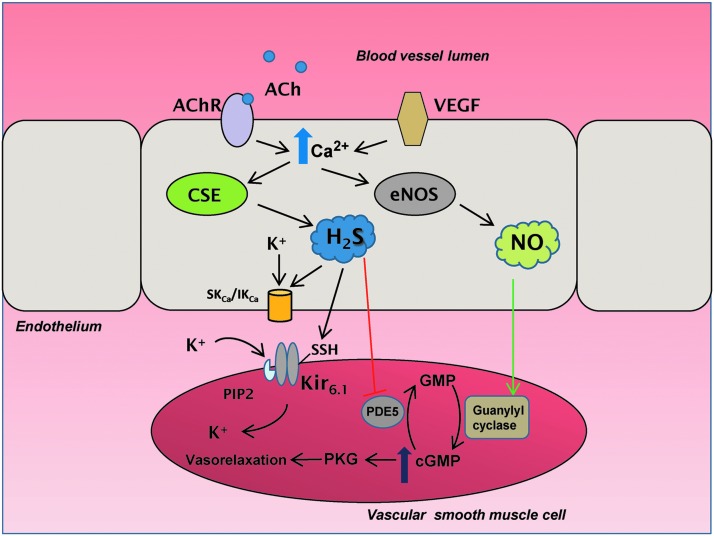
Similar to NO, H2S can mediate vasorelaxation with H2S acting on smaller resistance vessels such as the mesenteric artery and NO on larger vessels such as the aorta (101). Cholinergic stimuli induce release of intracellular calcium, which binds to the calcium binding protein, calmodulin. Yang and associates showed that calcium-calmodulin binds CSE and activates it to produce H2S (101), although a separate study reported no effect of calmodulin on CSE activity (54). Similar to NO, H2S acts as an EDRF. Similar to eNOS, CSE is present in the endothelium of blood vessels (101). Mice deleted for CSE exhibit impaired vasorelaxation, with the mesenteric artery EDRF activity diminished by 75% as compared with the wild-type mice. While NO and CO mediate vasorelaxation by stimulating guanylyl cyclase to produce cGMP, H2S elicits vasorelaxation by acting on KATP on smooth muscle cells (Fig. 2). The effect of H2S on vasodilation occurs in part by the sulfhydration of the reactive Cys43 on the Kir6.1 subunit of the KATP channel on smooth muscle cells in the vasculature (62). Sulfhydration of Cys43 prevents its association with ATP, and promotes its binding to phosphatidylinositol-4,5-bisphosphate (PIP2), thereby opening the channel and influx of K+. In addition, H2S acts on the small conductance Ca2+-activated potassium channels (SKCa) and intermediate conductance Ca2+-activated potassium channels (IKCa) on the endothelial membrane to regulate calcium influx (Fig. 2). H2S also inhibits cGMP phosphodiesterases to elevate cGMP levels and promote vasorelaxation.
FIG. 2.
Hydrogen sulfide (H2S) mediates vasorelaxation by sulfhydration. During cholinergic stimulation, when acetylcholine (ACh) binds to its receptor (AChR), calcium ions (Ca2+) increase in the cells, which activates cystathionine γ-lyase (CSE) to produce H2S. H2S sulfhydrates the small and intermediate conductance potassium channels (SKCa and IKCa) to regulate K+ disposition. In addition, H2S sulfhydrates the Cys43 residue of the Kir6.1 subunit of ATP-dependent K+ channel (KATP) channels on vascular smooth muscle cells, causing its dissociation from adenosine triphosphate (ATP) and association with phosphatidylinositol 4,5-bisphosphate (PIP2), causing the channel to open and allow K+ entry to mediate vasorelaxation. H2S also inhibits phosphodiesterase 5 (PDE5), which prevents it from degrading cyclic guanosine monophosphate (cGMP). Elevated cGMP levels activate protein kinase G (PKG) to act on downstream targets to mediate vasorelxation. H2S production also results from the activation of the vascular endothelial growth factor (VEGF) via elevated Ca2+ concentrations in the endothelium. Augmented Ca2+ levels stimulate the production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS), which activates guanylyl cyclase to synthesize more cGMP.
H2S is also involved in blood clotting. H2S modulates clotting and prevents the aggregation of platelet proteins. The expression of prothrombotic adhesion molecules was also decreased by the H2S donor, GYY4137 in a dose-dependent manner in platelets activated by thrombin-receptor activating peptide. The antithrombotic effects of H2S might be mediated by sulfhydration of platelet proteins (25). Treating platelets with H2S prevents platelet aggregation and increases sulfhydration of platelet proteins as monitored by the modified biotin-switch assay (25).
Sulfhydration in Inflammation and Stress Responses
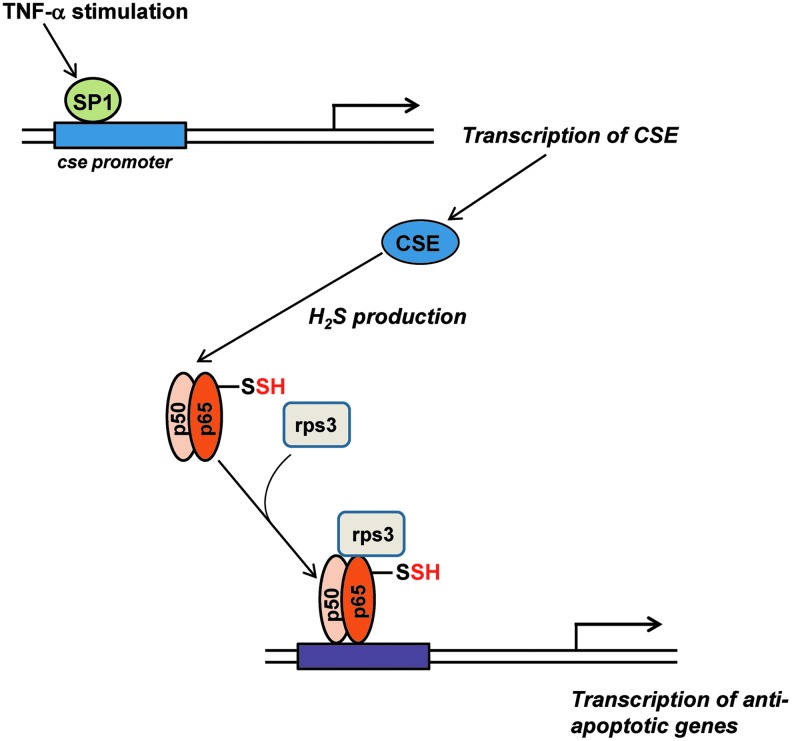
H2S participates in several signaling pathways that are activated by stress. For instance, during inflammation that involves the tumor necrosis factor-α (TNF-α), production of the gasotransmitter by CSE is stimulated by the transcription factor specificity protein 1 (SP1) (78). The generated H2S sulfhydrates the p65 subunit of nuclear factor-κB (NF-κB), which increases its interaction with ribosomal protein S3 (rps3), resulting in enhanced transcription of anti-apoptotic genes (Fig. 3). In CSE knockout mice, the anti-apoptotic function of NF-κB is markedly diminished. In addition to controlling signaling by NF-κB, sulfhydration modulates endoplasmic reticulum (ER) stress (43). Protein tyrosine phosphatase 1B (PTP1B), a key player in ER stress, is sulfhydrated at Cys215 by H2S, resulting in inhibition of its catalytic activity. As a result, protein kinase RNA-like ER kinase (PERK), a target of the phosphatase activity of PTP1B, retains its active, phosphorylated status and stimulates the unfolded protein response (UPR). Activated PERK phosphorylates the eukaryotic translational initiation factor 2α, which shuts down global protein synthesis. During ER stress, certain mRNAs such as the activating transcription protein 4 (ATF4) are selectively translated. An independent study revealed that CSE expression increases in response to ER stress via the action of the transcription factor ATF4 and disruption of ATF4 leads to decreased expression of CSE (16). Thus, elevated ATF4 expression induces CSE expression and, consequently, increases H2S production and stimulation of the ER stress response. These observations further confirm a role for CSE in the regulation of ER stress response.
FIG. 3.
H2S in stress signaling. During inflammation induced by tumor necrosis factor-α (TNF-α), the transcription factor specificity protein 1 (SP1) is recruited to the CSE promoter to stimulate transcription of CSE. CSE generates H2S, which sulfhydrates the p65 subunit of the transcription factor nuclear factor-κB (NF-κB), whose interaction with its coactivator ribosomal protein S3 (rps3) is enhanced, resulting in the transcription of anti-apoptotic genes.
Sulfhydration in Induction of Phase II Cytoprotective Enzymes
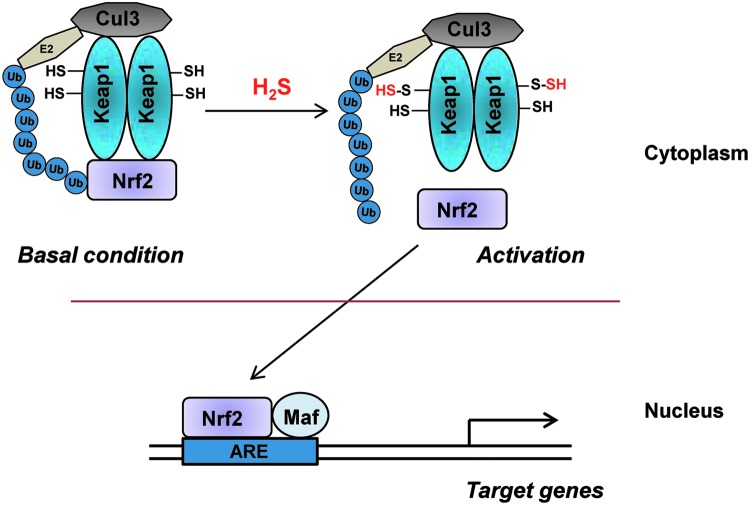
The transcriptional regulator nuclear factor erythroid 2-related factor 2 (Nrf2) coordinately regulates the genes encoding the phase II cytoprotective enzymes (4, 49). Phase II cytoprotective enzymes are involved in the synthesis of reduced glutathione, response to oxidative stress, and drug metabolism. At least ten chemical classes of compounds can induce this pathway. Under basal conditions, Nrf2 binds to kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and targets it for degradation by the Cullin-E3 proteosomal pathway. Keap1 has multiple reactive cysteines that are potential targets for post-translational modifications. Several inducers of the cytoprotective pathway influenced by Nrf2 covalently modify reactive cysteine residues on Keap1, causing it to dissociate from Nrf2. Nrf2 translocates to the nucleus, where it heterodimerizes with other coactivators such as Maf to elicit transcription of the phase II genes such as NADPH:quinone oxidoreductase, glutathione S transferase, and HO-1. H2S protects against ischemic injury and mediates cardioprotection by influencing the nuclear translocation of Nrf2 (11). H2S delays cellular senescence via this pathway (102) by sulfhydrating Keap1 at Cys151 to stimulate its dissociation from Nrf2 and enabling it to translocate to the nucleus and regulate the expression of cytoprotective genes (Fig. 4). Sulfhydration of Keap1, which occurs basally, is augmented by treatment with cysteine and is diminished in CSE knockout mice, which exhibit elevated oxidative stress and accelerated senescence. Sulforaphane, a thiol present in cruciferous plants, and a well-known inducer of the phase II genes, elicits the production of H2S (70), which may mediate sulforaphane's anticancer effect in prostate tumor cells.
FIG. 4.
H2S and the phase II cytoprotective pathways. Under basal conditions, Kelch-like ECH-associated protein 1 (Keap1) binds to nuclear factor erythroid 2-related factor 2 (Nrf2) and sequesters it, leading to its ubiquitination by cullin 3 (Cul3). When H2S levels increase in the cell in response to stress, Keap1 is sulfhydrated, leading to the dissociation of Nrf2. Nrf2 translocates to the nucleus and activates the transcription of target genes harboring the antioxidant response element (ARE) in association with coactivator proteins such as Maf.
H2S in the Nervous System
H2S plays important roles in the central nervous system. Although the prevalent belief is that CBS is the major H2S-generating enzyme, CSE and 3-MST are also present in the brain and could contribute to H2S production. There appears to be a functional compartmentalization of these enzymes in the brain. CBS is present predominantly in the Bergmann glia and astrocytes (19, 59) and CSE is present in neurons (59). Immunohistochemical staining reveals 3-MST mostly in neurons (82). At the subcellular level, under basal conditions, CSE and CBS are cytosolic; whereas 3-MST is both cytosolic and mitochondrial (40). 3-MST operates under alkaline conditions and generates sulfane sulfur, which releases H2S in the presence of reducing agents. The endogenous reductants that facilitate H2S production by 3-MST are thioredoxin and dihydrolipoic acid (33, 53). The intracellular pH of astrocytes can become alkaline during neuronal depolarization, which, in turn, can result in the generation of H2S. Thus, multiple mechanisms for the production of H2S exist in vivo. The diverse cellular localizations and modes of production of H2S ensure a steady supply of the gas under different conditions, further underscoring the importance of this gasotransmitter in the cell.
Functions of H2S in the central nervous system were first studied by Kimura and associates (1), who showed that H2S acts on N-methyl-D-aspartate (NMDA)-glutamate receptors to augment long-term potentiation (LTP). Interestingly, the other gasotransmitters, NO and CO also facilitate LTP but in an NMDA-independent manner. Inhibiting NMDA receptors abrogates LTP induction by H2S. H2S alone does not induce LTP but instead synergizes with weak electrical stimulation. H2S acts on active but not quiescent synapses, suggesting an involvement in associative learning (1). Although not demonstrated experimentally, activation of the NMDA receptor most likely occurs by sulfhydration of cysteine residues.
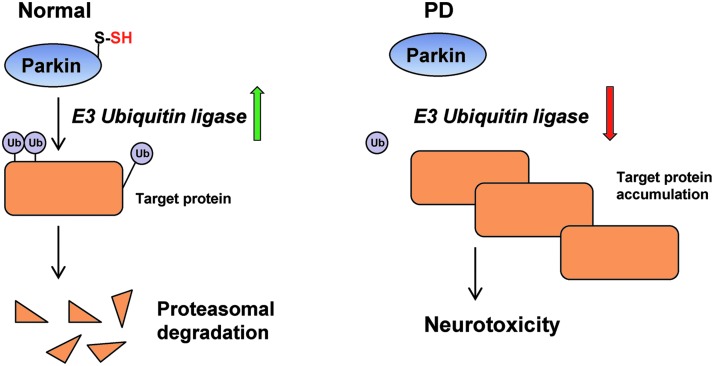
H2S impacts diverse aspects of brain function. Dysregulation of H2S metabolism occurs in neurodegenerative disorders such as Alzheimer's disease (AD) and Parkinson's disease (PD). AD is characterized by the formation of neurofibrillary tangles and deposition of amyloid plaques in the brain, which leads to cognitive dysfunction and dementia. H2S donors exert beneficial effects on spatial memory and cognitive functions in mouse models of AD (98). A common feature of several neurodegenerative diseases such as AD and PD is elevated oxidative stress. H2S supplementation prevents build-up of ROS and neuronal cell death under oxidative stress (42, 95, 96). H2S also stimulates glutathione biosynthesis to alleviate oxidative stress (41). In PD, a disease affecting the substantia nigra leads to motor abnormalities and tremor, one of the proteins affected is parkin, an E3 ubiquitin ligase. Mutations in the E3 ubiquitin ligase parkin decrease its catalytic activity, leading to accumulation of toxic targets, such as α-synuclein, a constituent of Lewy bodies that contributes to neurodegeneration (Fig. 5). In normal subjects, parkin is sulfhydrated, which enhances its activity (90). The principal site of sulfhydration on parkin is Cys95, with Cys59 and Cys182 also being modified. An analysis of patient striata reveals diminished sulfhydration of parkin with reduced catalytic activity (90); whereas nitrosylation of parkin, which inactivates it, is elevated (14). H2S donors are protective in rodent models of PD and could offer avenues for therapeutics (30, 39, 48). Recently, we showed that H2S production is diminished in Huntington's disease (HD), which could play a role in the pathophysiology of the disease (68). HD is a devastating neurodegenerative disease that targets the corpus striatum and is caused by expansion of polyglutamine repeats in the protein huntingtin. Mutant huntingtin (mHtt) forms aggregates, leading to disruption of multiple cellular functions ranging from transcriptional dysregulation to cognitive and motor functions. The transcriptional factor Sp1, which regulates the expression of CSE (35, 100), is sequestered by mHtt, leading to its depletion (18) and redox imbalance in the cell due to decreased cysteine biosynthesis. H2S also plays a neuroprotective role in mouse models of traumatic brain injury by reducing brain edema and facilitating the restoration of cognitive and motor deficits (105).
FIG. 5.
Sulfhydration of the E3 ubiquitin ligase parkin is affected in Parkinson's disease (PD). Under normal conditions, parkin, an E3 ubiquitin ligase, ubiquitinates diverse substrates and targets them for proteosomal degradation. The activity is enhanced by sulfhydration. In PD, diminished sulfhydration of parkin results in lower catalytic activity, leading to accumulation of target proteins and neurotoxicity.
An emerging theme in the biology of the gasotransmitter is that regulated H2S levels are crucial for optimal cellular processes. Too little or too much H2S can have deleterious effects. In the case of Down's syndrome caused by the trisomy of chromosome 21, excess H2S production occurs as the gene encoding CBS, the main H2S-generating enzyme, is present on chromosome 21 (38). An intriguing observation in Down's syndrome was that the overexpression of CBS was far greater than that expected from trisomy of chromosome 21. Immunohistochemical staining revealed the presence of CBS in astrocytes surrounding senile plaques, implicating a role for excess H2S production in cognitive impairment in the disease (31). The urine of Down's syndrome patients has high levels of thiosulfate, a catabolic product of H2S. In addition to mediating neuroprotection, H2S enhances neurogenesis and the proliferation and differentiation of neural stem cells in the dentate gyrus of the adult hippocampus. Cognitive deficits induced by hypoxia are reversed by H2S via unclear mechanisms. Perhaps sulfhydration of key proteins involved in learning and memory modulates their activity to maintain optimal cognitive responses.
Protective Effect of Sulfhydration in Redox Homeostasis
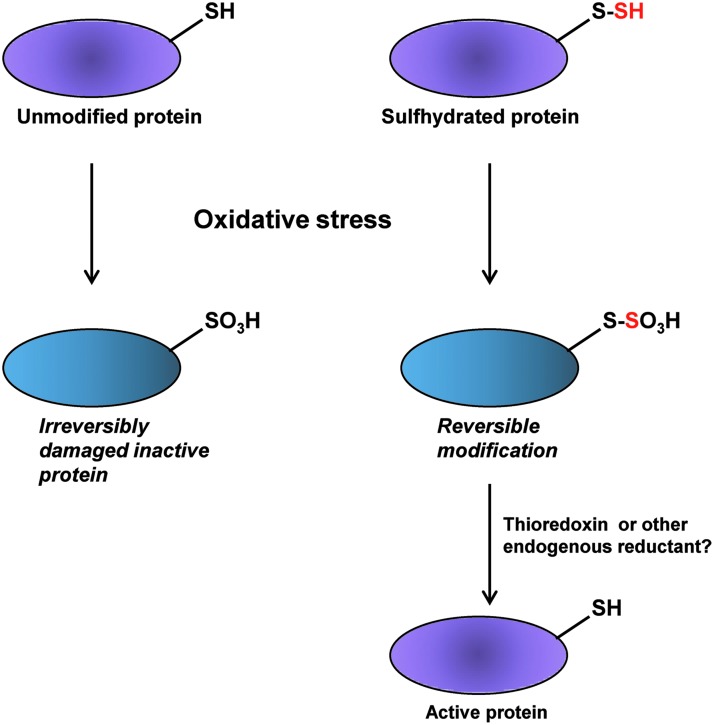
H2S affords protection against oxidative stress in multiple ways. H2S exists at physiologic pH in the dissociated state as H+ and HS− ions (17). HS− can sulfhydrate a variety of electrophiles such as 8-nitro-cGMP, 15-deoxy-Δ12, 14-prostaglandin J2 (15d-PGJ2), 4-hydroxy-2-nonenal (HNE), acrolein, and fatty acid nitroalkene derivatives to modulate signaling pathways or defend cells against oxidative damage (63). The electrophile 8-nitro-cGMP can covalently modify reactive cysteine residues in a process termed S-guanylation. In a screen for modulators of protein S-guanylation, HS− was found to influence the metabolism of 8-nitro-cGMP. Knockdown of CSE and CBS expression elevates the levels of protein S-guanylation in cultured mammalian cells treated with exogenous 8-nitro-cGMP. The basal level of protein S-guanylation is also elevated in cells where either CSE or CBS were depleted. HS− derived from H2S directly reacts with 8-nitro-cGMP and forms 8-SH-cGMP, which arises from the nucleophilic substitution of the nitro moiety with HS−. Sulfhydration of 8-nitro-cGMP to form 8-SH-cGMP not only prevents the nitration of proteins, but also imparts PDE resistance to 8-nitro-cGMP. The 8-SH-cGMP molecule still retains cGMP activity and can activate PKG. Thus, the deleterious effect of 8-nitro-cGMP is converted to a beneficial function via sulfhydration. H2S reduces iNOS-induced protein S-guanylation, which is a post-translational modification of cysteine residues on proteins caused by 8-nitro-cGMP. S-guanylation of H-Ras activates it and triggers cell senescence pathways via the ERK and p38 MAPK cascade. Continued activation of ERK and p38 MAPK via their phosphorylation leads to stimulation of p53 and Rb, which promotes senescence. The protective effects of H2S donors on myocardial infarction occur in part due to the neutralization of 8-nitro-cGMP by sulfhydration and concomitant reduction in H-Ras activation. Treatment with the H2S donor, NaHS suppresses phosphorylation of ERK and MAPK and the ensuing p53/Rb activation. H2S can also mitigate the effects of 4HNE by causing its sulfhydration. 4HNE is a potent inducer of lipid peroxidation in cells so that sulfhydration of 4HNE should lower lipid peroxidation. Thus, sulfhydration of electrophiles provides a strategy to terminate signaling induced by these molecules and contributes to redox homeostasis. H2S can indirectly protect against oxidants by preventing oxidation of cysteines via sulfhydration. We had earlier proposed that by sulfhydrating thiol groups of cysteine residues, H2S could protect these moieties from irreversible damage (69) as depicted (Fig. 6). The protective effect of H2S has been verified experimentally using the lipid phosphatase and tensin homolog (PTEN). PTEN, pretreated with the H2S donor, NaHS and then exposed to the oxidant H2O2, induces oxidation of reactive cysteine residues. In this case, polysulfides generated in the solution of NaHS could mediate the reported effects. Consistent with a role for sulfhydration, in preserving thiol groups of cysteine residues, the reductant DTT restores activity of PTEN only when samples are pretreated with NaHS (26).
FIG. 6.
Protection of reactive cysteine thiols. During oxidative stress, cysteine residues can undergo irreversible oxidation. However, in the case of proteins whose cysteine residues are sulfhydrated, although oxidation can occur, endogenous reductants can cleave the S-S bond and regenerate the active thiol group of the cysteine residues.
Reciprocity of Sulfhydration and Nitrosylation
An emerging theme in thiol modification by H2S and NO by sulfhydration and nitrosylation is their reciprocity. In a vast majority of regulatory events, sulfhydration and nitrosylation are antagonistic. For instance, nitrosylation of the E3 ubiquitin ligase parkin suppresses its activity (14), whereas sulfhydration stimulates its activity (90). Similarly, nitrosylation inhibits the glycolytic activity of GAPDH (27) and sulfhydration enhances it (60). Since sulfhydration and nitrosylation frequently occur on the same residue, there could be competition between the two modifications. Both nitrosylation and sulfhydration can be readily reversed providing scope for fine-tuning of regulatory responses to stress. In certain scenarios, sulfhydration precedes nitrosylation. During inflammatory conditions, the p65 subunit of NF-κB is sulfhydrated within the first 2 h of TNF-α treatment, followed by nitrosylation (78). In the context of PD, parkin undergoes waves of sulfhydration and nitrosylation (90). It is conceivable that sequential modifications may confer additional regulatory mechanisms to fine tune signal transduction.
Conclusions and Emerging Perspectives
H2S has now been recognized as an endogenous signaling molecule with multiple modes of action in diverse tissues ranging from the vasculature to the central nervous system. Similar to NO and CO, H2S was regarded as a noxious molecule until its roles in physiologic systems were discovered. This ancient signaling messenger has protective functions across different phyla. In bacteria, H2S defends against oxidative stress and toxic effects of antibiotics (79). In C. elegans, H2S influences longevity by reducing oxidative stress (76). Deletion of cysl-2, a cysteine synthase, reduces the lifespan of the nematode, which is partially rescued by the H2S donor GYY4137. In general, H2S is cytoprotective at lower doses and toxic at higher levels. Several mechanisms of H2S signaling have been elucidated. However, the mechanisms and conditions under which physiologic signals can mobilize H2S from bound stores such as sulfane sulfur and acid-labile pools are yet to be resolved. To ensure a constant and readily available supply of this important gasotransmitter under different conditions, the cells evidently maintain different types of sulfur stores. For example the acid-labile pool can only be released under acidic conditions and the sulfane sulfur pool can be released under more basic conditions in the presence of reductants. Another area that merits study is the interplay between the three gasotransmitters, NO, CO and H2S, to arrive at therapeutics for diseases involving dysregulated gasotransmitter signaling.
Abbreviations Used
- 3-MST
3-mercaptopyruvate sulfurtransferase
- ACh
acetylcholine
- AChR
acetylcholine receptor
- AD
Alzheimer's disease
- ARE
antioxidant response element
- ATP
adenosine triphosphate
- CaM
calmodulin
- cAMP
cyclic adenosine monophosphate
- CBS
cystathionine β-synthase
- cGMP
cyclic guanosine monophosphate
- CO
carbon monoxide
- CSE
cystathionine γ-lyase
- Cul3
cullin 3
- Cys
cysteine
- EDRF
endothelium-derived relaxing factor
- eNOS
endothelial nitric oxide synthase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- H2S
hydrogen sulfide
- HNE
4-hydroxy-2-nonenal
- HO-2
heme oxygenase-2
- HUVECs
human umbilical vascular endothelial cells
- IKCa
intermediate conductance Ca2+-activated potassium channels
- KATP
ATP-dependent K+ channel
- Keap1
kelch-like ECH-associated protein 1
- Kir6.1
inwardly rectifying potassium channel subunit 6.1
- LPS
lipopolysaccharides
- LTP
long-term potentiation
- MCA
methyl cyanoacetate
- mHtt
mutant huntingtin
- MMTS
methyl methanethiosulfonate
- MSBT
methylsulfonyl benzothiazole
- NaHS
sodium hydrosulfide
- NF-κB
nuclear factor-κB
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- PAG
D,L-propargylglycine
- PDE2A
phosphodiesterase 2A
- PERK
protein kinase RNA-like ER kinase
- PIP2
phosphatidylinositol-4,5-bisphosphate
- pKa
acid dissociation constant
- PKG
protein kinase G
- PTEN
phosphatase and tensin homolog
- PTP1B
protein tyrosine phosphatase 1B
- ROS
reactive oxygen species
- rps3
ribosomal protein S3
- Siah1
seven in absentia homolog 1
- SKCa
Ca2+-activated potassium channels
- SP1
specificity protein 1
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Acknowledgment
This work was supported by USPHS grants DA 000266 and MH18501 to S.H.S.
References
- 1.Abe K. and Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Magableh MR, Kemp-Harper BK, Ng HH, Miller AA, and Hart JL. Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn Schmiedebergs Arch Pharmacol 387: 67–74, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Andersson DA, Gentry C, and Bevan S. TRPA1 has a key role in the somatic pro-nociceptive actions of hydrogen sulfide. PLoS One 7: e46917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird L. and Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol 85: 241–272, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Benhar M, Forrester MT, and Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 10: 721–732, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal E, Weaver AL, Visscher DW, Cliby W, Sood AK, Bhattacharya R, and Mukherjee P. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 8: e79167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackstone E, Morrison M, and Roth MB. H2S induces a suspended animation-like state in mice. Science 308: 518, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Pyriochou A, Roussos C, Roviezzo F, Brancaleone V, and Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 30: 1998–2004, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, Cantalupo A, Dhayade S, Karalis KP, Wang R, Feil R, and Cirino G. cGMP-dependent protein kinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One 7: e53319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, and Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, and Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, and Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol 3: 448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan MV. and Wallace JL. Hydrogen sulfide-based therapeutics and gastrointestinal diseases: translating physiology to treatments. Am J Physiol Gastrointest Liver Physiol 305: G467–G473, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, and Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304: 1328–1331, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, and Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 109: 9161–9166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, and Austin RC. Integrated stress response modulates cellular redox state via induction of cystathionine gamma-lyase: cross-talk between integrated stress response and thiol metabolism. J Biol Chem 287: 7603–7614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dombkowski RA, Russell MJ, and Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol 286: R678–R685, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Dunah AW, Jeong H, Griffin A, Kim YM, Standaert DG, Hersch SM, Mouradian MM, Young AB, Tanese N, and Krainc D. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science 296: 2238–2243, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, and Kimura H. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J 13: 1854–1856, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Filipovic MR, Miljkovic JLj, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, and Ivanović-Burmazović I. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J Am Chem Soc 134: 12016–12027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannigan KL, Ferraz JG, Wang R, and Wallace JL. Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: a site-specific, pro-resolution mechanism. PLoS One 8: e71962, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu M, Zhang W, Wu L, Yang G, Li H, and Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A 109: 2943–2948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuto JM, Chiang K, Hszieh R, Wong P, and Chaudhuri G. The pharmacological activity of nitroxyl: a potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J Pharmacol Exp Ther 263: 546–551, 1992 [PubMed] [Google Scholar]
- 24.Gallego D, Clave P, Donovan J, Rahmati R, Grundy D, Jimenez M, and Beyak MJ. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil 20: 1306–1316, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Grambow E, Mueller-Graf F, Delyagina E, Frank M, Kuhla A, and Vollmar B. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets 2013. [Epub ahead of print]; DOI: 10.3109/09537104.2013.786823 [DOI] [PubMed] [Google Scholar]
- 26.Greiner R, Pálinkás Z, Bäsell K, Becher D, Antelmann H, Nagy P, and Dick TP. Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19: 1749–1765, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, and Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7: 665–674, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hill BC, Woon TC, Nicholls P, Peterson J, Greenwood C, and Thomson AJ. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem J 224: 591–600, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosoki R, Matsuki N, and Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, and Bian JS. Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell 9: 135–146, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, and Kimura H. Cystathionine beta-synthase is enriched in the brains of Down's patients. Biochem Biophys Res Commun 338: 1547–1550, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ise F, Takasuka H, Hayashi S, Takahashi K, Koyama M, Aihara E, and Takeuchi K. Stimulation of duodenal HCO3− secretion by hydrogen sulphide in rats: relation to prostaglandins, nitric oxide and sensory neurones. Acta Physiol (Oxf) 201: 117–126, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, and Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205–214, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, and Suematsu M. Cystathionine gamma-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem 285: 26358–26368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, and Kimura H. Murine cystathionine γ-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J 381: 113–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, and Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3: 193–197, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Jaffrey SR. and Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 86: pl1, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Kamoun P, Belardinelli MC, Chabli A, Lallouchi K, and Chadefaux-Vekemans B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am J Med Genet A 116A: 310–311, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Kida K, Yamada M, Tokuda K, Marutani E, Kakinohana M, Kaneki M, and Ichinose F. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson's disease. Antioxid Redox Signal 15: 343–352, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids 41: 113–121, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Kimura Y, Goto Y, and Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12: 1–13, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Kimura Y. and Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Krishnan N, Fu C, Pappin DJ, and Tonks NK. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kruszyna H, Kruszyna R, and Smith RP. Cyanide and sulfide interact with nitrogenous compounds to influence the relaxation of various smooth muscles. Proc Soc Exp Biol Med 179: 44–49, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang LH, and Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal 12: 1179–1189, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Linden DR. Hydrogen Sulfide Signaling in the Gastrointestinal Tract. Antioxid Redox Signal 20: 818–830, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Kalogeris T, Wang M, Zuidema MY, Wang Q, Dai H, Davis MJ, Hill MA, and Korthuis RJ. Hydrogen sulfide preconditioning or neutrophil depletion attenuates ischemia-reperfusion-induced mitochondrial dysfunction in rat small intestine. Am J Physiol Gastrointest Liver Physiol 302: G44–G54, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu M, Zhao FF, Tang JJ, Su CJ, Fan Y, Ding JH, Bian JS, and Hu G. The neuroprotection of hydrogen sulfide against MPTP-induced dopaminergic neuron degeneration involves uncoupling protein 2 rather than ATP-sensitive potassium channels. Antioxid Redox Signal 17: 849–859, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401–426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mard SA, Neisi N, Solgi G, Hassanpour M, Darbor M, and Maleki M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig Dis Sci 57: 1496–1503, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Matsunami M, Tarui T, Mitani K, Nagasawa K, Fukushima O, Okubo K, Yoshida S, Takemura M, and Kawabata A. Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut 58: 751–761, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Medeiros JV, Bezerra VH, Gomes AS, Barbosa AL, Lima-Junior RC, Soares PM, Brito GA, Ribeiro RA, Cunha FQ, and Souza MH. Hydrogen sulfide prevents ethanol-induced gastric damage in mice: role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther 330: 764–770, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, and Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 439: 479–485, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Mikami Y, Shibuya N, Ogasawara Y, and Kimura H. Hydrogen sulfide is produced by cystathionine γ-lyase at the steady-state low intracellular Ca2+ concentrations. Biochem Biophys Res Commun 431: 131–135, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, and Ichinose F. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 120: 888–896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Módis K, Coletta C, Erdélyi K, Papapetropoulos A, and Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J 27: 601–611, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Módis K, Panopoulos P, Coletta C, Papapetropoulos A, and Szabo C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharmacol 86: 1311–1319, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Módis K, Wolanska K, and Wozdek R. Hydrogen sulfide in cell signaling, signal transduction, cellular bioenergetics and physiology in C. elegans. Gen Physiol Biophys 32: 1–22, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, Ishii I, Matsubara K, Kabe Y, Uchiyama S, Nagata E, Gadalla MM, Snyder SH, and Suematsu M. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A 109: 1293–1298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, and Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mustafa AK, Gadalla MM, and Snyder SH. Signaling by gasotransmitters. Sci Signal 2: re2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, and Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, and Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8: 714–724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogawa H, Takahashi K, Miura S, Imagawa T, Saito S, Tominaga M, and Ohta T. H2S functions as a nociceptive messenger through transient receptor potential ankyrin 1 (TRPA1) activation. Neuroscience 218: 335–343, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Olson KR. and Whitfield NL. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid Redox Signal 12: 1219–1234, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey JM, Fukuto M, Feelisch DA, Wink DA, and Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A 98: 10463–10468, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, and Szabó C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paul BD, Sbodio JI, Xu R, Vandiver MS, Cha JY, Snowman AM, and Snyder SH. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington's disease. Nature 2014. [Epub ahead of print]; DOI: 10.1038/nature13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul BD. and Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Pei Y, Wu B, Cao Q, Wu L, and Yang G. H2S mediates the anti-survival role of sulforaphane on human prostate cancer cells. Toxicol Appl Pharmacol 257: 420–428, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Peng YJ, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, and Prabhakar NR. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci U S A 111: 1174–1179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, and Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A 107: 10719–10724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petersen LC. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta 460: 299–307, 1977 [DOI] [PubMed] [Google Scholar]
- 74.Pfennig N. and Widdel F. The bacteria of the sulphur cycle. Phil Trans R Soc Lond B Biol Sci 298: 433–441, 1982 [DOI] [PubMed] [Google Scholar]
- 75.Prathapasinghe GA, Siow YL, Xu Z, and OK Inhibition of cystathionine-beta-synthase activity during renal ischemia-reperfusion: role of pH and nitric oxide. Am J Physiol Renal Physiol 295: F912–F922, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Qabazard B, Ahmed S, Li L, Arlt VM, Moore PK, and Stürzenbaum SR. C. elegans aging is modulated by hydrogen sulfide and the sulfhydrylase/cysteine synthase cysl-2. PLoS One 8: e80135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De GR, Campi B, and Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology 131: 1542–1552, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, and Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shatalin K, Shatalina E, Mironov A, and Nudler E. H2S: a universal defense against antibiotics in bacteria. Science 334: 986–990, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Shibuya N. and Kimura H. Production of hydrogen sulfide from D-cysteine and its therapeutic potential. Front Endocrinol (Lausanne) 4: 87, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N, and Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun 4: 1366, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, and Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, and Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A 89: 444–448, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, and Szabo C. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A 108: 13829–13834, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, and Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A 110: 12474–12479, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, and Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and Physiological Mechanisms. Br J Pharmacol 171: 2099–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takano N, Peng Y-J, Kumar GK, Luo W, Hu H, Shimoda LA, Suematsu M, Prabhakar NR, and Semenza GL. Hypoxia-inducible factors regulate human and rat cystathionine β-synthase gene expression. Biochem J 458: 203–211, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taoka S. and Banerjee R. Characterization of NO binding to human cystathionine beta-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem 87: 245–251, 2001 [DOI] [PubMed] [Google Scholar]
- 89.Teng H, Wu B, Zhao K, Yang G, Wu L, and Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc Natl Acad Sci U S A 110: 12679–12684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Ko HS, Lee YI, Dawson VL, Dawson TM, Sen N, and Snyder SH. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun 4: 1626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vandiver MS. and Snyder SH. Hydrogen sulfide: a gasotransmitter of clinical relevance. J Mol Med (Berl). 90: 255–963, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, Baily J, Miller MR, Cudmore M, Hadoke PW, Wang R, Gratacós E, Buhimschi IA, Buhimschi CS, and Ahmed A. Dysregulation of hydrogen sulfide producing enzyme cystathionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 127: 2514–2522, 2013 [DOI] [PubMed] [Google Scholar]
- 93.Wang R. Hydrogen sulfide: a new EDRF. Kidney Int 76: 700–704, 2009 [DOI] [PubMed] [Google Scholar]
- 94.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, and Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem 90: 765–768, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, and Moore PK. Hydrogen sulphide: a novel inhibitor of hypochlorous acid mediated oxidative damage in the brain? Biochem Biophys Res Commun 326: 794–798, 2005 [DOI] [PubMed] [Google Scholar]
- 97.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, and Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun 343: 303–310, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, and Liu J. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer's disease. J Neuroinflammation 9: 202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan H, Du J, and Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun 313: 22–27, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Yang G, Pei Y, Teng H, Cao Q, and Wang R. Specificity protein-1 as a critical regulator of human cystathionine γ-lyase in smooth muscle cells. J Biol Chem 286: 26450–26460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, and Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, and Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18: 1906–1919, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Yusof M, Kamada K, Kalogeris T, Gaskin FS, and Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol 296: H868–H876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang D, Macinkovic I, Devarie-Baez NO, Pan J, Park CM, Carroll KS, Filipovic MR, and Xian M. Detection of protein S-sulfhydration by a tag-switch technique. Angew Chem Int Ed Engl 53: 575–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang M, Shan H, Chang P, Wang T, Dong W, Chen X, and Tao L. Hydrogen sulfide offers neuroprotection on traumatic brain injury in parallel with reduced apoptosis and autophagy in mice. PLoS One 9: e87241, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao W, Ndisang J, and Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81: 848–853, 2003 [DOI] [PubMed] [Google Scholar]
- 107.Zuidema MY, Yang Y, Wang M, Kalogeris T, Liu Y, Meininger CJ, Hill MA, Davis MJ, and Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol 299: H1554–H1567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]