Abstract
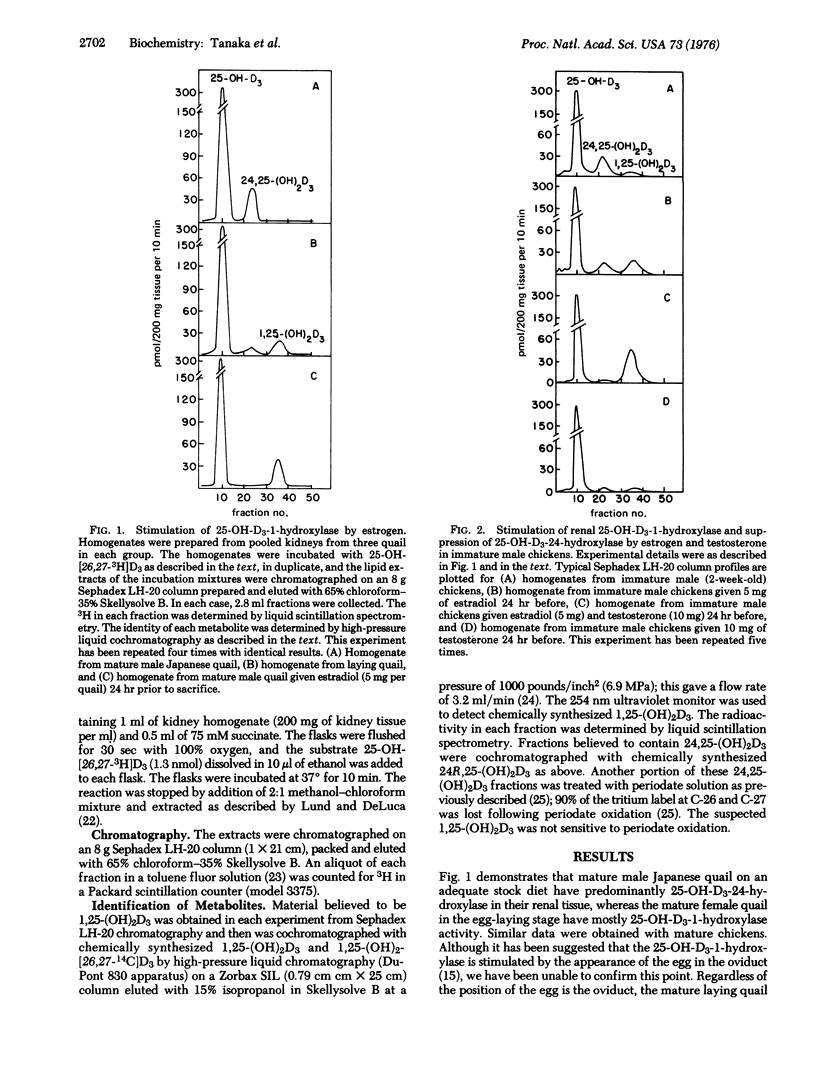
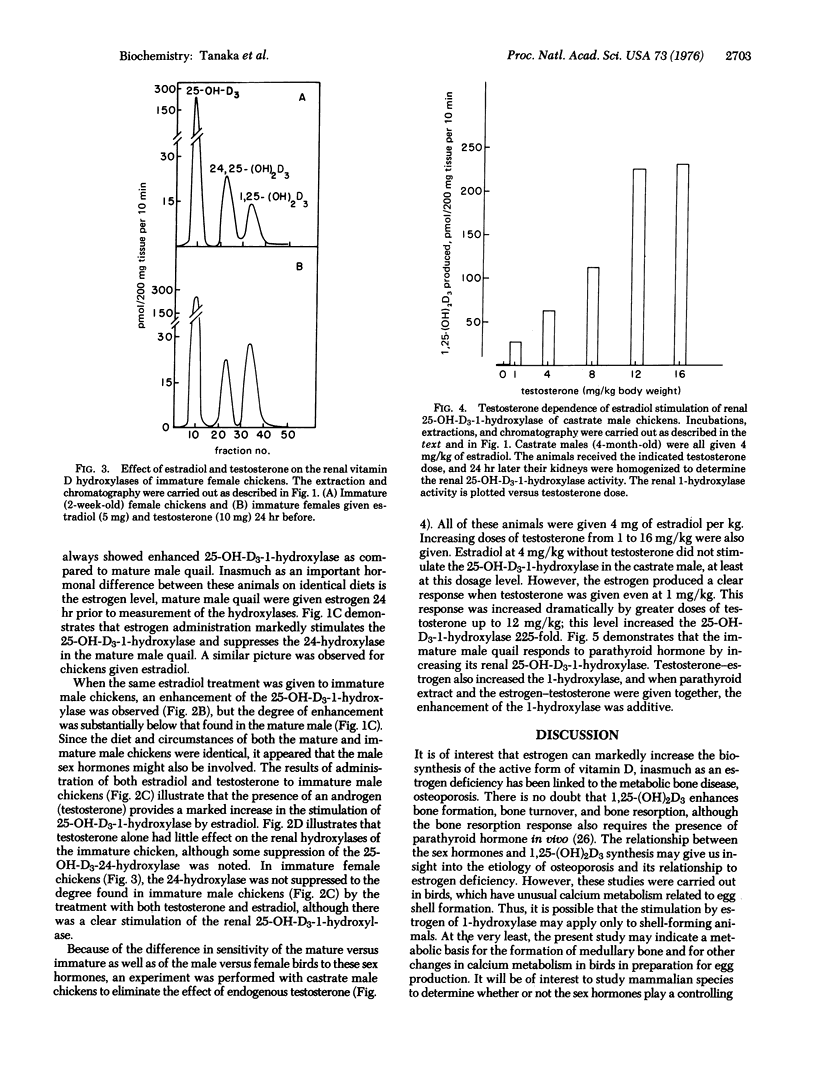
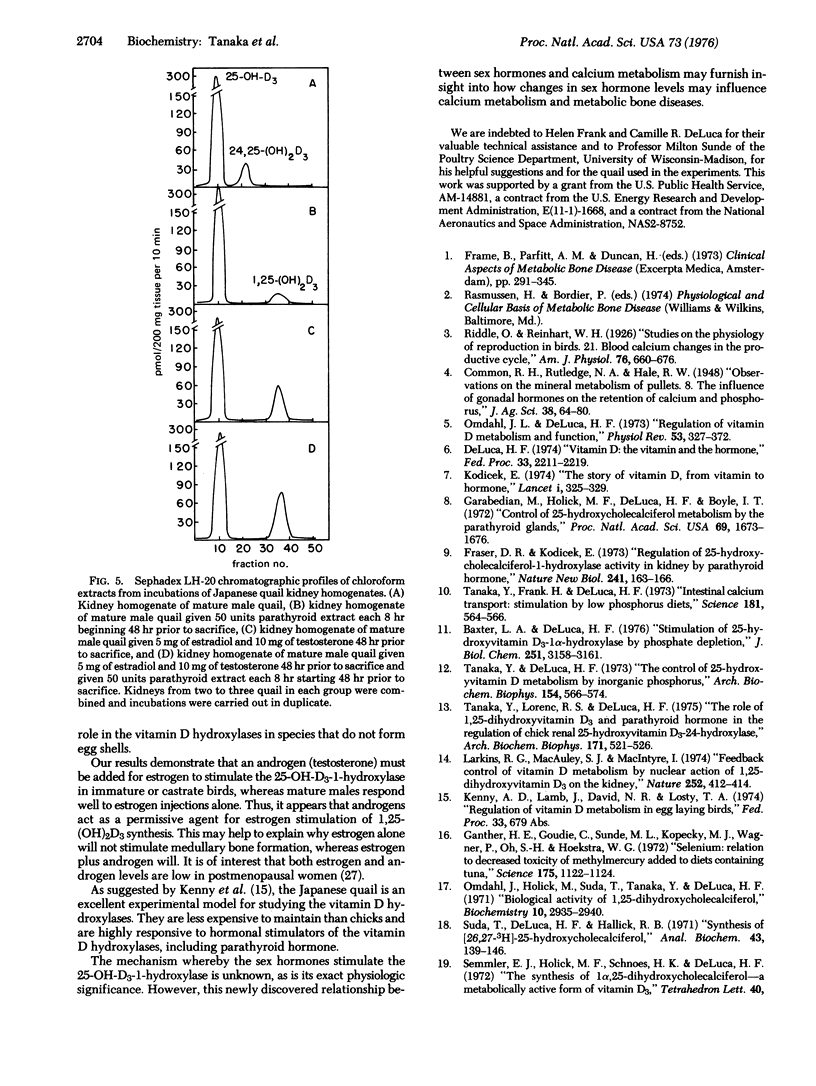
Kidney homogenates from adult male Japanese quail or chickens demonstrate hydroxylase activity predominantly for the 24 rather than the 1 position of 25-hydroxyvitamin D3 (25-hydroxycholecalciferol). A single injection of 5 mg of estradiol-17beta into a male bird completely suppresses the 24-hydroxylase and greatly increases the 1-hydroxylase activity. Immature males do not respond well to estrogen alone, but they do respond well to estradiol plus testosterone. Testosterone alone has little or no effect on the hydroxylases of either species. Castrated male chickens show an estradiol response only when testosterone is also given. Optimal 24 hr responses to 5 mg of estradiol per kg in the castrate male were obtained with about 12 mg of testosterone per kg. These optimal amounts of estradiol and testosterone increased the activity of 25-hydroxyvitamin D3-1-hydroxylase approximately 225-fold (this enzyme is also known as 25-hydroxycholecalciferol 1-monooxygenase; 25-hydroxycholecalciferol, NADPH: oxygen oxidoreductase (hydroxylating), EC 1.14.13.13). These results demonstrate a strong regulation by the sex hormones of the renal vitamin D hydroxylases in birds.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter L. A., DeLuca H. F. Stimulation of 25-hydroxyvitamin D3-1alpha-hydroxylase by phosphate depletion. J Biol Chem. 1976 May 25;251(10):3158–3161. [PubMed] [Google Scholar]
- DeLuca H. F. Vitamin D: the vitamin and the hormone. Fed Proc. 1974 Nov;33(11):2211–2219. [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Ganther H. E., Goudie C., Sunde M. L., Kopecky M. J., Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972 Mar 10;175(4026):1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M., Tanaka Y., Holick M. F., Deluca H. F. Response of intestinal calcium transport and bone calcium mobilization to 1,25-dihydroxyvitamin D3 in thyroparathyroidectomized rats. Endocrinology. 1974 Apr;94(4):1022–1027. doi: 10.1210/endo-94-4-1022. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972 Dec 10;247(23):7528–7532. [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Gray R. W., Boyle I. T., Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972 Nov 7;11(23):4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- Knutson J. C., DeLuca H. F. 25-Hydroxyvitamin D3-24-hydroxylase. Subcellular location and properties. Biochemistry. 1974 Mar 26;13(7):1543–1548. doi: 10.1021/bi00704a034. [DOI] [PubMed] [Google Scholar]
- Larkins R. G., MacAuley S. J., MacIntyre I. Feedback control of vitamin D metabolism by a nuclear action of 1,25-dihydroxycholecalciferol on the kidney. Nature. 1974 Nov 29;252(5482):412–414. doi: 10.1038/252412a0. [DOI] [PubMed] [Google Scholar]
- Lund J., DeLuca H. F. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966 Nov;7(6):739–744. [PubMed] [Google Scholar]
- Mason J. B., Hay R. W., Leresche J., Peel S., Darley S. The story of vitamin D: from vitamin to hormone. Lancet. 1974 Mar 2;1(7853):325–329. [PubMed] [Google Scholar]
- Neville P. F., DeLuca H. F. The synthesis of [1,2-3H]vitamin D3 and the tissue localization of a 0.25-mu-g (10 IU) dose per rat. Biochemistry. 1966 Jul;5(7):2201–2207. doi: 10.1021/bi00871a007. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L., DeLuca H. F. Regulation of vitamin D metabolism and function. Physiol Rev. 1973 Apr;53(2):327–372. doi: 10.1152/physrev.1973.53.2.327. [DOI] [PubMed] [Google Scholar]
- Omdahl J., Holick M., Suda T., Tanaka Y., DeLuca H. F. Biological activity of 1,25-dihydroxycholecalciferol. Biochemistry. 1971 Jul 20;10(15):2935–2940. doi: 10.1021/bi00791a022. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Hallick R. B. Synthesis of (26,27- 3 H)-25-hydroxycholecalciferol. Anal Biochem. 1971 Sep;43(1):139–146. doi: 10.1016/0003-2697(71)90118-7. [DOI] [PubMed] [Google Scholar]
- Tahaka Y., Lorenc R. S., DeLuca H. F. The role of 1,25-dihydroxyvitamin D3 and parathyroid hormone in the regulation of chick renal 25-hydroxyvitamin D3-24-hydroxylase. Arch Biochem Biophys. 1975 Dec;171(2):521–526. doi: 10.1016/0003-9861(75)90061-2. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Frank H., DeLuca H. F. Intestinal calcium transport: stimulation by low phosphorus diets. Science. 1973 Aug 10;181(4099):564–566. doi: 10.1126/science.181.4099.564. [DOI] [PubMed] [Google Scholar]



