Abstract
Significance: Cancer represents a major socioeconomic problem; there is a significant need for novel therapeutic approaches targeting tumor-specific pathways. Recent Advances: In colorectal and ovarian cancers, an increase in the intratumor production of hydrogen sulfide (H2S) from cystathionine β-synthase (CBS) plays an important role in promoting the cellular bioenergetics, proliferation, and migration of cancer cells. It also stimulates peritumor angiogenesis inhibition or genetic silencing of CBS exerts antitumor effects both in vitro and in vivo, and potentiates the antitumor efficacy of anticancer therapeutics. Critical Issues: Recently published studies are reviewed, implicating CBS overexpression and H2S overproduction in tumor cells as a tumor-growth promoting “bioenergetic fuel” and “survival factor,” followed by an overview of the experimental evidence demonstrating the anticancer effect of CBS inhibition. Next, the current state of the art of pharmacological CBS inhibitors is reviewed, with special reference to the complex pharmacological actions of aminooxyacetic acid. Finally, new experimental evidence is presented to reconcile a controversy in the literature regarding the effects of H2S donor on cancer cell proliferation and survival. Future Directions: From a basic science standpoint, future directions in the field include the delineation of the molecular mechanism of CBS up-regulation of cancer cells and the delineation of the interactions of H2S with other intracellular pathways of cancer cell metabolism and proliferation. From the translational science standpoint, future directions include the translation of the recently emerging roles of H2S in cancer into human diagnostic and therapeutic approaches. Antioxid. Redox Signal. 22, 424–448.
Introduction
Hydrogen sulfide (H2S), a colorless, flammable, water-soluble gas with the characteristic smell of rotten eggs, is now widely recognized as an endogenous biological mediator. It is synthesized by mammalian tissues via two cytosolic pyridoxal-5′-phosphate-dependent enzymes: cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), and a mitochondrial enzyme 3-mercaptopyruvate sulfurtransferase (3-MST) to mediate diverse biological functions [reviewed in Refs. (54, 59, 60, 114–116, 132)]. Similar to the other two gasotransmitters (nitric oxide [NO] and carbon monoxide [CO]), many of the biological responses to H2S follow a biphasic dose-response: The effects of H2S range from physiological, cytoprotective effects (which occur at low concentrations) to cytotoxic effects (which are typically apparent only at higher concentrations) [reviewed in Ref. (120)].
The goals of the current article are to provide a summary of selected biological effects of H2S that are relevant in cancer cell biology, to review the experimental evidence on the role of endogenous cancer cell-derived H2S in cancer biology, and to overview the therapeutic potential of CBS inhibition for cancer therapy.
Biological Effects of H2S with Relevance for Cancer Biology
H2S, as a vasodilator and pro-angiogenic mediator
Vasorelaxation is one of the first recognized biological effects of H2S. Often compared with NO, H2S exerts a concentration-dependent vasodilatory effect in blood vessels. The mechanisms of H2S-mediated vasodilation include the activation of KATP channels, a variety of other channels, inhibition of phosphodiesterases, and a synergy with NO (132). The physiological vasodilatory effect, which appears to be more prominent in the microvasculature than in large resistance vessels, is evidenced by the development of progressive hypertension in mice deficient in CSE (142), although it should be also noted that the hypertension was not observed in another strain of CSE-deficient mice (49).
In the late 2000s, the pro-angiogenic effect of H2S was recognized. This effect involves all prototypical hallmarks of angiogenesis, such as endothelial cell proliferation, migration, and stimulation of the formation of tube-like structures. The pathways involved in this effect include KATP channel activation, Akt activation, and a synergistic interaction with NO through a cooperative inhibition of phosphodiesterases and the consequent activation of the cGMP/protein kinase G pathway (14, 20, 90, 119, 131). Endogenous production of H2S by endothelial CSE is also involved in the angiogenic effects of vascular endothelial growth factor (VEGF), a key endogenous growth factor and tumor-derived angiogenic hormone (90, 94).
H2S, as a bioenergetic stimulator
Although initially H2S was solely considered a mitochondrial “poison” via the inhibition of cytochrome c oxidase (mitochondrial complex IV), more recent studies unveiled a more complex, concentration-dependent modulation of mitochondrial function and cellular bioenergetics by H2S. In various cell types (including intestinal epithelial cells and hepatocytes), low concentrations of H2S act as mitochondrial electron donor, resulting in bioenergetic stimulation (36, 67, 120). Endogenous H2S produced by 3-MST or by CSE can also serve a role as a bioenergetic stimulator (31, 80, 81). The stimulatory effects of H2S on mitochondrial electron transport occur at the level of mitochondrial complex II through the interaction of H2S with the redox-sensitive protein sulfur-quinone-oxidoreductase. At higher concentrations, the stimulatory effects of H2S are superceded by an inhibitory effect at complex IV (87, 120).
H2S, as promoter of proliferation and cell survival pathways
H2S stimulates the proliferation of endothelial cells, fibroblasts, hepatocytes, and various cancer cells (8). The signaling mechanisms involved include activation of specific kinase pathways (e.g., MAPK and PI3K/Akt) and inhibition of selective phosphatases such as PTEN and PTP1B (8, 14, 47, 75, 144). The underlying molecular mechanisms include post-transcriptional protein modification by H2S (sulfhydration) (e.g., in the inhibition of PTEN and PTP1B) (65, 75) and intracellular formation of polysulfides from H2S followed by oxidative inactivation of the proteins (38, 61). Of potential relevance to cancer cell biology, the sulfhydration of nuclear factor kappa B (NF-κB) by H2S has also been shown to inhibit apoptosis (103).
Enzymology, expression, and transcriptional regulation of CBS
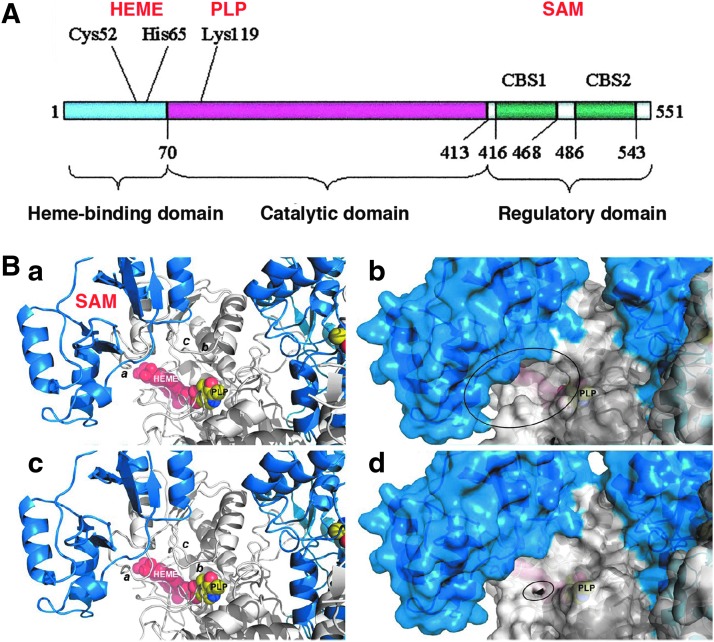
For detailed information on the enzymology, tissue expression levels, and transcriptional and post-transcriptional regulation of CBS, we refer the reader to multiple specialized reviews (7, 51, 76, 77, 107). CBS (EC 4.2.1.22) is traditionally introduced as the first enzyme of the transsulfuration pathway. Its physiological function is the elimination of homocysteine by conversion to cysteine. CBS deficiency is the most common cause of homocystinuria, an inherited metabolic disease, manifested from infancy, where many inactivating CBS mutations have been identified (76, 141). The catalytic activity and crystal structure of human CBS, a homotetramer consisting of 63-kDa subunits, has been characterized in substantial detail. The enzyme binds two cofactors, pyridoxal-phosphate (PLP) and heme. Each CBS monomer binds two substrates (homocysteine and serine), and its activity is allosterically modulated by S-adenosyl-L-methionine (SAM), which binds to and induces a rotation of the C-terminal CBS motifs and relaxation of the loops delineating the entrance to the catalytic site of the enzyme (Fig. 1). A truncated form of CBS (a 45 kDa “active core”) that exhibits increased catalytic activity due to a loss of the C-terminal CBS motifs has also been described (28, 58, 148).
FIG. 1.
Domains and prosthetic groups of cystathionine β-synthase (CBS). (A) The modular domain structure of human CBS showing the N-terminal domain that binds heme, the catalytic domain, and the C-terminal regulatory domain which contains two “CBS” domains, CBS1 and CBS2. Reproduced by permission (77). (B) A model of hCBS activation, as recently proposed by Ereño-Orbea et al. Ribbon (a) and surface (b) representations of a model of the activated form of hCBS on binding of S-adenosyl-L-methionine (SAM) at site S2. The loops controlling the access to the pyridoxal-phosphate (PLP) cavity (a: L191–202; b: L171–174; c: L145–148) are open, thus enabling the access of substrates. Ribbon (c) and surface (d) representations of the basal form of hCBS obtained from the crystals. The loops controlling access to the PLP cavity are closed and occlude the entrance of substrates into the catalytic cavity. Reproduced by permission (28). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
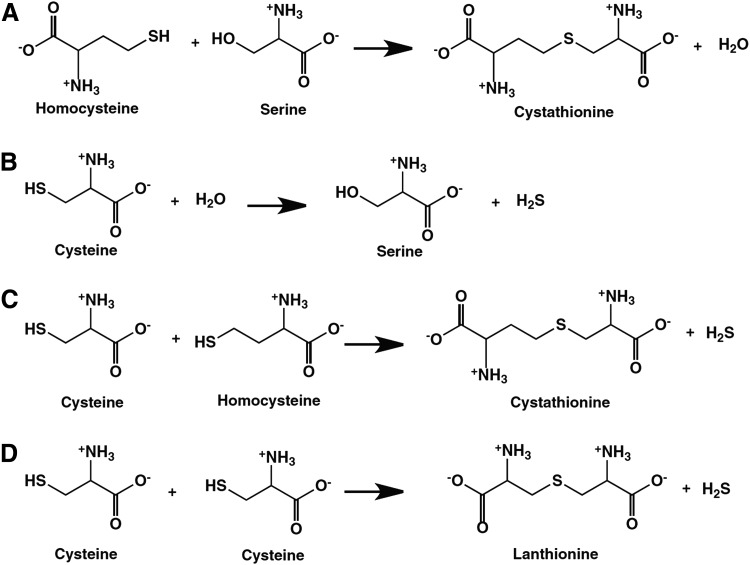
The reactions catalyzed by CBS are shown in Figure 2. In addition to the “canonical” pathway that is considered the major elimination route of homocysteine (Fig. 2A), CBS can produce H2S via at least three pathways, by the conversion of cysteine+H2O to serine+H2S (Fig. 2B), by the condensation of cysteine+homocysteine to yield Cystathionine+H2S (Fig. 2C), and by the condensation of two cysteine molecules to lanthionine+H2S (Fig. 2D). The catalysis involves two separate pockets of CBS, one, where the PLP external aldimine is formed and a separate one, where the nucleophilic amino acid is bound (107, 108).
FIG. 2.
Reactions catalyzed by CBS. Part (A) represents a β-replacement reaction, which is estimated to exhibit a turnover number (v/[E]), estimated at physiological substrate concentrations, [10 μM homocysteine, 100 μM cysteine, 560 μM serine, and 5μM cystathionine]) at 18.5×10−3 s−1. Part (B) represents an α,β-elimination reaction, with an estimated turnover number of 8.1×10−3 s−1 under the conditions outlined in part (A). Part (C) represents a β-replacement reaction, with an estimated turnover number of 1.8×10−6 s−1 under the conditions outlined in part (A). Part (D) represents a β-replacement reaction, with an estimated turnover number of 0.029×10−6 s−1 under the conditions outlined in part (A). Modified from (108) by permission.
CBS is physiologically a cytosolic enzyme, with highest expression in the brain [where H2S has been implicated as a neurotransmitter (1, 60, 100)], the liver, where the majority of physiological homocysteine “detoxification” takes place (29, 99) (via the reaction shown in Fig. 2A), and the kidney (29, 127). The cellular levels of CBS are regulated by transcriptional (29, 32, 73, 132), epigenetic (96), and post-translational (41, 107, 123) mechanisms. CBS expression is also under the control of hormonal regulation. For example, glucocorticoids and insulin regulate its expression in the liver and testosterone regulates its expression in the kidney (99, 127). Although normally cytosolic, CBS can also translocate into the nuclear and mitochondrial compartments under certain conditions (55, 123).
Role of the CBS/H2S Axis in Colorectal Cancer
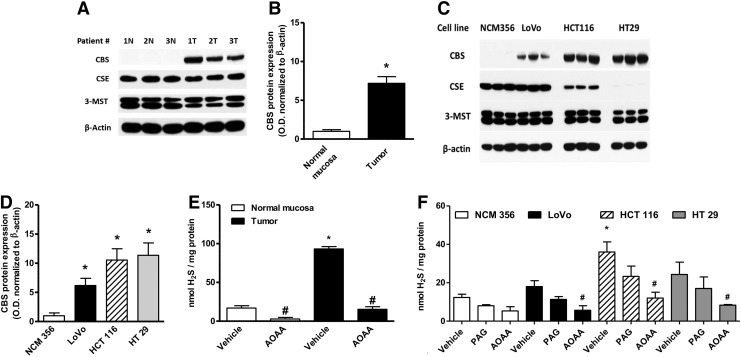
Colorectal cancer cells have a substantially higher expression of CBS than the surrounding normal mucosal margin (Fig. 3A, B) (117). Similarly, various human colon cancer-derived cell lines exhibit high expression of CBS relative to a normal colon cancer cell line (NCM356), whereas the expression levels of CSE are variable (Fig. 3C, D). The increased CBS expression correlates with increased capacity for H2S production, as measured in homogenates from human colon tumor tissue and colon cancer cell lines in vitro (Fig. 3E, F). The expression of the other H2S-producing enzymes (CSE and 3-MST) is not up-regulated in the colorectal cancer cells or the tumor tissue; the expression of 3-MST was fairly uniform in all cells and tissues studied, while CSE expression levels show more variability (Fig. 3A, C).
FIG. 3.
CBS is highly expressed in human colorectal cancer. (A) Representative Western blot of CBS, cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) protein expression in human colorectal cancer specimens, paired with the corresponding normal mucosa tissues. Polyvinylidene difluoride membranes were probed with rabbit polyclonal antibodies against CBS, CSE, and 3-MST. (B) Densitometric analyses of CBS expression, in seven pairs of human colorectal cancers and the patient-matched normal mucosa, showed an approximately sevenfold increase in CBS protein expression in colon cancer (arbitrary relative densitometric units were normalized with β-actin using image analysis software) (*p<0.05 vs. normal mucosa). (C, D) CBS was highly expressed in three different colon cancer cell lines (LoVo, HCT116, and HT29), while low expression was detected in the nontumorigenic normal colon mucosa cells (NCM356) (arbitrary relative densitometric units were normalized with β-actin using image analysis software) (*p<0.05 vs. NCM356 cells). Hydrogen sulfide (H2S) production was measured in human colorectal cancer specimens (E) and in colon cancer cell lines (F) by the methylene blue method. H2S production was stimulated in tissue or cell lysates by incubation at 37°C (30 min) in the presence of the CBS substrates L-cysteine (3 mM) and L-homocysteine (0.5 mM). CBS activity was significantly higher in colon cancer tissues, compared with their corresponding controls. Aminooxyacetic acid (AOAA) (1 mM) blocked the H2S-producing activity of CBS in the tissue extracts (*p<0.05 vs. corresponding from normal mucosa and #p<0.05 vs. vehicle), whereas the CSE inhibitor propargylglycine (PAG) (3 mM) had no significant effect. HCT116 cells exhibited the highest rate of H2S production, as measured by the methylene blue method in cell lysates (*p<0.05 vs. corresponding values in NCM356 and #p<0.05 vs. vehicle). Reproduced by permission (117).
To address the function of CBS in colon cancer cell biology, we use the shRNA-mediated gene silencing to achieve >50% reduction in CBS protein expression in the human colonic epithelial cancer cell line HCT116 (Fig. 4A). For comparison, cells expressing a non-targeting lentiviral vector (shNT) were used. We also compared a separate HCT116 cell line with silencing of CSE, another H2S-producing enzyme, in order to verify the specificity of CBS in colorectal cancer.
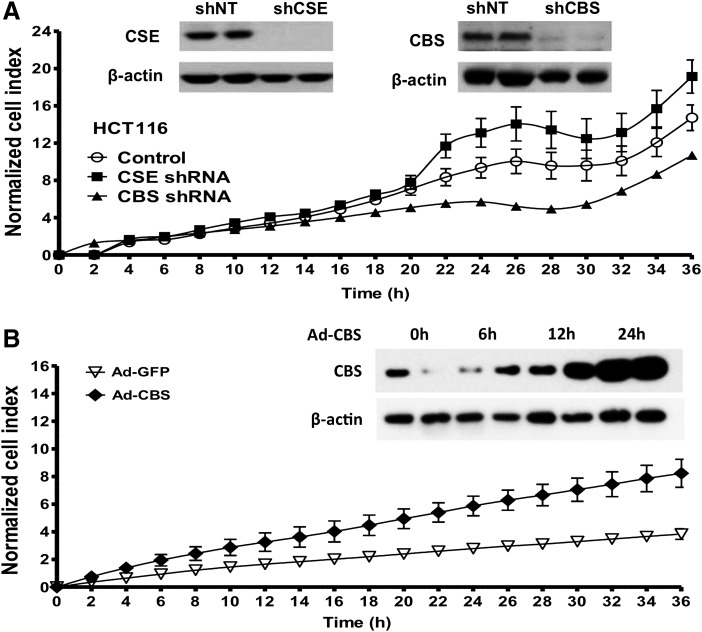
FIG. 4.
CBS silencing inhibits the proliferation of human colorectal cancer cells, while CBS overexpression stimulates the proliferation of nontumorigenic normal colonic mucosa cells. (A) The lentiviral shRNA vectors targeting CBS (shCBS) and CSE (shCSE) were transfected into HCT116 cells. A nontargeting sequence was used as control (shNT). The shRNA approach inhibited the expression of both CBS and CSE genes at the protein level, as shown by Western blotting (inset). After CBS and CSE silencing, cells were seeded at the density of 3000 cells per well in xCELLigence plates and proliferation was monitored for 36 h. Down-regulation of CBS, but not CSE, significantly reduced HCT116 proliferation rate. (B) Adenoviral-mediated CBS overexpression enhances the proliferation rate of NCM356 cells. The NCM356 cells were infected overnight with a CBS expressing adenovirus (Ad-CBS, 10 multiplicities of infection) or its control, a green fluorescent protein (Ad-GFP). The culture medium was then replaced, and cells were seeded in XCELLigence plates at 3000 cells per well. Cell proliferation was then measured in real-time over 36 h. Effective overexpression of CBS was detected within 12–24 h after infection (inset). Adenoviral-mediated CBS overexpression significantly enhanced NCM356 cell proliferation. Reproduced by permission (117).
CBS silencing resulted in a significant inhibition of HCT116 cell proliferation; whereas CSE silencing did not inhibit it, and, in fact, it tended to enhance it at later time points (Fig. 4A). Conversely, adenoviral overexpression of CBS into non-tumorigenic colonic epithelial cells (NCM356) resulted in the stimulation of cell proliferation (Fig. 4B) (117). These findings indicated a proliferation-stimulating role for endogenously produced H2S in colon cancer cells.
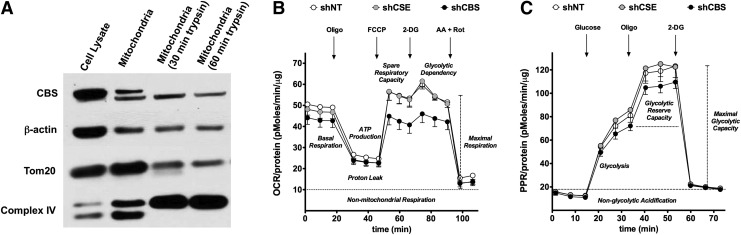
We next investigated the potential role of the CBS/H2S in the regulation of the bioenergetic status of the colorectal cancer cell. Although CBS is physiologically a cytosolic enzyme, we were surprised to find that a substantial proportion of CBS is localized to the mitochondria of HCT116 cells (Fig. 5A) (117). We speculated that translocation of CBS to the mitochondria places the enzyme to close proximity of one of its cellular targets (mitochondrial electron chain proteins), perhaps enabling a more effective transmission of H2S. Consistent with this hypothesis, CBS silencing suppressed both basal oxygen consumption and stimulated oxygen consumption induced by the administration of a mitochondrial-uncoupling agent (Fig. 5B) and also attenuated the glycolytic activity of the cancer cell (Fig. 5C) (117). We hypothesize that this is related to the known stimulatory effect of H2S on GAPDH (84). In contrast to CBS silencing, shRNA-mediated suppression of CSE failed to significantly affect either the proliferation or bioenergetics of HCT116 cells (Fig. 5B, C) (117).
FIG. 5.
CBS is present in the mitochondria of human colorectal cancer cells and stimulates cellular bioenergetics. (A) Western blot shows the presence of CBS in mitochondrial isolates of HCT116 cells. Limited trypsin digestion of isolated mitochondria (30–60 min) reduced mitochondrial CBS, as well as the mitochondrial outer membrane protein Tom20, while enriching complex IV (an inner membrane protein). (B) ShRNA-mediated down-regulation of CBS suppresses cellular bioenergetics in HCT116 cells. Oxygen consumption rate (OCR) in HCT116 cells subjected to either nontargeting (shNT, control) or stable lentiviral silencing of CBS or CSE (shCBS, shCSE). shCBS enzyme significantly decreased basal OCR, calculated ATP production, maximal respiration, and spare respiratory capacity whereas CSE silencing had no effect on the bioenergetic profile. (C) CBS silencing attenuates glycolysis in HCT116 cells. The figure shows time-dependent Extracellular Flux Analysis. shCBS significantly diminished the maximal glycolytic capacity and the glycolytic reserve capacity, whereas CSE silencing had no effect on the glycolytic parameters. Reproduced by permission (117).
We also explored the potential role of L-cystathionine (the other product of CBS). HCT116 cells contained high levels of L-cystathionine, which was further stimulated by the addition of L-cysteine/homocysteine. However, the addition of 1 mM L-cystathionine to HCT116 cells failed to increase their proliferation rate or bioenergetic function (117). Since the various biological responses (proliferation, mitochondrial electron transport) that were inhibited by CBS silencing and can be recapitulated by the administration of H2S donors (but not of L-cystathionine), we conclude that H2S is an independent product of CBS which is primarily responsible for the proliferative and bioenergetic actions observed.
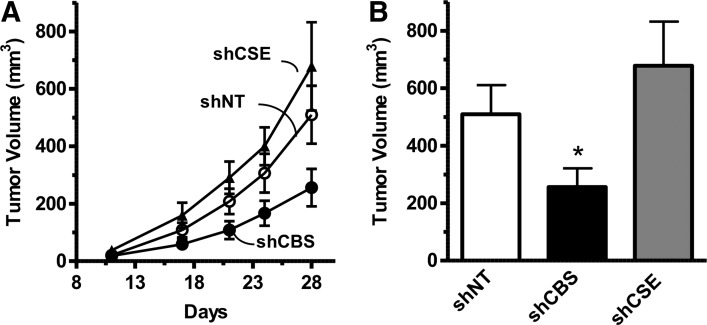
Consistent with the in vitro findings, shRNA-mediated knockdown of CBS reduced the growth rate of HCT116 tumor xenografts, while CSE silencing had no effect (Fig. 6A, B) (117). CBS suppression significantly reduced the density of CD31-positive blood vessels within the tumor tissue, and reduced both the prevalence of larger blood vessels and vessel branching (117). These data are consistent with the hypothesis that CBS-derived H2S, in addition to acting as an autocrine mediator, also acts locally in the tumor microenvironment in a paracrine manner to stimulate tumor angiogenesis. Finally, direct intra-tumor administration of the CBS substrate L-cysteine increased peritumoral blood flow, while pharmacological inhibition of CBS decreased it (117).
FIG. 6.
ShRNA-mediated down-regulation of CBS inhibits colon cancer growth in vivo. (A) Effects of shRNA-mediated gene silencing of CBS (shCBS) and CSE (shCSE) on HCT116 tumor xenograft when compared with the control response (shNT, nontargeting shRNA control). (B) CBS silencing resulted in a reduction of tumor volume at harvest (*p=0.04). Reproduced by permission (117).
The autocrine and paracrine functions of colon cancer-derived H2S are also supported by an independent study of Yamagishi et al. (140). These investigators used HT29 cells [another human colon cancer line in which we have found high expression of CBS (117)]. When implanted into nude mice, Yamagishi et al. observed that the proliferation of HT29 cells was inhibited by a sodium hyaluronate-based physical barrier, which was designed to prevent the effusion of gaseous mediators (such as H2S) from the tumor. They also detected significant amounts of H2S inside the colon cancer tissue, although its enzymatic sources have not been identified. Based on their in vitro experiments in cell-free systems, the authors hypothesized that H2S is produced by the “Maillard reaction” (L-cysteine+D-glucose) (140), although it is currently unclear whether this reaction, in fact, takes place in tumor cells.
We have studied the proliferative and bioenergetic effects of the CBS activator, SAM, in HCT116 cells and the non-tumorigenic colonic epithelial cell line NCM356 (79). As expected, SAM exerted only minor stimulatory effects on the proliferation and energetics of NCM356 cells, which expressed low levels of CBS and produced low levels of H2S, which was increased by SAM to a slight degree. On the other hand, SAM induced a pronounced, concentration-dependent stimulation of H2S production in HCT116 cells, which was converted into an inhibition at the highest SAM concentration tested. The stimulatory effects of SAM-induced were absent in HCT116 cells with CBS silencing, indicating that these effects, indeed, are related to the allosteric stimulation of CBS, and the consequent enhancement of intracellular H2S levels. With longer SAM exposure times, however, the inhibitory effects of SAM became more prominent (on both proliferation and energetics/cell survival). These effects were comparable in HCT116 and NCM356 cells, and were not attenuated by CBS silencing. Taken together, these data indicate that the stimulatory effects of SAM are mainly related to the stimulation of the CBS pathway, while the inhibitory effects seen with higher concentrations/longer exposures of SAM are due to a significant CBS/H2S-independent cytotoxic component. Based on these data, inhibition of cell proliferation by SAM does not appear to represent a tumor cell-selective approach (79).
Role of the CBS/H2S Axis in Ovarian Cancer
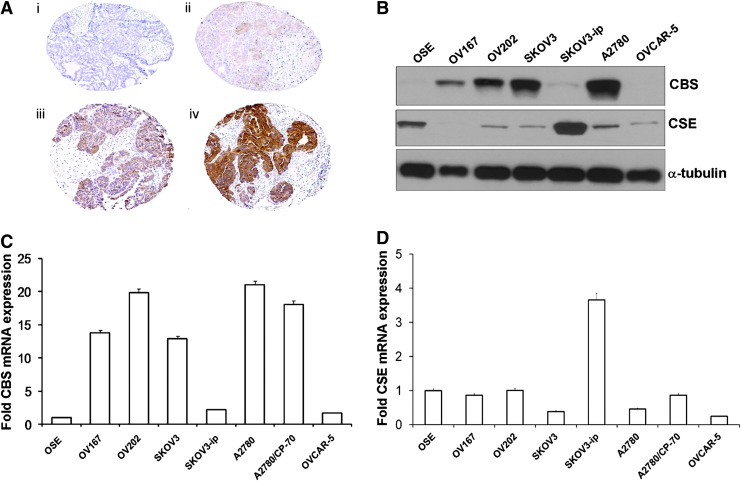
Our findings of colorectal cancers have recently been confirmed in ovarian cancer (10). These investigators assessed the expression level of CBS in primary ovarian cancer specimens, and found high expression of CBS in the cytosol of primary ovarian tumors, especially in serous carcinoma, as well as in various ovarian cancer cell lines (Fig. 7). CBS was already present in early stages, but its levels further increased in more advanced forms of ovarian cancer (10).
FIG. 7.
CBS is highly expressed in human ovarian cancer. (A) Immunohistochemical staining of a tissue microarray of epithelial ovarian cancer samples. Representative images are shown of none (i), weak (ii), moderate (iii), and (iv) strong staining. (B) Expression of CBS and CSE in various ovarian cell lines as determined by immunoblotting. α-Tubulin was used as the loading control. (C) Real-time polymerase chain reaction (RT-PCR) data showing the expression of CBS mRNA in various ovarian cell lines. (D) RT-PCR data showing the expression of CSE mRNA in various ovarian cell lines. Reproduced by permission (10). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
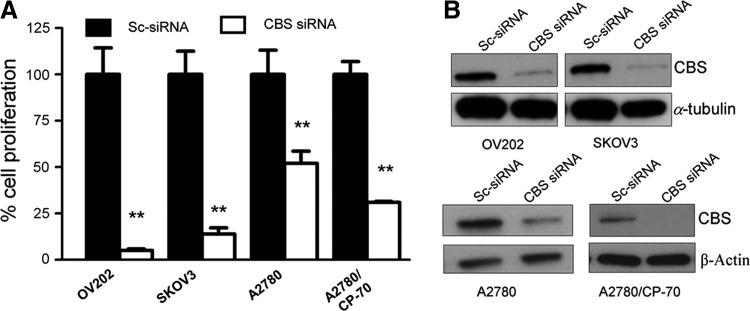
Similar to our studies in HCT116 cells, Bhattacharyya et al. determined the functional effects of CBS silencing on ovarian cancer cell biology using CBS-specific siRNA-mediated gene knockdown. To exclude nonspecific targeting, two different siRNAs were used. The efficiency of the silencing was determined as 80% using Western blotting. CBS silencing resulted in a significant decrease in proliferation in all ovarian cancer cell lines studied (Fig. 8) (10). Similar to HCT116 cells, CBS silencing in A2870 ovarian cancer cells markedly suppressed cellular metabolism (mitochondrial electron transport); both basal oxygen consumption responses and the enhanced respiratory responses induced by a mitochondrial uncoupler were inhibited (10). The effects of CBS silencing were also associated with a decrease in cellular NAD/NADH ratio, a partial suppression of cellular ATP levels, and a significant decrease in cell viability (10). Similar to our earlier findings in colon cancer cells, a large proportion of CBS in ovarian cancer cells was localized to the mitochondrial compartment (Fig. 9A) (10). As expected, silencing of CBS significantly decreased total cellular glutathione levels in the ovarian cancer cells, while exogenous administration of GSH improved cell viability (10). Moreover, CBS silencing resulted in an increase in cellular reactive oxygen species (ROS) levels (Fig. 9B) (10), consistent with the role of H2S as an endogenous mitochondrial antioxidant. Since H2S exerts a protective/antioxidant effect on mitochondria (93, 113, 135), we speculate that this effect may be especially relevant for cancer cells, which often contain partially uncoupled mitochondrial electron transport and produce high levels of mitochondrial ROS (102, 134).
FIG. 8.
CBS silencing inhibits the proliferation of human ovarian cancer cells. (A) Effect of CBS knockdown on the proliferation of OV202, SKOV3, A2780, and A2780/CP-70 cells. (B) Immunoblotting data to determine the extent of siRNA-mediated knockdown. (**p<0.01 shows significant inhibition of cell proliferation by CBS siRNA.) Reproduced by permission (10).
FIG. 9.
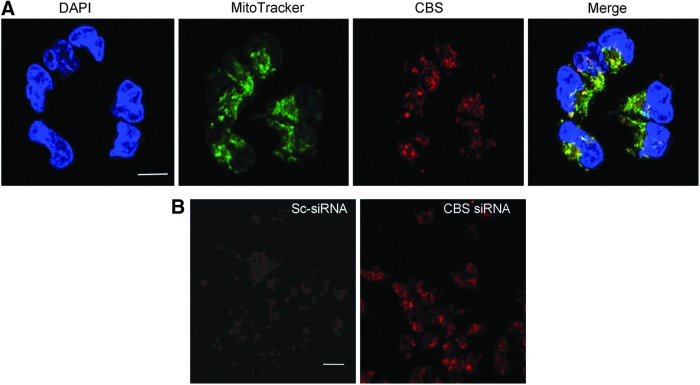
CBS is present in the mitochondria of human ovarian cancer cells, and its silencing increases mitochondrial reactive oxygen species production. (A) Localization of CBS in A2780 cells determined by immunofluorescence using confocal microscopy. Nuclear stain with DAPI (blue channel), CBS (red channel), and MitoTracker green (green channel) was used to label mitochondria. Scale bar is 10 μm. (B) MitoSOX staining in live A2780 cells shows the buildup of mitochondrial superoxide on silencing CBS. Scale bar is 30 μm. Reproduced by permission (10). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The effect of CBS silencing was also evaluated with regard to the activity of two critical redox-sensitive signaling pathways, p53 and NF-κB. CBS silencing in A2780 cells increased the expression of p53, while the expression of the RelA/p65 subunit of NF-κB decreased and the activation of NF-κB was attenuated (10). Although the exact connection of these responses to H2S and to cell proliferation and cell viability remains to be determined, these data confirm earlier suggestions (117, 118, 120) on the existence of an intricate interplay between H2S production, cellular bioenergetics, cellular redox status, and intracellular signaling pathways.
Consistent with our findings in colon cancer cells, the other product of CBS, L-homocysteine, failed to affect cell viability or proliferation in ovarian cancer cells, while “rescue” experiments with an authentic H2S donor (Na2S) restored cell proliferation in CBS-silenced ovarian cancer cells (10).
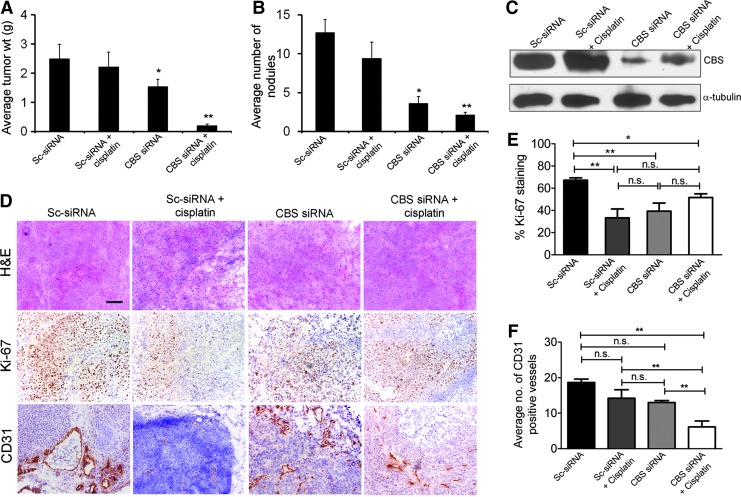
Nanoliposomal siRNA silencing of CBS in ovarian cancer xenografts significantly reduced tumor growth, with a 40% reduction in tumor burden and a 70% reduction in the number of nodules (Fig. 10A, B) (10). CBS silencing resulted in a significant growth inhibition in vivo, as assessed by Ki-67 staining, a nuclear proliferation marker (Fig. 10D). Finally, the number of CD31-positive blood vessels in the tumor was decreased, consistent with a paracrine angiogenic effect of tumor-derived H2S (10), similar to our earlier observations (117) in colon cancer cells. In the ovarian cancer model, the potential additive or synergistic effect of CBS silencing with cisplatin was also explored. In vitro, CBS silencing produced a slight potentiation of cisplatin cytotoxicity in A2780 cells; while in the in vivo experiments, the combination of cisplatin and CBS silencing produced a near-complete inhibition of ovarian cancer cell volume and nodule number (Fig. 10A, B) (10). Based on in vitro data, it is conceivable (although it remains to be directly tested) that the depletion of glutathione plays a role in the CBS-silencing mediated enhancement of cisplatin cytotoxicity.
FIG. 10.
SiRNA-mediated down-regulation of CBS inhibits ovarian cancer growth in vivo and synergizes with cisplatin therapy. To assess the effects of siRNA therapy on tumor growth, treatment was initiated at 1 week after i.p. injection of tumor cells. Mice were divided into four groups: (i) control siRNA-dioleoyl phosphatidylcholine (DOPC) (150 mg/kg i.p. twice weekly), (ii) control siRNA-DOPC (150 mg/kg i.p. twice weekly)+cisplatin (160 mg/mouse i.p. weekly), (iii) CBS siRNA-DOPC (150 mg/kg i.p. twice weekly), and (iv) CBS siRNA-DOPC (150 mg/kg i.p. twice weekly)+cisplatin ((160 mg/mouse i.p. weekly). Treatment was continued until 4 weeks after tumor inoculation before sacrifice. (A) Mouse and tumor weights and (B) the number of tumor nodules for each group were compared using Student's t-test with p<0.05 considered statistically significant. (C) Immunoblotting of tumor samples for confirmation of CBS knockdown. One animal from each group was selected for immunoblotting analysis. (D) Representative histology of tumors from mice xenografts of A2780/CP-20 cells with Ki-67 expression (middle row) and CD31 expression (lower row) acquired at 20×magnification. Scale bar represents 100 μm. (E) and (F) Quantification of Ki-67 staining and CD31 staining in the mouse xenografts, respectively (n=4). Statistical analysis was determined using one-way ANOVA with *p<0.05 and **p<0.01. Reproduced by permission (10). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Taken together, the studies in colon and ovarian cancer cells clearly establish CBS and CBS-produced H2S as a novel, endogenous, regulator of tumor progression.
Increased Expression of CBS and/or Increased Production of H2S in Other Forms of Cancer
Previous studies suggested increased expression of CBS and/or the increased production of H2S in various cancers. The earliest published report dates back to 1987 when Klein et al. detected elevated CBS activity in neuroblastoma surgical resection homogenates; these increases were also associated with elevated urinary cystathionine levels in the patients (62). In fact, the observation that cancer patients present with cystathionuria dates back as far as the early 1960s (34, 35); detection of cystathionine in the urine of patients was considered of diagnostic potential (33, 128). Stabler et al. showed markedly elevated urinary levels of cystathionine in the urine of prostate cancer patients; the levels correlated with the progression of the disease (111). While there are multiple pathways that produce cystathionine, an intriguing possibility may be the contribution of CBS up-regulation in cancer cells to this response. However, this hypothesis remains to be experimentally tested.
In myeloma samples, oligonucleotide microarray analysis showed a 16-fold increase in CBS gene expression (23) and a 7-fold up-regulation of CBS transcripts in biliary cancers (43). In an expression profiling survey, conducted in 2005, most cell lines of the NCI60 collection of human cancer cell exhibited increased CBS expression (assessed by Western blot analysis), with the highest expression levels noted in breast, ovarian, and renal cancer lines (146). In a more recent study, the prostate cancer cell lines LNCaP, PBH-1, and DU145 showed a marked increase in CBS expression, which correlated with increased H2S production (39).
Several lines of evidence from recent studies demonstrate significant increases in exhaled H2S levels in cancer patients (4, 48, 66, 140); however, the identification of its biological source was not investigated. In addition, elevated urinary thiosulfate (the stable breakdown product of H2S) levels were found in prostate cancer patients; the mean thiosulfate levels in prostate cancer patients were almost 50 times higher than in the control groups and 5 times higher than in patients with benign prostatic hyperplasia (19). Again, the cellular sources of thiosulfate were not defined in this study.
We speculate that there may be three potential mechanisms of the increased H2S levels in cancer patients. (i) Tumor cell-derived H2S may be absorbed into systemic circulation and exhaled through the lungs and/or excreted into the urine via its oxidative degradation to thiosulfate. (ii) CBS may be up-regulated as a consequence of local or systemic inflammation [as reviewed in Refs. (29, 132)]; thus, cancers develop in this pre-inflammatory microenvironment). (iii) Normal intestinal microbiota produces significant amounts of H2S; however, in the presence of cancer, additional tumor-derived H2S may contribute to elevate basal systemic H2S levels [as reviewed in Ref. (120)].
Effect of CBS Inhibition in Preclinical Models of Cancer
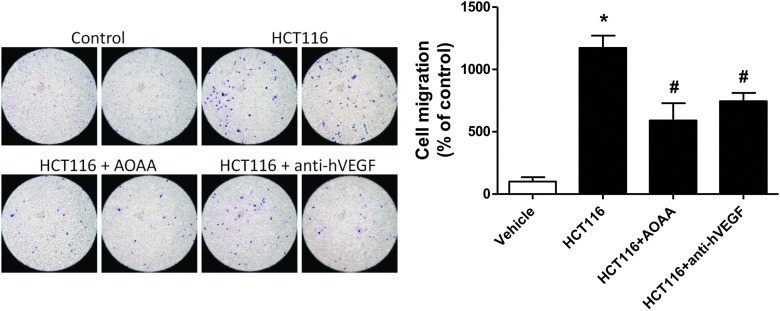
Aminooxyacetic acid (AOAA) is a prototypical pharmacological inhibitor of CBS that is widely used in H2S biology. Although the selectivity of AOAA is limited, it is currently the best pharmacological tool that is used to inhibit CBS-derived H2S production in vitro or in vivo. Using HCT116 cells, we and others have shown that AOAA treatment recapitulates all of the effects of CBS gene silencing, including the inhibition of cellular bioenergetics, proliferation, and H2S production (117). AOAA treatment also suppressed the migration and invasion of HCT116 cells toward fibroblast conditioned media and reduced endothelial cell migration toward HCT116 colon cancer cells in a multi-chamber co-cultures assay (Fig. 11). The efficacy of AOAA was comparable to that of a VEGF neutralizing antibody in this experimental system (Fig. 11). Furthermore, studies conducted in ovarian cancer cell lines (10), the breast adenocarcinoma cell line MDA-MB-231 (124), and pancreatic ductal adenocarcinoma cells (109) showed that AOAA treatment attenuated cellular proliferation, viability, and bioenergetic functions in vitro.
FIG. 11.
Both AOAA and vascular endothelial growth factor (VEGF) neutralization inhibit tumor-induced endothelial cell migration. Tumor-induced endothelial cell migration was tested in vitro in a co-culture assay involving human umbilical endothelial cells (EAhy926) and human colon cancer cells (HCT116). HCT116 cells were seeded to confluence in the lower chamber of a 6.5 mm Transwell insert (8.0 μm pore); while EAhy926 were serum starved for 5 h, detached by Trypsin-EDTA, re-suspended in serum-free media, and added to the upper chamber (105 cells/well). Cells were allowed to migrate for 4 h. Migrated cells were fixed with Carson's fixative, stained by 0.33% Toluidine blue, and quantified by visual counting. AOAA (1 mM) and antihuman VEGF polyclonal antibody (5 μg/ml) were added to the HCT116 culture at 45 min (37°C) before the addition of the endothelial cells to the upper chamber (*p<0.05 vs. vehicle and #p<0.05 vs. HCT116). The comparable degree of inhibition of endothelial cell migration by AOAA and of VEGF neutralization should be noted. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
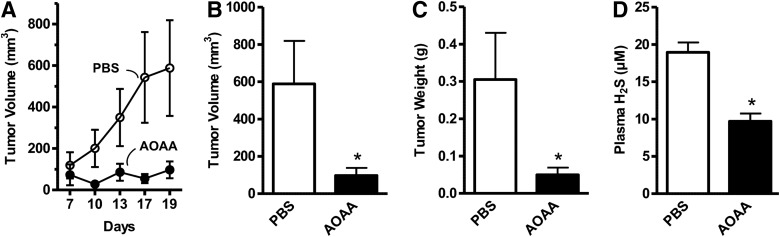
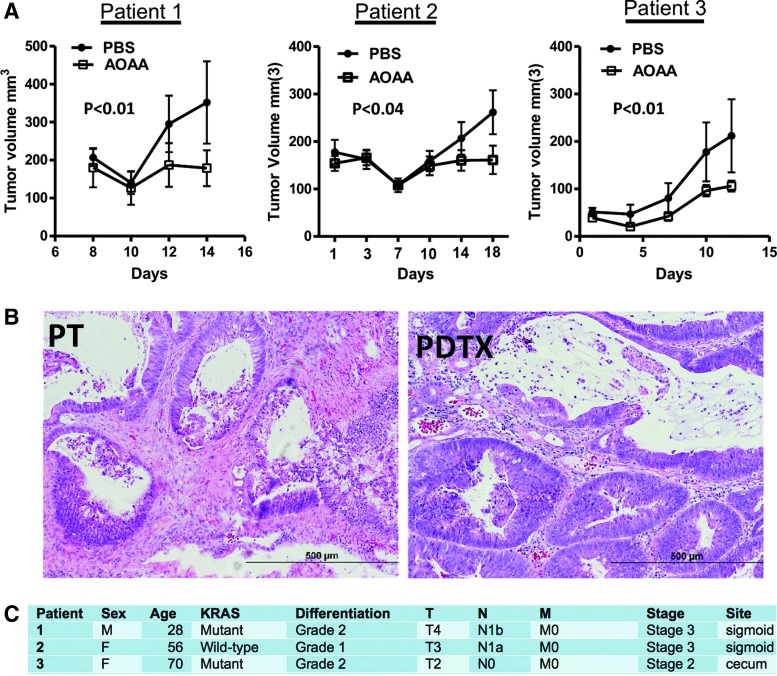
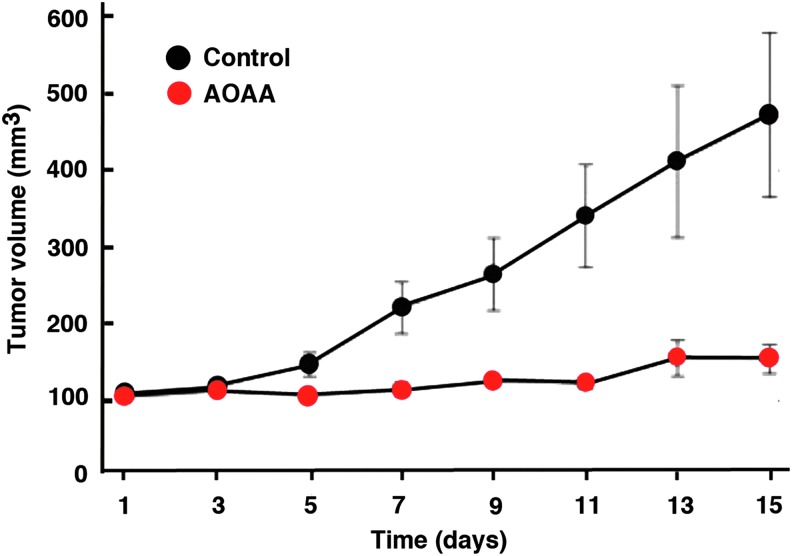
In vivo, AOAA exerted a marked inhibition of subcutaneous HCT116 cell tumor xenografts in athymic-nude mice (Fig. 12) (117), and the growth of patient-derived tumor xenografts (PDTXs) from three different colon cancer patients (Fig. 13). Moreover, in vivo treatment of mice with AOAA also attenuated the growth of MDA-MB-231 cell xenografts in nude mice (Fig. 14) (124).
FIG. 12.
AOAA inhibits HCT116 colon cancer growth in vivo. Effects of AOAA or its vehicle (phosphate-buffered saline [PBS]) on HCT116 tumor xenografts' (A) growth rate, (B) tumor volume (*p=0.02), (C) wet weight (*p=0.001), and (D) plasma concentrations of H2S (*p=0.0005). Reproduced by permission (117).
FIG. 13.
AOAA inhibits colon cancer growth in patient-derived tumor xenografts (PDTX) in vivo. (A) Effects of AOAA or its vehicle (PBS) on PDTX growth rate in three different patients. p<0.05 was considered statistically significant. (B) Photomicrographs of H&E-stained formalin-fixed paraffin-embedded sections (5 μm) of the primary colon adenocarcinoma from a patient with stage III disease and Kras mutation (PT), and the corresponding PDTX. The similar morphology of both specimens should be noted. (C) Patient information with regard to sex, age, KRAS mutation status, differentiation, tumor severity grade/stage, and tumor localization. PDTX data from patients 1 and 2 are reproduced by permission (117). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 14.
AOAA inhibits breast cancer growth in vivo. MDA-MB-231 xenografts were initiated in nude mice. Tumors were measured daily using blunt end Vernier calipers, and mice with established tumors (130–190 mg) were blindly randomized into either PBS control (black symbols) or AOAA treatment (red symbols) groups. Experimental mice were weighed, and they were given daily intraperitoneal injections of either PBS or 0.2 mg AOAA. Data are plotted as the mean±SD. It should be noted that AOAA markedly suppressed the growth of established breast cancer tumors. Reproduced by permission (124). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
CBS inhibition also attenuated metastasis formation, as demonstrated in our recent studies, and enhanced the effect of oxaliplatin therapy (16a). Taken together with the studies demonstrating the synergy between cisplatin and CBS silencing in the ovarian cancer cells (10), CBS inhibition may enhance the efficacy of standard-of-care chemotherapeutic agents to reduce both recurrent and metastatic disease.
Pharmacological Inhibitors of CBS
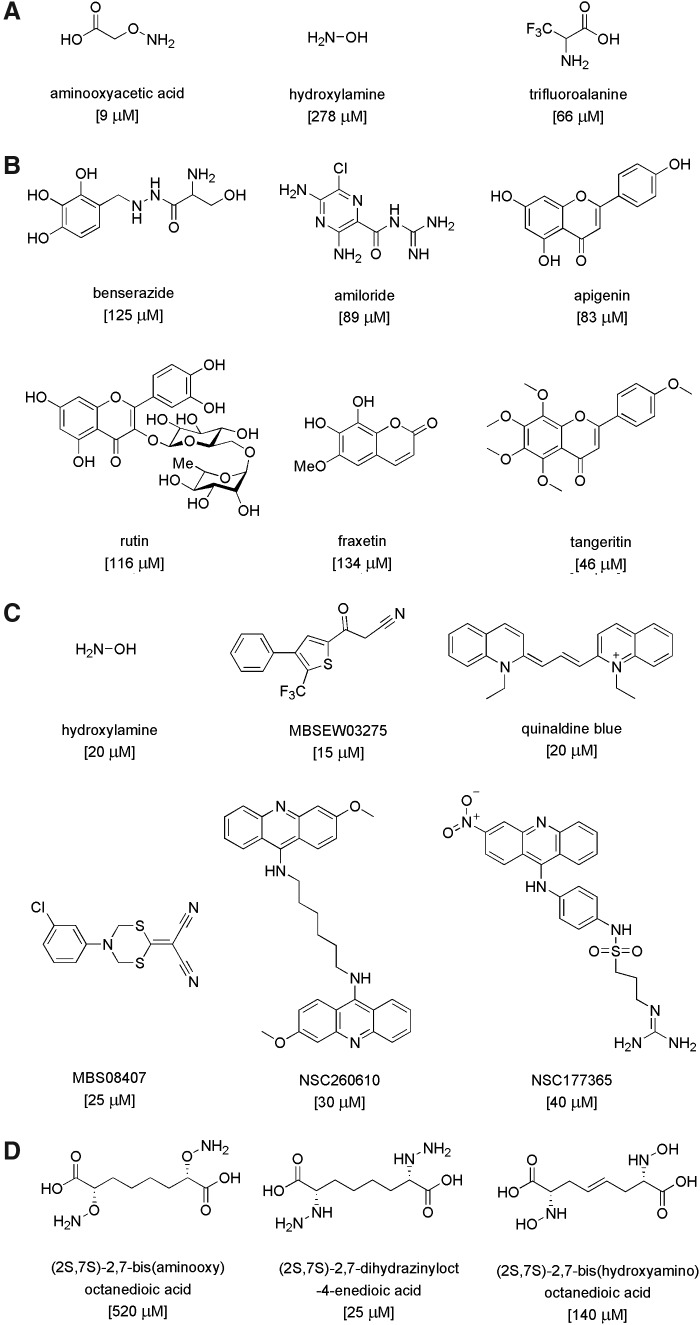
The field of CBS inhibitors is in its infancy, because before the discovery of the role of CBS in cancer, there was no biomedical reason to synthesize or develop CBS inhibitors. In a recent study conducted in a collaborative effort between the Papapetropoulos group and our own group (5), a number of previously identified H2S biosynthesis inhibitors were tested with regard to the enzymatic activity of human recombinant GST-tagged CBS and CSE enzymes. For CBS inhibition, the potency was the highest for AOAA (IC50=9 μM), while trifluoroalanine and hydroxylamine showed weaker inhibition (IC50=66 and 278 μM, respectively) (Fig. 15A). Propargylglycine (PAG) and L-aminoethoxyvinylglycine were confirmed as fairly selective CSE inhibitors without any inhibitory effect on CBS for approximately 1 mM. Beta-cyanoalanine was also a preferential CSE inhibitor, which only exerted a slight inhibitory effect on CBS at 1 mM and above. The known PLP-dependent enzyme inhibitors D-cycloserine, isoniazid, parsalmide, pargyline, and methylseleno-cysteine exhibited a 6%–26% inhibition of CBS activity at 100 μM and a 10%–21% inhibition at 1 mM. None of the compounds tested showed selectivity to CBS compared with CSE (5).
FIG. 15.
Pharmacological inhibitors of CBS. (A) shows the comparative potency of previously known CBS inhibitors, as determined by the “methylene blue” assay by Asimakopoulou and colleagues at the University of Patras and UTMB Galveston (5). (B) shows newly identified CBS inhibitors identified by a screen utilizing a fluorescent H2S detection method by Thornburg et al. at the University of Utah and the University of Colorado at Denver (125). (C) shows newly identified CBS inhibitors identified by Zhao and colleagues at the Shanghai Jiao Tong University [Shanghai, China (147)] using a fluorescent H2S detection method, and the potency of the previously known inhibitor, hydroxylamine, in the same assay. (D) shows the most potent CBS inhibitors synthesized by rational design by Shen at the University of Nebraska (105) and tested in a radioactive CBS assay. All assays used human recombinant CBS enzyme. Structures of the inhibitors, names, and IC50 values in brackets are shown. Due to differences in the assay conditions, inhibitory potencies of the compounds should be compared within the same assay, but not within assays.
In late 2013, two articles were published on small-molecule screening for CBS inhibitors. Both projects used cell-free assays utilizing recombinant CBS enzymes and employed fluorescent H2S readouts (125, 147). Thorson et al., using a truncated version of human CBS (D414-551), screened a composite library of 1900 compounds (including compounds with known biological activity, clinically used drugs, and natural products) from the Microsource Spectrum Collection. An initial screen showed that twelve compounds at a concentration of 150 μM exhibited significant inhibitory activity in their fluorescence assay. Subsequent evaluations confirmed, the activity of several inhibitors included benserazide, a clinically used compound and known inhibitor of L-3,4-dihydroxyphenylalanine decarboxylase (a PLP-dependent enzyme), amiloride (a potassium-sparing diuretic and clinically used drug), and several flavonoids, including apigenin, tangeritin, and rutin. The structures of the compounds mentioned earlier and their reported IC50 are shown in Figure 15B. However, the relative potency of these compounds, compared with prototypical CBS inhibitors (e.g., AOAA or hydroxylamine) in their assay, is unknown.
Zhou et al. used recombinant human full-length CBS in a tandem microplate assay (147) to screen a chemical library consisting of ∼20,000 compounds assembled from NCI, FDA, and the Johns Hopkins Clinical Compound Library, Maybridge, ChemBridge, and other sources. Hit confirmation assays included selectivity testing against CSE, as well as the confirmation of direct binding by the hit molecules using a surface plasmon resonance assay, followed by in silico docking analysis. Controls included hydroxylamine (which, as expected, inhibited both CBS and CSE) and PAG, which, [in accordance with earlier studies (5)] was selective for CSE).
The screening yielded eleven CBS inhibitor compounds with IC50 values lower than 50 μM. Almost all of these inhibitors belonged to different structural classes, with NSC111041 and NSC67078 being the most potent inhibitors (IC50: 4 and 12 μM, respectively). Five compounds: MBSEW03275, quinaldine blue (also known as pinacyanol; code name in the screen: JHU-8555), MBS08407, NSC260610, and NSC177365 showed some selectivity for CBS compared with CSE, with MBS08407 showing the highest (>16-fold) selectivity, and an IC50 of 25 μM (147) (Fig. 15C). The potency of AOAA was not reported; however, another CBS inhibitor (hydroxylamine) had an IC50 of 20 μM under the assay conditions employed for the screening.
Others have used a structure-function paradigm to develop potential CBS inhibitors (105). Their efforts focused on three series of putative CBS inhibitors, featuring oxyamino, hydrazino, and hydroxylamino functionalities, designed to engage the active site cofactor PLP in stable oxime, hydrazone, and nitrone adducts, respectively. Since these molecules are functionalized analogues of CBS product L-cystathionine, the assays were designed such that the compounds competed with endogenous (L,L)-cystathionine at the active site of CBS via CBS “reverse reaction” involving the hydrolysis of (L,L)-cystathionine to L-serine and L-homocysteine in the presence of CBS. The L-homocysteine formed was trapped and measured with Ellman's reagent. The most potent inhibition (25–30 μM) was observed with some of the hydroxyamino compounds. The oxamino and hydrazino derivatives exhibited low potency (IC50 between 500 and 3000 μM), although the inhibition became more potent (140–1200 μM) when the compounds were pre-incubated with the enzyme, indicating a slow binding kinetic. The most potent compounds from each class were (2S,7S)-2,7-bis(aminooxy)octanedioic acid, (2S,7S)-2,7-bis(aminooxy)octanedioic acid, and (2S,7S)-2,7-bis(hydroxyamino)octanedioic acid (Fig. 15D). Direct measurements demonstrated that these compounds form complexes with the CBS prosthetic group PLP as expected.
In the same work (105), an additional approach was also described, aimed at synthesizing and testing quartenary α-branched amino acids as a new class of potential electrophile-releasing triggers for CBS (as well as other PLP-dependent enzymes). For this series of compounds, CBS inhibitory effects were assessed using a radioactive assay. The inhibitors were preincubated with hCBS dimer (D,L)-homocysteine followed by the addition of [14C]L-serine. The radioactive product of the reaction was chromatographically separated and quantified by scintillation counting. These compounds were characterized as weak inhibitors; 30 mM O-difluromethyl L-serine or S-difluromethyl L-cysteine produced an ∼50% inhibition of catalytic activity after 60 min of pre-incubation with the enzyme.
To be complete, the gaseous transmitters CO and NO should also be mentioned, as they represent a special “class” of endogenous CBS inhibitors. These molecules bind to the heme group of CBS. The biochemical characteristics of these interactions have been explored in significant detail (16, 95, 112).
Different experimental conditions yield different relative IC50 values for CBS inhibition. For instance, CBS activity and the inhibitory potency of various compounds are influenced by the source and structure of the recombinant CBS used (i.e., species, full length vs. truncated etc.), as well as by the assay conditions (e.g., pH, buffer, time of pre-incubation of test compounds with the enzyme, read-out of the assay, plate format, etc.). As an example, a slight modification in the composition of the assay buffer produced a shift in the IC50 of hydroxylamine from 20 μM to above 200 μM (147). Therefore, inclusion of reference compounds/standards (e.g., hydroxylamine, AOAA) in the screening assays is essential to enable comparisons between different screening campaigns conducted in different laboratories. In the absence of such reference standards, the IC50 values reported in Figure 15A–D only can be compared within each screen, but not between different screens. For example, in our previous screen in human CBS, AOAA and hydroxylamine were determined to have respective IC50 values of 9 and 278 μM (5). In contrast, Zhou et al. established the IC50 of hydroxylamine as 20 μM under the conditions applied for screening (147); in other words, all of the identified hits in this screening campaign were comparable, in terms of potency, to hydroxylamine (Fig. 15C). Thus, there is a high likelihood that according to the present state of the art, all currently known CBS inhibitors lay in the AOAA-hydroxylamine range, perhaps with AOAA remaining the most potent molecule. However, this remains to be directly tested in future studies.
With regard to therapeutic repurposing of existing FDA-approved drugs, it appears that those identified as CBS inhibitors to date lack the necessary potency and/or selectivity for CBS-targeted therapy. For example, inhibition of CBS by amiloride or benserazide requires concentrations of 100 μM or higher, which would be difficult to achieve therapeutically, as the achievable plasma concentration of these drugs in humans is in the low nM range (52, 53). Based on the published database of clinically used compounds that are candidates for repurposing (18), Zhou et al. referred to quinalidine blue/pinacyanol chloride as an FDA-approved anticancer compound (147). However, other than the fact that the compound used to be a part of in vitro staining techniques for chromosome banding or beta-amyloid staining (86, 101), we were unable to find any in vivo studies or clinically relevant information on this compound in the literature. Therefore, the status of this compound as a potential human therapeutic agent remains unclear.
Taken together, recent work in the field of CBS inhibitors may have identified potentially useful chemical scaffolds for future medicinal chemistry optimization. It is likely that some of these compounds target the PLP site of CBS, increasing the potential for cross-reactivity with other PLP-dependent enzymes.
The Pharmacological Profile of AOAA: Implications for Cancer Therapy
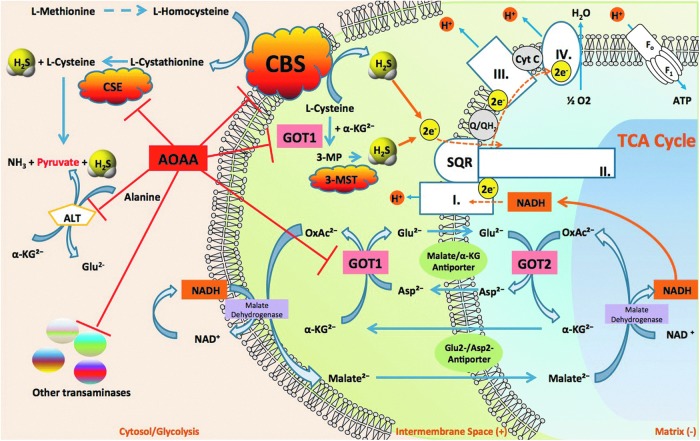
Although referred to as the prototypical CBS inhibitor, the pharmacology of AOAA is complex (78) and not entirely specific. Its mode of action entails an irreversible binding to the prosthetic group PLP in CBS and therefore other PLP-dependent enzymes. For example, AOAA inhibits CSE (5). However, when compared under the same assay conditions, AOAA is a much weaker inhibitor of CSE (IC50=1 μM) when compared with CBS (IC50=9 μM) (5). In isolated rat aortic rings assay (a tissue with low levels of CBS), pretreatment with AOAA inhibits L-cysteine-induced smooth muscle relaxations to the same extent as the CSE inhibitor PAG; while the combined treatment of PAG and AOAA exerted no greater inhibition of relaxation, suggesting that AOAA can inhibit CSE in vascular tissue (5) (Fig. 16). Thus, the use of AOAA as an anticancer therapy may potentially cause hemodynamic side effects due to the physiological actions of CSE/H2S on vasodilator tone.
FIG. 16.
Multiple pharmacological actions of AOAA. By simultaneously inhibiting CBS activity and inhibiting glutamate oxaloacetate transaminase 1 (GOT1), a key enzyme of the malate/aspartate shuttle, AOAA acts as an inducer of “synthetic lethality” in cancer cells. CBS-derived H2S supports mitochondrial electron transport and cancer cell bioenergetics by donating electrons at complex II. By inhibiting CBS, AOAA suppresses this bioenergetic pathway. The malate-aspartate shuttle translocates electrons that are produced in glycolysis across the semipermeable inner membrane of the mitochondrion, in order to support oxidative phosphorylation. These electrons enter the electron transport chain at complex I. The shuttle system is required, because the mitochondrial inner membrane is impermeable to NADH (a primary reducing equivalent of the electron transport chain). In humans, the cytoplasmic enzyme (GOT1) is one of the key enzymes in the malate shuttle: It functions to catalyze the interconversion of aspartate and α-ketoglutarate to oxaloacetate and glutamate using PLP as a cofactor. By inhibiting GOT1, AOAA reduces the transfer of electron donors to the mitochondria, thereby suppressing cancer cell bioenergetics. By the simultaneous inhibition of CBS and GOT1, AOAA interferes with two key pathways of cancer cell mitochondrial function. In addition, by inhibiting G0T1 (also termed CAT), AOAA may have additional inhibitory effects on mitochondrial H2S production by reducing the substrate level of 3-MST and thereby indirectly inhibiting its activity. Moreover, AOAA may also inhibit several additional PLP-dependent enzymes in the cell, including the cytosolic enzyme CSE. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
It is possible that AOAA exerts additional inhibitory effects on H2S production through the indirect inhibition of 3-MST. 3-MST generates H2S from 3-mercaptopyruvate (3-MP), which is produced by the cysteine aminotransferase (CAT, EC 2.6.1.3)-dependent condensation of cysteine and α-ketoglutarate through the following reaction: L-cysteine+2-oxoglutarate→mercaptopyruvate+L-glutamate. CAT is identical to cytosolic aspartate aminotransferase (EC 2.6.1.1, also known as glutamate oxaloacetate transaminase 1 [GOT1], aspartate aminotransferase, or aspartate transaminase [AST]). In its latter (GOT or AST) function, the enzyme catalyzes the following reaction: L-aspartate+2-oxoglutarate→oxaloacetate+L-glutamate. Inhibition of CAT by AOAA would be expected to inhibit the formation of 3-MP and therefore, it would be expected to inhibit 3-MST-induced H2S production (Fig. 16).
To further increase the complexity of the subject, we also have to mention that GOT1 is an essential component of the malate/aspartate shuttle. In cancer cells, this mechanism has been shown to link glycolysis to the transfer of electron donors into the mitochondria and this transfer is inhibited by AOAA (22, 37, 124, 133). Moreover, the function of GOT1 is linked to glutaminolysis, another partially cancer-selective metabolic pathway and anticancer target (109). Knockdown of GOT1 and other essential enzymes in the glutaminolysis pathway have recently been shown to suppress cancer cell metabolism (109). Therefore, we have recently suggested (78) that AOAA may exert its anticancer effects through the simultaneous suppression of the malate/aspartate shuttle, glutaminolysis, and the CBS/H2S axis. In effect, through these diverse, but interrelated pharmacological actions in the cancer cell, AOAA can be viewed as an inducer of “synthetic lethality” (Fig. 16).
Although not an FDA-approved drug, AOAA was given to patients with Huntington's disease and tinnitus in several non-randomized clinical trials in the 1970s and 1980s (42, 91, 92). In these disorders, AOAA was used to inhibit an enzyme in neurons, glutamic acid decarboxylase or GABA transaminase (GABA-T), a PLP-dependent transaminase. These clinical studies established dose-limiting toxicities in human subjects and suggested that AOAA can be reasonably well tolerated. However, the study design and drug formulation would not be acceptable if we applied today's regulatory standards. Nevertheless, with appropriate chemical and regulatory preparations, AOAA or closely related compounds may be amenable for re-introduction for cancer therapy in the future.
H2S Donation Versus H2S Biosynthesis Inhibition in Cancer
Literature searches focusing on “hydrogen sulfide” and “cancer” keywords will identify a significant number of articles which support the concept that cancer cells can be killed via H2S donation. On the surface, these studies appear to contradict key concepts outlined earlier. However, many of the contradictory findings regarding the effects of H2S on cancer cells may be explained by a careful consideration of a bell-shaped dose-response curve that characterizes H2S activity in biological systems.
Some of the published reports use complex molecules, where the H2S donor group is linked with a larger molecule, which also has its own independent pharmacological action. Prime examples of such parent molecules include non-steroidal anti-inflammatory drugs (NSAIDs) such as diclofenac, naproxen, or sulindac (17, 30, 57, 63). In these studies, typically no information is presented on the relative contribution of H2S versus the parent molecule to the anticancer effects, even though it is well known that NSAIDs exert anticancer effects in many preclinical models of cancer through inhibition of the pro-inflammatory cyclo-oxygenase signaling pathways. In fact, the beneficial property of the H2S donating group is likely related, at least in part due to its vasodilatory/cytoprotective effects on the gastric mucosa, which permits higher doses of the parent NSAIDs without gastric irritation. Moreover, simple calculations of the H2S “content” of these molecules, and a comparison of these numbers with the effects of authentic H2S donors makes it unlikely that the H2S contained in these structures can reach sufficiently high levels to induce cytotoxicity in the cancer cell. For these reasons, we have excluded the combined molecules mentioned earlier from our present analysis. In another study, Ma et al. report on the anticancer effect of the garlic extract component S-propargyl-cysteine (72). Although this compound is often discussed as a H2S pathway modulator, its pharmacological profile is extremely complex, as it includes kinase activation, up-regulation of CSE, and many other actions (9, 72). Thus, while the compound can induce apoptosis in vitro and may reduce tumors in vivo, we believe that its actions are too complex to be evaluated in the current review.
We will focus solely on studies where the effect of H2S donors was directly tested on the viability and/or proliferation of various cancer cell lines in vitro (6, 8, 13, 85, 139). For instance, Wu et al. using colon cancer cells (includig HT29 and HCT116) reported that H2S (400–1000 μM) decreased cell proliferation and induced autophagy in a concentration-dependent manner (139). Moore and colleagues used the slow-release H2S donor GYY4137 in multiple human cancer cell lines (HeLa, HCT116, Hep G2, HL-60, MCF-7, MV4-11, and U2OS) and found that the H2S donor decreased cancer cell survival at 400–800 μM (68). Similarly, Attene-Ramos et al. used CHO cells and HT29 cells, and showed a concentration-dependent decrease in cell viability at concentrations above 100 μM (6) and also when used in combination with caustic agents such as hydroxylurea (6). Finally, Cai et al. evaluated a wide range of H2S concentrations in HCT116 and SW480 cells and reported a concentration-dependent stimulation of cell proliferation between 10 and 50 μM H2S, a plateauing of the effect at 200 μM, and an inhibition of proliferation at 1000 μM (13).
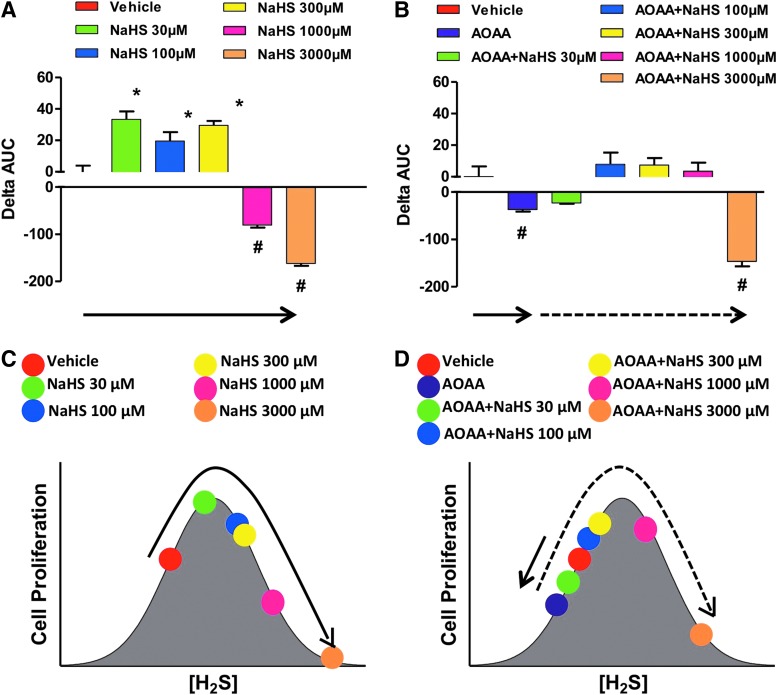
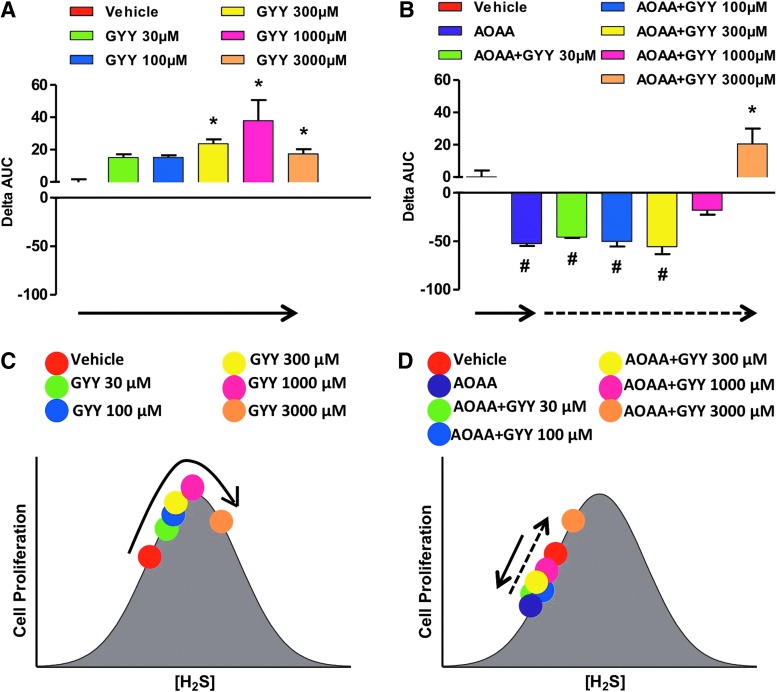
Other studies reported diverse effects of H2S and cell growth/cell death in various transformed and non-transformed cell lines in vitro (6, 8, 13, 83, 85, 121, 139). Many of these reports are summarized in a review article by Baskar and Bian (8). A critical evaluation of the conditions and the concentrations of the H2S donors suggests that the effects of H2S are bi-phasic, as in a bell-shaped curve, where lower concentrations of H2S are pro-survival and proliferative, while higher concentrations of H2S become cytostatic, cytotoxic, and antiproliferative. We also hypothesize that the net effects seen in cell proliferation are the result of the net balance of endogenously produced and exogenously administered H2S. We assessed the proliferation rates of HCT116 colon cancer cells in the presence of different concentrations (0.03–3 mM) of either NaHS (fast-release) or GYY4137 (slow-release) H2S donors, with or without pretreatment with the CBS inhibitor AOAA. In the absence of AOAA, low concentrations (0.03–0.3 mM) of NaHS stimulated proliferation whereas higher concentrations (1–3 mM) inhibited growth (Fig. 17A). CBS inhibition with AOAA shifted the dose response to the left, not only decreasing basal proliferation but also limiting toxicity only to cells exposed to 3 mM NaHS (Fig. 17B). In the absence of AOAA, all concentrations of GYY4137 (0.03–3 mM) stimulated HCT116 cell proliferation over the basal growth rate (Fig. 18). However, only the highest concentration of GY4137 (3 mM) stimulated HCT116 cells when CBS was inhibited (Fig. 18). These data demonstrate that colon cancer cell proliferation exhibits a biphasic (bell-shaped) dose response to H2S. The precise nature of the cellular response (increased or decreased growth) is determined by the rate of H2S production (fast- vs. slow-release H2S donors) as well as by the concentration of donor relative to the endogenous (basal) level of CBS-dependent H2S production. Our model provides a useful framework to reconcile some of the controversies in the literature regarding the role of H2S in the regulation of cancer cell proliferation.
FIG. 17.
Bell-shaped effect of the fast-acting H2S donor NaHS on the proliferation of HCT116 cells in the absence or presence of AOAA. (A, B) show the effect of various concentrations of NaHS on proliferation. The HCT116 cell proliferation was assessed in real time for approximately 72 h using the xCELLigence system as described (117), and the effect of the H2S donor was calculated as the change in the area under the curve (0–72 h) relative to the proliferation of vehicle-treated cells. In this analysis, the ΔAUC of the cells in the presence of vehicle only is defined as zero. Columns that are oriented in the positive direction represent increases in proliferation in response to the H2S donor compared with vehicle (*p<0.05); negative columns represent the inhibitory effect of NaHS on cell proliferation (#p<0.05). (C, D) show the interpretation of the findings based on a bell-shaped dose-response model. (A, C) as well as (B, D) are color-coordinated to represent identical experimental groups. See “H2S Donation vs. H2S Biosynthesis Inhibition in Cancer” for additional explanation of the model. Data represent mean±SEM of n=4 independent determinations. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 18.
Bell-shaped effect of the slow-acting H2S donor GYY4137 on the proliferation of HCT116 cells in the absence or presence of AOAA. The effect of GYY4137 on HCT116 cell proliferation is shown (A, B). Cell proliferation was monitored for approximately 72 h by xCELLigence system as described (117). The xCELLigence enables real-time monitoring of the cell status and is carried out in a specially designed 96-well plate containing microelectrodes to measure the impedance of the cell monolayer, also called cell index (CI). Curves of the CI over time were obtained, and the effect of the H2S donor was calculated as relative change in the area under the curve (0–72 h) over the vehicle-treated cells. In this analysis, the ΔAUC of vehicle-treated cells is defined as zero. Positive or negative values represent, respectively, increases or decreases in HCT116 cell proliferation in response to the H2S donor compared with vehicle (*p<0.05 and #p<0.05). (C, D) shows the interpretation of the findings based on a bell-shaped dose-response model. (A, C) as well as (B, D) are color-coordinated to represent identical experimental groups. See “H2S Donation Versus H2S Biosynthesis Inhibition in Cancer” for additional explanation of the model. Data represent mean±SEM of n=4 independent determinations. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
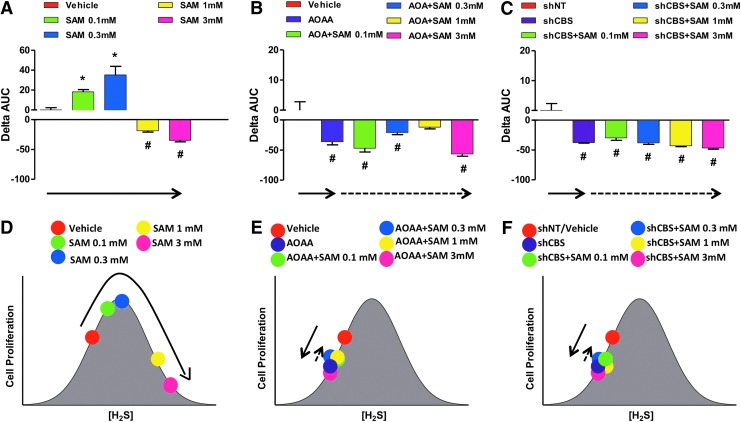
Similar to the bell-shaped pharmacological effects of the H2S donors, lower concentrations of the allosteric CBS activator, SAM increase proliferation; whereas higher concentrations (3 mM) inhibit it (Fig. 19A). When CBS was catalytically inhibited or physically absent, the allosteric activator was unable to modulate cellular H2S production or cancer cell proliferation (Fig. 19B, C, bottom cartoons).
FIG. 19.
Bell-shaped effect of the allosteric CBS activator SAM on the proliferation of HCT116 cells, and loss of the pharmacological effect of SAM in cells pretreated with AOAA or in cells with stable lentiviral silencing of CBS (shCBS). (A–C) show the effect of various concentrations of SAM on colon cancer cell proliferation either in basal conditions or on pharmacological inhibition/shRNA-mediated silencing of CBS. The assay was conducted using the xCELLigence system as described (117), and the effect of the CBS allosteric modulator was calculated as relative change in the area under the curve of the index of cell proliferation over time (0–72 h). In this analysis, the ΔAUC for the vehicle-treated cells is defined as zero. Columns that are oriented in the positive direction represent increases in proliferation in response to SAM compared with vehicle (*p<0.05); negative columns represent inhibition of proliferation compared with the vehicle-treated control cells (#p<0.05). (D–F) show the interpretation of the findings based on a bell-shaped dose-response model. (A, D), (B, E) as well as (C, F) are color-coordinated to represent identical experimental groups. See “H2S Donation Versus H2S Biosynthesis Inhibition in Cancer” for an additional explanation of the model. Data represent mean±SEM of n=4 independent determinations. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
We should emphasize that the earlier schemes only depict a reductionist model, as the model only considers ambient (endogenous/exogenous) H2S concentrations and simple outcome measures (such as survival or proliferation). The terms “local concentration of H2S” and the “rate of release of H2S from H2S donors” are often used interchangeablely. Thus, in most cases, donors with higher H2S release rates produce higher ambient H2S concentrations. In other situations, H2S donors with different rates can produce not only quantitative but also qualitative differences in cellular responses (68, 136). This aspect of H2S biology remains to be further explored and integrated into our model. In addition, the complexities of the temporal relationship between H2S donation and its effect should be considered. For example, pretreatment of cells or animals with H2S can induce the expression of protective genes and pathways (e.g., ones governed by the antioxidant master switch Nrf2), resulting in altered cellular phenotypes at later time points (i.e., where ambient H2S levels are no longer detectable, the classical phenomenon of “preconditioning”) (15, 143). While these mechanisms are primarily studied in the context of cardiovascular biology, Koike et al. have shown that pretreatment of cancer cells with polysulfides (which have H2S-donating properties, as well as many other pharmacological actions) can induce Nrf2-dependent protective phenotypes (64).
In summary, the simple bell-shaped model outlined in the current section is consistent with the majority of published data and provides a useful framework to reconcile some of the controversies regarding H2S donation versus H2S biosynthesis inhibition.
Unanswered Questions and Future Directions
The field of H2S and cancer is a new and expanding research area. The later sections represent a partial list of open questions and briefly outline potential future research directions.
Why have the cancer cells chosen such an unusual route to support themselves?
We speculate that H2S-mediated metabolism/signaling may represent a “regressive response” by the tumor cell, similar to the Warburg effect, another cancer-specific metabolic alteration, where the tumor cells can switch to anaerobic metabolism. As previously discussed (120), billions of years ago, before the presence of atmospheric oxygen on Earth, H2S was a common metabolic “fuel” for bacteria and other simple organisms. In fact, even present-day organisms living in the oxygen-poor but H2S-rich environment of deep-sea vents utilize H2S to support their metabolic functions. By synthesizing large amounts of intracellular H2S, tumor cells may regress back to such primordial metabolic pathways. One important difference between the H2S-dependent metabolism of the tumor cells and the Warburg effect is that based on what we know about H2S-dependent mitochondrial mechanisms, we do not believe that H2S-dependent metabolism will be maintained in severe hypoxia, because the H2S-fueled electron transport requires oxygen as a terminal electron acceptor at complex IV.
Is CBS-derived H2S the reason that dogs can smell cancer?
Two decades of literature show that dogs can be trained to detect cancer patients, in some cases with astonishing precision (46, 110, 137). The dog's nose can detect H2S down to parts per billion concentrations. When coupled with multiple lines of recent data showing that exhaled H2S and urinary H2S is increased in cancer patients (as discussed earlier), it may possible that dogs sense cancer patients by detecting H2S. However, we believe that dogs detect a complex “signature” of various volatile molecules (including H2S). Such volatile signatures may be useful for the future detection of cancer, either by dogs or by various “artificial nose” systems currently under development (21, 70).
What is the mechanism of up-regulation of CBS in cancer?
Currently, it is not known whether the mechanism involves transcriptional, post-transcriptional mechanisms, or both; further, the trigger/signals that up-regulate CBS are unknown. When CBS expression was studied in the NCI60 panel of tumor cells, the increased CBS protein expression statistically correlated with increased CBS mRNA levels (146), suggesting that transcriptional regulation is a distinct possibility. In addition, both Wang and Kruger have recently published on post-transcriptional regulatory mechanisms of CBS expression, and discovered that CBS is subject to intracellular degradation by various proteases (e.g., the Lon protease) (41, 106, 123), suggesting that perhaps CBS protein stability may be mediated by decreased CBS-degrading proteases in the cancer cell. Bhattacharyya et al. found that the up-regulation of CBS appears to be a relatively early event in ovarian cancer progression (10). However, for colorectal cancer, the temporal sequence of CBS up-regulation, in relation to the progression of colorectal cancer (expression levels in premalignant intestinal polyps vs. early-stage cancers), remains to be further studied.
What are the local and systemic levels of H2S in biological fluids in normal biological conditions and in cancer patients?
A significant controversy in the field of H2S relates to quantification methods for biological levels of H2S. The absolute value of the H2S level in extracellular fluids or in plasma depends on the experimental method used (e.g., derivatization of H2S with monobromobimane, or other methods, or measurement using the “methylene blue” assay, or free H2S gas measurements using headspace analysis, or readings taken by H2S electrode), as well as the tissue type studied, with vascular tissues exhibiting substantially high levels than many other tissues (29, 89, 120, 138). Further work is needed to address this question. No reports exist on circulating H2S levels in cancer patients. In our nude mice studies xenografted with human HCT116 colon cancer cells, we were unable to detect significant alterations in plasma H2S levels using the “methylene blue” method. Tissue levels of H2S are determined by both H2S production and H2S degradation (26). In cancer cells, partially uncoupled mitochondria may produce increased levels of ROS, and the increased cancer cell-derived H2S may be accompanied by increased H2S degradation due to increased oxidative stress, resulting in the primary accumulation of H2S metabolites, rather than H2S. A full analysis of the levels of H2S and its metabolites (e.g., thiosulfate) in cancer remains to be conducted in future studies.
How does the CBS/H2S pathway in cancer cells relate to various known pathways of cellular metabolism or cell proliferation?
It is not yet known whether up-regulation of CBS in cancer cells is a part of a global reprogramming of the trans-sulfuration pathway, which subsequently affects cellular metabolism and signaling. Future studies need to characterize these complex alterations. Ramasamy et al. characterized the expression of thiosulfate sulfurtransferase (TST, an isoform of the H2S-detoxifying enzyme family rhodanese) in HT29 colon cancer cells and in biopsies of colon cancer and showed that TST expression increases in proliferating HT29 cells can be induced by low-to-intermediate concentrations of authentic H2S; while TST is down-regulated by higher H2S concentrations (98). In addition, the same authors reported that there is a reduction in TST expression in sections of human colon cancer (98). Although the exact pathophysiological role of these alterations remains to be explored, the cellular effect is the net result of H2S production and its degradation and so, it is expected to be regulated by both changes in the expression/activity of enzymes that produce it (such as CBS) and enzymes that degrade it (such as TST).
Deplancke and Gaskins conducted a microarray analysis of gene expression changes in response to H2S exposure in the colonic epithelial cell line IEC-18 (25) and found that H2S increased the expression of cytochrome c oxidase subunit V, perhaps as a compensatory response to alterations in mitochondrial electron transport. Importantly, VEGF expression was also found to be up-regulated. There are several studies in the literature showing that VEGF up-regulates H2S production and/or that H2S up-regulates VEGF expression (11, 45, 71, 90, 94, 97, 103, 122, 130, 131). Thus, the possibility of a positive feedback cycle (with increased tumor angiogenesis, as a possible outcome) needs to be investigated in future studies.
What are the interactions of the CBS/H2S system with other gaseous mediators in cancer?
There are three gaseous mediators, NO, CO, and H2S, that utilize partially overlapping, and partially synergistic pathways (56, 93, 114). Many known interactions exist between the three transmitters and the respective enzyme systems that generate and metabolize the gases. As previously noted, one example is the direct inhibition of CBS by NO and CO via binding to its heme group. Although multiple previous studies have explored the potential role of NO in cancer, its exact role remains unclear. Since all three gaseous transmitters can travel through cell membranes, in theory, all of them have the potential to affect cell–cell communications in the tumor microenvironment. The interactions of the three gaseous transmitters, both in the regulation of physiological processes in general and in cancer biology remains to be explored in the future.
Outlook: The Therapeutic Potential of H2S Biosynthesis Inhibition in Cancer
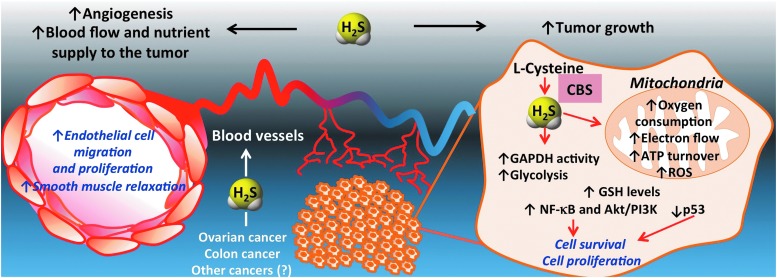
The realization of the functional importance of CBS-derived H2S production in the biology of the cancer cell is a very recent one. Our current model, supported by several lines of experimental data, is shown in Figure 20. CBS up-regulation results in enhanced H2S production in cancer cells, which, in turn, stimulate its mitochondrial function and activate proliferative and pro-inflammatory signaling. In addition, H2S acts as a mitochondrial antioxidant and cytoprotectant for the cancer cell. Moreover, in a paracrine fashion, H2S diffuses out from the cancer cells into the tumor microenvironment, where it stimulates angiogenesis and local vasodilatation, which, in turn, provides the tumor with nutrients and oxygen.
FIG. 20.
Summary: role of CBS/H2S axis in the biology of colorectal and ovarian cancer cells. CBS is highly expressed in colon cancer cells, ovarian cancer cells (and likely in other forms of cancer as well). It is located partially in the cytosol and partially in the mitochondria. H2S produced from it serves as an inorganic electron donor, stimulating mitochondrial electron transport, increasing ATP turnover. In addition, H2S increases the glycolytic activity of the tumor cell, presumably by activating GAPDH. Moreover, it exerts antioxidant effects in the mitochondria. In addition, it contributes to the maintenance of GSH in the cancer cell. Via autocrine bioenergetic effects, and/or via direct effects on multiple signaling pathways (Akt/PI3K, nuclear factor kappa B [NF-κB] etc.), endogenously produced H2S stimulates cancer cell proliferation, migration, and invasion. In addition, H2S diffuses into the surrounding cells and tissues, stimulating angiogenesis, as well as acting as a vascular relaxant. Via these paracrine effects, CBS-derived H2S promotes the supply of blood and nutrients to the tumor. Genetic silencing or pharmacological inhibition of CBS exerts inhibitory effects on tumor bioenergetics, cellular signaling, resulting in inhibition of proliferation, growth, migration, and metastasis. CBS inhibition also enhances the antitumor effect of various chemotherapeutic agents such as cisplatin and oxaliplatin both in vitro and in vivo. Modified from Szabo et al. (117). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
To progress this novel concept into clinical testing, significant further basics, as well as translational research are required. Colorectal and ovarian cancer are the two diseases with promising preclinical data. Thus far, the role of CBS/H2S in the translationally highly predictive PDTX model has only been confirmed in the colorectal cancer model. In these experiments, the CBS inhibitor AOAA showed consistent and potent anti-tumor growth effects independent of the cancer's KRAS status (Fig. 13) (117). Based on preclinical data (10, 16a), a combination of CBS inhibitors with existing antitumor agents is a distinct possibility, even though additional work is needed to identify the most effective combinations and the best sequence of their administration.
Finally, further development of pharmacological inhibitors of CBS are needed, including work to improve the potency and the selectivity of the currently known CBS inhibitors. All available published data on screening and medicinal chemistry studies in this area are covered in the current review.
Even with the most selective and most potent inhibitors, we predict that some mechanism-based side effects will remain unavoidable, as CBS is physiologically expressed in some non-tumor tissues, such as the liver, the kidney, and the nervous system. The main physiological role of CBS is to metabolize homocysteine. Thus, we predict that treatment with any pharmacological CBS inhibitor will be associated with some accumulation of plasma homocysteine, a known cytotoxic molecule and cardiovascular risk factor (12, 24, 27, 50, 104). Accordingly, CBS knockout animals have a limited life span, which is a consequence of severe hyperhomocysteinemia (3, 74). Patients who completely lack CBS exhibit severe pathophysiological alterations, including thrombosis, osteoporosis, and mental retardation (82). However, while extremely high levels of circulating homocysteine are dangerous, there may be a threshold effect with homocysteine. Moderate homocysteinemia is well tolerated in animal models (40). For example, CBS−/− mice that receive a transgene expressing a catalytically deficient form of human CBS which only partially normalizes circulating homocysteine levels are viable (129). In addition, CBS+/− heterozygote mice have significantly elevated homocysteine levels, but are fully viable (69, 126, 145).
Correlative data linking hyperhomocysteinemia and cardiovascular risk may be of concern with the use of CBS inhibitors. Recent clinical trials studied patients taking vitamin B12 and folate to facilitate the conversion of homocysteine to methionine. Although the intervention successfully reduced circulating levels of homocysteine, it was not effective in reducing the cardiovascular adverse events in the patients (2, 88), questioning whether hyperhomocysteinemia causes cardiovascular disease.
Taken together, in our view, CBS is a partially selective novel target for cancer therapy. Although we have shown that the CBS inhibitor AOAA was tolerated in mice at therapeutically effective doses, this does not imply that CBS inhibitors will be well tolerated by cancer patients. We predict that administration of CBS inhibitor anticancer drugs will present a risk of hyperhomocysteinemia, which will have to be monitored and clinically managed. Perhaps measurement of circulating homocysteine levels may be used in future anticancer clinical trials as a biomarker to estimate the extent of CBS inhibition and/or to assess patient compliance. Finally, a possible way of reducing the therapeutic dose of CBS inhibitors in human trials may be to use them in combination with appropriately selected standard anticancer agents.
Abbreviations Used
- 3-MP
3-mercaptopyruvate
- 3-MST
3-mercaptopyruvate sulfurtransferase
- AOAA
aminooxyacetic acid
- AST
aspartate transaminase
- CAT
cysteine aminotransferase
- CBS
cystathionine β-synthase
- CI
cell index
- CO
carbon monoxide
- CSE
cystathionine γ-lyase
- DOPC
dioleoyl phosphatidylcholine
- GABA-T
glutamic acid decarboxylase or GABA transaminase
- GOT1
glutamate oxaloacetate transaminase 1
- H2S
hydrogen sulfide
- NF-κB
nuclear factor kappa B
- NO
nitric oxide
- NSAID
non-steroidal anti-inflammatory drug
- OCR
oxygen consumption rate
- PAG
propargylglycine
- PBS
phosphate-buffered saline
- PDTX
patient-derived tumor xenograft
- PLP
pyridoxal-phosphate
- ROS
reactive oxygen species
- RT-PCR
real-time polymerase chain reaction
- SAM
S-adenosyl-L-methionine
- TST
thiosulfate sulfurtransferase
- VEGF
vascular endothelial growth factor
Acknowledgments
The work was supported by National Institutes of Health (R01CA175803, K08CA125209) and the Shriners Hospitals for Children, the John Sealy Memorial Foundation, the Institute for Translational Sciences at the University of Texas Medical Branch, and a Clinical and Translational Science Award (8UL1TR000071).
Author Disclosure Statement
M.R.H., C. Chao, and C.S. have stock ownership in CBS Therapeutic Inc., a for-profit organization involved in the development of CBS inhibitor therapies for cancer.
References
- 1.Abe K. and Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham JM. and Cho L. The homocysteine hypothesis: still relevant to the prevention and treatment of cardiovascular disease? Cleve Clin J Med 77: 911–918, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Akahoshi N, Kobayashi C, Ishizaki Y, Izumi T, Himi T, Suematsu M, and Ishii I. Genetic background conversion ameliorates semi-lethality and permits behavioral analyses in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. Hum Mol Genet 17: 1994–2005, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Altomare DF, Di Lena M, Porcelli F, Trizio L, Travaglio E, Tutino M, Dragonieri S, Memeo V, and de Gennaro G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg 100: 144–150, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, and Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169: 922–932, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attene-Ramos MS, Wagner ED, Plewa MJ, and Gaskins HR. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res 4: 9–14, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Banerjee R, Evande R, Kabil O, Ojha S, and Taoka S. Reaction mechanism and regulation of cystathionine beta-synthase. Biochim Biophys Acta 1647: 30–35, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Baskar R. and Bian J. Hydrogen sulfide gas has cell growth regulatory role. Eur J Pharmacol 656: 5–9, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, and Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A 104: 17977–17982, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal E, Weaver AL, Visscher DW, Cliby W, Sood AK, Bhattacharya R, and Mukherjee P. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 8: e79167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, and Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 1: e004093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boushey CJ, Beresford SA, Omenn GS, and Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. J Am Med Assoc 274: 1049–1057, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Cai WJ, Wang MJ, Ju LH, Wang C, and Zhu YC. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol Int 34: 565–572, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, and Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, and Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Chao C, Bohannon FJ, Mrazek A, Coletta C, Szabo C, Hellmich MR. Cysthionine-β-synthetase inhibition in combination with standard-chemotherapy decreases colorectal cancer metastasis to the liver. Nitric Oxide 39: S21, 2014 [Google Scholar]
- 16.Carballal S, Cuevasanta E, Marmisolle I, Kabil O, Gherasim C, Ballou DP, Banerjee R, and Alvarez B.Kinetics of reversible reductive carbonylation of heme in human cystathionine β-synthase. Biochemistry 52: 4553–4562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay M, Nath N, Kodela R, Sobocki T, Metkar S, Gan ZY, and Kashfi K.Hydrogen sulfide-releasing aspirin inhibits the growth of leukemic Jurkat cells and modulates β-catenin expression. Leuk Res 37: 1302–1308, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong CR. and Sullivan DJ., Jr.New uses for old drugs. Nature 448: 645–646, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Chwatko G, Forma E, Wilkosz J, Głowacki R, Jóźwiak P, Różański W, Bryś M, and Krześlak A.Thiosulfate in urine as a facilitator in the diagnosis of prostate cancer for patients with prostate-specific antigen less or equal 10 ng/mL. Clin Chem Lab Med 51: 1825–1831, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I, Martin E, and Szabo C.Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 109: 9161–9166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Amico A, Di Natale C, Falconi C, Martinelli E, Paolesse R, Pennazza G, Santonico M, and Sterk PJ.U-BIOPRED study. Detection and identification of cancers by the electronic nose. Expert Opin Med Diagn 6: 175–185, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Dang CV, Hamaker M, Sun P, Le A, and Gao P.Therapeutic targeting of cancer cell metabolism. J Mol Med (Berl) 89: 205–212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vos J, Thykjaer T, Tarte K, Ensslen M, Raynaud P, Requirand G, Pellet F, Pantesco V, Rème T, Jourdan M, Rossi JF, Ørntoft T, and Klein B.Comparison of gene expression profiling between malignant and normal plasma cells with oligonucleotide arrays. Oncogene 21: 6848–6857, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Den Heijer M, Lewington S, and Clarke R.Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost 3: 292–299, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Deplancke B. and Gaskins HR.Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J 17: 1310–1312, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR, Jr., Darley-Usmar VM, and Kraus DW.Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341: 40–51, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Duell PB. and Malinow MR.Homocyst(e)ine: an important risk factor for atherosclerotic vascular disease. Curr Opin Lipidol 8: 28–34, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Ereño-Orbea J, Majtan T, Oyenarte I, Kraus JP, and Martínez-Cruz LA.Structural basis of regulation and oligomerization of human cystathionine β-synthase, the central enzyme of transsulfuration. Proc Natl Acad Sci U S A 110: E3790–E3799, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiorucci S, Distrutti E, Cirino G, and Wallace JL.The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology 131: 259–271, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Frantzias J, Logan JG, Mollat P, Sparatore A, Del Soldato P, Ralston SH, and Idris AI.Hydrogen sulphide-releasing diclofenac derivatives inhibit breast cancer-induced osteoclastogenesis in vitro and prevent osteolysis ex vivo. Br J Pharmacol 165: 1914–1925, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu M, Zhang W, Wu L, Yang G, Li H, and Wang R.Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A 109: 2943–2948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge Y, Jensen TL, Matherly LH, and Taub JW.Synergistic regulation of human cystathionine-beta-synthase-1b promoter by transcription factors NF-YA isoforms and Sp1. Biochim Biophys Acta 1579: 73–80, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Geiser CF. and Efron ML.Cystathioninuria in patients with neuroblastoma or ganglioneuroblastoma. Its correlation to vanilmandelic acid excretion and its value in diagnosis and therapy. Cancer 22: 856–860, 1968 [DOI] [PubMed] [Google Scholar]
- 34.Geiser CF. and Shih VE.Cystathioninuria and its origin in children with hepatoblastoma. J Pediatr 96: 72–75, 1980 [DOI] [PubMed] [Google Scholar]
- 35.Gjessing LR.Cystathioninuria and vanil-lactic-aciduria in neuroblastoma and argentaffinoma. Lancet 2: 1281–1282, 1963 [DOI] [PubMed] [Google Scholar]
- 36.Goubern M, Andriamihaja M, Nübel T, Blachier F, and Bouillaud F.Sulfide, the first inorganic substrate for human cells. FASEB J 21: 1699–1706, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Greenhouse WV. and Lehninger AL.Magnitude of malate-aspartate reduced nicotinamide adenine dinucleotide shuttle activity in intact respiring tumor cells. Cancer Res 37: 4173–4181, 1977 [PubMed] [Google Scholar]
- 38.Greiner R, Pálinkás Z, Bäsell K, Becher D, Antelmann H, Nagy P, and Dick TP.Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19: 1749–1765, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo H, Gai JW, Wang Y, Jin HF, Du JB, and Jin J.Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology 79: 483, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Kühnisch J, Mustafa A, Lhotak S, Schlachterman A, Slifker MJ, Klein-Szanto A, High KA, Austin RC, and Kruger WD.Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J 23: 883–893, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta S, Wang L, Anderl J, Slifker MJ, Kirk C, and Kruger WD.Correction of cystathionine β-synthase deficiency in mice by treatment with proteasome inhibitors. Hum Mutat 34: 1085–1093, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guth PS, Risey J, Briner W, Blair P, Reed HT, Bryant G, Norris C, Housley G, and Miller R.Evaluation of amino-oxyacetic acid as a palliative in tinnitus. Ann Otol Rhinol Laryngol 99: 74–79, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Hansel DE, Rahman A, Hidalgo M, Thuluvath PJ, Lillemoe KD, Shulick R, Ku JL, Park JG, Miyazaki K, Ashfaq R, Wistuba II, Varma R, Hawthorne L, Geradts J, Argani P, and Maitra A.Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol 163: 217–229, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.This reference has been deleted. [Google Scholar]
- 45.Holwerda KM, Burke SD, Faas MM, Zsengeller Z, Stillman IE, Kang PM, van Goor H, McCurley A, Jaffe IZ, Karumanchi SA, and Lely AT.Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol 25: 717–725, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horvath G, Andersson H, and Nemes S.Cancer odor in the blood of ovarian cancer patients: a retrospective study of detection by dogs during treatment, 3 and 6 months afterward. BMC Cancer 13: 396, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Kumar S, Abbassi-Ghadi N, Spaněl P, Smith D, and Hanna GB.Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal Chem 85: 3409–3416, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Huang Y, Li F, Tong W, Zhang A, He Y, Fu T, and Liu B.Hydrogen sulfide, a gaseous transmitter, stimulates proliferation of interstitial cells of Cajal via phosphorylation of AKT protein kinase. Tohoku J Exp Med 221: 125–132, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, and Suematsu M. Cystathionine gamma-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury J Biol Chem 285: 26358–26368, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobsen DW, Catanescu O, Dibello PM, and Barbato JC.Molecular targeting by homocysteine: a mechanism for vascular pathogenesis. Clin Chem Lab Med 43: 1076–1083, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Jhee KH. and Kruger WD.The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal 7: 813–822, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Jones KM, Liao E, Hohneker K, Turpin S, Henry MM, Selinger K, Hsyu PH, Boucher RC, Knowles MR, and Dukes GE.Pharmacokinetics of amiloride after inhalation and oral administration in adolescents and adults with cystic fibrosis. Pharmacotherapy 17: 263–270, 1997 [PubMed] [Google Scholar]
- 53.Jorga KM, Larsen JP, Beiske A, Schleimer M, Fotteler B, Schmitt M, and Moe B.The effect of tolcapone on the pharmacokinetics of benserazide. Eur J Neurol 6: 211–219, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Kabil O. and Banerjee R.Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20: 770–782, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kabil O, Zhou Y, and Banerjee R.Human cystathionine beta-synthase is a target for sumoylation. Biochemistry 45: 13528–13536, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Kajimura M, Nakanishi T, Takenouchi T, Morikawa T, Hishiki T, Yukutake Y, and Suematsu M.Gas biology: tiny molecules controlling metabolic systems. Respir Physiol Neurobiol 184: 139–148, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Kashfi K.Anti-cancer activity of new designer hydrogen sulfide-donating hybrids. Antioxid Redox Signal 20: 831–846, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kery V, Poneleit L, and Kraus JP.Trypsin cleavage of human cystathionine beta-synthase into an evolutionarily conserved active core: structural and functional consequences. Arch Biochem Biophys 355: 222–232, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Kimura H.Hydrogen sulfide: its production, release and functions. Amino Acids 41: 113–121, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Kimura H.The physiological role of hydrogen sulfide and beyond. Nitric Oxide 2014. [Epub ahead of print]; DOI: 10.1016/j.niox.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 61.Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, and Kimura H.Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J 27: 2451–2457, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Klein CE, Roberts B, Holcenberg J, and Glode LM.Cystathionine metabolism in neuroblastoma. Cancer 62: 291–298, 1988 [DOI] [PubMed] [Google Scholar]
- 63.Kodela R, Chattopadhyay M, and Kashfi K.Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. Med Chem Comm 4: 1039, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koike S, Ogasawara Y, Shibuya N, Kimura H, and Ishii K.Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett 587: 3548–3555, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Krishnan N, Fu C, Pappin DJ, and Tonks NK.H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar S, Huang J, Cushnir JR, Španěl P, Smith D, and Hanna GB.Selected ion flow tube-MS analysis of headspace vapor from gastric content for the diagnosis of gastro-esophageal cancer. Anal Chem 84: 9550–9557, 2012 [DOI] [PubMed] [Google Scholar]
- 67.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, and Bouillaud F.Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta 1797: 1500–1511, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, and Deng LW.The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 6: e21077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, and Faraci FM.Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient mice. Am J Physiol Heart Circ Physiol 279: H970–H975, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Leunis N, Boumans ML, Kremer B, Din S, Stobberingh E, Kessels AG, and Kross KW.Application of an electronic nose in the diagnosis of head and neck cancer. Laryngoscope 2014. [Epub ahead of print]; DOI: doi: 10.1002/lary.24463 [DOI] [PubMed] [Google Scholar]
- 71.Lin VS, Lippert AR, and Chang CJ.Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc Natl Acad Sci U S A 110: 7131–7135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan BK, and Zhu YZ.H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS One 6: e20525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maclean KN, Kraus E, and Kraus JP.The dominant role of Sp1 in regulating the cystathionine beta-synthase-1a and -1b promoters facilitates potential tissue-specific regulation by Kruppel-like factors. J Biol Chem 279: 8558–8566, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Maclean KN, Sikora J, Kožich V, Jiang H, Greiner LS, Kraus E, Krijt J, Crnic LS, Allen RH, Stabler SP, Elleder M, and Kraus JP.Cystathionine beta-synthase null homocystinuric mice fail to exhibit altered hemostasis or lowering of plasma homocysteine in response to betaine treatment. Mol Genet Metab 101: 163–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manna P. and Jain SK.Hydrogen sulfide and L-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J Biol Chem 286: 39848–39859, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meier M, Oliveriusova J, Kraus JP, and Burkhard P.Structural insights into mutations of cystathionine beta-synthase. Biochim Biophys Acta 1647: 206–213, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Miles EW. and Kraus JP.Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem 279: 29871–29874, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Módis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, Hellmich MR, Radermacher P, Bouillaud F, and Szabo C.Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol 171: 2123–2146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Módis K, Coletta C, Asimakopoulou A, Szczesny B, Chao C, Papapetropoulos A, Hellmich MR, and Szabo C.Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide 2014. [Epub ahead of print]; DOI: 10.1016/j.niox.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Módis K, Coletta C, Erdélyi K, Papapetropoulos A, and Szabo C.Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J 27: 601–611, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Módis K, Panopoulos P, Coletta C, Papapetropoulos A, and Szabo C.Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharmacol 86: 1311–1319, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R, Fowler B, Grobe H, Schmidt H, and Schweitzer L.The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet 37: 1–31, 1985 [PMC free article] [PubMed] [Google Scholar]
- 83.Murata T, Sato T, Kamoda T, Moriyama H, Kumazawa Y, and Hanada N.Differential susceptibility to hydrogen sulfide-induced apoptosis between PHLDA1-overexpressing oral cancer cell lines and oral keratinocytes: Role of PHLDA1 as an apoptosis suppressor. Exp Cell Res 320: 247–257, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, and Snyder SH.H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muthuramu I, Jacobs F, Singh N, Gordts SC, and De Geest B.Selective homocysteine lowering gene transfer improves infarct healing, attenuates remodelling, and enhances diastolic function after myocardial infarction in mice. PLoS One 8: e63710, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Narayan RK.A technique for C-banding chromosomes with pinacyanol chloride. Stain Technol 55: 9–11, 1980 [DOI] [PubMed] [Google Scholar]
- 87.Nicholls P.Inhibition of cytochrome c oxidase by sulphide. Biochem Soc Trans 3: 316–319, 1975 [DOI] [PubMed] [Google Scholar]
- 88.Nursalim A, Siregar P, and Widyahening IS.Effect of folic acid, vitamin B6 and vitamin B12 supplementation on mortality and cardiovascular complication among patients with chronic kidney disease: an evidence-based case report. Acta Med Indones 45: 150–156, 2013 [PubMed] [Google Scholar]
- 89.Olson KR.Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787: 856–863, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, and Szabo C.Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perry TL. and Hansen S.Biochemical effects in man and rat of three drugs which can increase brain GABA content. J Neurochem 30: 679–684, 1978 [DOI] [PubMed] [Google Scholar]
- 92.Perry TL, Wright JM, Hansen S, Allan BM, Baird PA, and MacLeod PM.Failure of aminooxyacetic acid therapy in Huntington disease. Neurology 30: 772–775, 1980 [DOI] [PubMed] [Google Scholar]
- 93.Pun PB, Lu J, Kan EM, and Moochhala S.Gases in the mitochondria. Mitochondrion 10: 83–93, 2010 [DOI] [PubMed] [Google Scholar]
- 94.Pupo E, Pla AF, Avanzato D, Moccia F, Cruz JE, Tanzi F, Merlino A, Mancardi D, and Munaron L.Hydrogen sulfide promotes calcium signals and migration in tumor-derived endothelial cells. Free Radic Biol Med 51: 1765–1773, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Puranik M, Weeks CL, Lahaye D, Kabil O, Taoka S, Nielsen SB, Groves JT, Banerjee R, and Spiro TG.Dynamics of carbon monoxide binding to cystathionine beta-synthase. J Biol Chem 281: 13433–13438, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi F, Zhou Y, Xiao Y, Tao J, Gu J, Jiang X, and Xu GY.Promoter demethylation of cystathionine-β-synthetase gene contributes to inflammatory pain in rats. Pain 154: 34–45, 2013 [DOI] [PubMed] [Google Scholar]
- 97.Qipshidze N, Metreveli N, Mishra PK, Lominadze D, and Tyagi SC.Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci 8: 430–441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramasamy S, Singh S, Taniere P, Langman MJ, and Eggo MC.Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am J Physiol Gastrointest Liver Physiol 291: G288–G296, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, and Brosnan JT.Hormonal regulation of cystathionine beta-synthase expression in liver. J Biol Chem 277: 42912–42918, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Régnier V, Billard JM, Gupta S, Potier B, Woerner S, Paly E, Ledru A, David S, Luilier S, Bizot JC, Vacano G, Kraus JP, Patterson D, Kruger WD, Delabar JM, and London J.Brain phenotype of transgenic mice overexpressing cystathionine β-synthase. PLoS One 7: e29056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sabaté R. and Estelrich J.Pinacyanol as effective probe of fibrillar beta-amyloid peptide: comparative study with Congo Red. Biopolymers 72: 455–463, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Samudio I, Fiegl M, and Andreeff M.Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res 69: 2163–2166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, and Snyder SH.Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sen U, Sathnur PB, Kundu S, Givvimani S, Coley DM, Mishra PK, Qipshidze N, Tyagi N, Metreveli N, and Tyagi SC.Increased endogenous H2S generation by CBS, CSE, and 3MST gene therapy improves ex vivo renovascular relaxation in hyperhomocysteinemia. Am J Physiol Cell Physiol 303: C41–C51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen W.Development of a new assay and inhibitors for human cystathionine beta-synthase. Asymmetric catalyst development guided by in situ enzymatic screening. PhD dissertation, University of Nebraska, 2007 [Google Scholar]
- 106.Singh LR, Gupta S, Honig NH, Kraus JP, and Kruger WD.Activation of mutant enzyme function in vivo by proteasome inhibitors and treatments that induce Hsp70. PLoS Genet 6: e1000807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh S. and Banerjee R.PLP-dependent H2S biogenesis. Biochim Biophys Acta 1814: 1518–1527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh S, Padovani D, Leslie RA, Chiku T, and Banerjee R.Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, and Kimmelman AC.Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496: 101–105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sonoda H, Kohnoe S, Yamazato T, Satoh Y, Morizono G, Shikata K, Morita M, Watanabe A, Morita M, Kakeji Y, Inoue F, and Maehara Y.Colorectal cancer screening with odour material by canine scent detection. Gut 60: 814–819, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stabler S, Koyama T, Zhao Z, Martinez-Ferrer M, Allen RH, Luka Z, Loukachevitch LV, Clark PE, Wagner C, and Bhowmick NA.Serum methionine metabolites are risk factors for metastatic prostate cancer progression. PLoS One 6: e22486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Su Y, Majtan T, Freeman KM, Linck R, Ponter S, Kraus JP, and Burstyn JN.Comparative study of enzyme activity and heme reactivity in Drosophila melanogaster and Homo sapiens cystathionine β-synthases. Biochemistry 52: 741–751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gero D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, and Szabo C.Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A 108: 13829–13834, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szabo C.Gaseotransmitters: new frontiers for translational science. Sci Transl Med 2: 59ps54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Szabo C.Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Szabo C.Medicinal chemistry and therapeutic applications of the gasotransmitters nitric oxide, carbon monoxide and hydrogen sulfide. Burgers Med Chem 5: 265–367, 2010 [Google Scholar]
- 117.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, and Hellmich MR.Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A 110: 12474–12479, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szabo C. and Hellmich MR.Endogenously produced hydrogen sulfide supports tumor cell growth and proliferation. Cell Cycle 12: 2915–2916, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szabo C. and Papapetropoulos A.Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol 164: 853–865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, and Bouillaud F.Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 171: 2099–2122, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Talaei F, Bouma HR, Van der Graaf AC, Strijkstra AM, Schmidt M, and Henning RH.Serotonin and dopamine protect from hypothermia/rewarming damage through the CBS/H2S pathway. PLoS One 6: e22568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, and Zhu YC.VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal 19: 448–464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Teng H, Wu B, Zhao K, Yang G, Wu L, and Wang R.Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc Natl Acad Sci U S A 110: 12679–12684, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, Eaton JW, Telang S, and Chesney J.Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res 10: R84, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thorson MK, Majtan T, Kraus JP, and Barrios AM.Identification of cystathionine β-synthase inhibitors using a hydrogen sulfide selective probe. Angew Chem Int Ed Engl 52: 4641–4644, 2013 [DOI] [PubMed] [Google Scholar]
- 126.Tyagi N, Qipshidze N, Sen U, Rodriguez W, Ovechkin A, and Tyagi SC.Cystathionine beta synthase gene dose dependent vascular remodeling in murine model of hyperhomocysteinemia. Int J Physiol Pathophysiol Pharmacol 3: 210–222, 2011 [PMC free article] [PubMed] [Google Scholar]
- 127.Vitvitsky V, Prudova A, Stabler S, Dayal S, Lentz SR, and Banerjee R.Testosterone regulation of renal cystathionine beta-synthase: implications for sex-dependent differences in plasma homocysteine levels. Am J Physiol Renal Physiol 293: F594–F600, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Voûte PA, Jr., and Wadman SK.Cystathioninuria in hepatoblastoma. Clin Chim Acta 22: 373–378, 1968 [DOI] [PubMed] [Google Scholar]
- 129.Wang L, Jhee KH, Hua X, DiBello PM, Jacobsen DW, and Kruger WD.Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res 94: 1318–1324, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Wang MJ, Cai WJ, Li N, Ding YJ, Chen Y, and Zhu YC.The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal 12: 1065–1077, 2010 [DOI] [PubMed] [Google Scholar]
- 131.Wang MJ, Cai WJ, and Zhu YC.Mechanisms of angiogenesis: role of hydrogen sulphide. Clin Exp Pharmacol Physiol 37: 764–771, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Wang R.Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92: 791–896, 2012 [DOI] [PubMed] [Google Scholar]
- 133.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, and Chandel NS.Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 107: 8788–8793, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wen S, Zhu D, and Huang P.Targeting cancer cell mitochondria as a therapeutic approach. Future Med Chem 5: 53–67, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wen YD, Wang H, Kho SH, Rinkiko S, Sheng X, Shen HM, and Zhu YZ.Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS One 8: e53147, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, and Moore PK.The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal 12: 1147–1154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Willis CM, Church SM, and Guest CM.Olfactory detection of human bladder cancer by dogs: proof of principle study. BMJ 329: 712–714, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, and Szabo C.A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol 160: 941–957, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu YC, Wang XJ, Yu L, Chan FK, Cheng AS, Yu J, Sung JJ, Wu WK, and Cho CH.Hydrogen sulfide lowers proliferation and induces protective autophagy in colon epithelial cells. PLoS One 7: e37572, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yamagishi K, Onuma K, Chiba Y, Yagi S, Aoki S, Sato T, Sugawara Y, Hosoya N, Saeki Y, Takahashi M, Fuji M, Ohsaka T, Okajima T, Akita K, Suzuki T, Senawongse P, Urushiyama A, Kawai K, Shoun H, Ishii Y, Ishikawa H, Sugiyama S, Nakajima M, Tsuboi M, and Yamanaka T.Generation of gaseous sulfur-containing compounds in tumor tissue and suppression of gas diffusion as an antitumour treatment. Gut 61: 554–561, 2012 [DOI] [PubMed] [Google Scholar]
- 141.Yamanishi M, Kabil O, Sen S, and Banerjee R.Structural insights into pathogenic mutations in heme-dependent cystathionine-beta-synthase. J Inorg Biochem 100: 1988–1995, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, and Wang R.H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, and Wang R.Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18: 1906–1919, 2013 [DOI] [PubMed] [Google Scholar]
- 144.Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y, Li N, Qi J, Wang L, Shi Y, Qiu S, Fan J, and Zha X.Sp1 is involved in regulation of cystathionine γ-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal 24: 1229–1240, 2012 [DOI] [PubMed] [Google Scholar]
- 145.Yu M, Sturgill-Short G, Ganapathy P, Tawfik A, Peachey NS, and Smith SB.Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp Eye Res 96: 124–131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang W, Braun A, Bauman Z, Olteanu H, Madzelan P, and Banerjee R.Expression profiling of homocysteine junction enzymes in the NCI60 panel of human cancer cell lines. Cancer Res 65: 1554–1560, 2005 [DOI] [PubMed] [Google Scholar]
- 147.Zhou Y, Yu J, Lei X, Wu J, Niu Q, Zhang Y, Liu H, Christen P, Gehring H, and Wu F.High-throughput tandem-microwell assay identifies inhibitors of the hydrogen sulfide signaling pathway. Chem Commun (Camb) 49: 11782–11784, 2013 [DOI] [PubMed] [Google Scholar]
- 148.Zou C-G. and Banerjee R.Tumor necrosis factor-alpha-induced targeted proteolysis of cystathionine beta-synthase modulates redox homeostasis. J Biol Chem 278: 16802–16808, 2003. 12615917 [Google Scholar]