Abstract
Background: The storage of adipose tissue in ectopic compartments is a hallmark attribute linking greater body mass index (BMI) with cardiometabolic diseases. Despite ample evidence to confirm that increased visceral adipose tissue (VAT) deposition occurs with obesity, the interrelations between altered fat partitioning and regional muscle and bone quality are less well understood.
Objective: We examined the association between adiposity and spinal muscle and bone quality across a large, heterogeneous cohort of adults.
Design: We identified 8833 thoracic or abdominal computed tomography scans from patients in the University of Michigan Health System who were aged 18–64.9 y. We measured trabecular bone densities, cortical bone densities, VAT areas, and subcutaneous adipose tissue (SAT) areas at vertebral levels T7 to L5. Psoas muscle attenuation (an indicator of fat infiltration in muscle) was measured at the L4 level.
Results: Muscle attenuation as well as trabecular and cortical bone densities revealed negative correlations with BMI, SAT, and VAT. The correlation between BMI and psoas attenuation was −0.321, between BMI and the density of cortical bone was −0.250, and between BMI and trabecular bone was −0.143 (all P < 0.001). However, correlations between VAT and lower muscle attenuation were stronger as were those between VAT and lower bone densities. Inverse correlations between VAT and densities of psoas muscle and cortical and trabecular bone were −0.460, −0.407, and −0.434, respectively (P < 0.001). Even after adjustment for age, sex, and BMI, partial correlations between VAT, muscle attenuation, and bone densities remained significant at −0.250, −0.119, and −0.216, respectively (P < 0.001).
Conclusions: Contrary to previous reports that high body mass is associated with increased bone quality, our data show a significant negative association between BMI and muscle and bone densities, suggesting fat infiltration into these tissues. More importantly, correlations between VAT and decreased bone and muscle densities remained statistically significant even after adjustment for age, sex, and BMI.
Keywords: analytic morphomics, bone density, computed tomography, muscle attenuation, visceral adiposity
INTRODUCTION
The accumulation of lipids in nonadipose tissues (i.e., ectopic adiposity) is a lipotoxic process that occurs as a result of disequilibrium between energy intake and energy expenditure and is strongly associated with cardiometabolic diseases (1–3). Reports pertaining to the negative impact of ectopic adiposity on chronic health have historically focused on visceral adipose tissue (VAT)5 (4) as the underlying driver linking high BMI and cardiometabolic abnormalities. However, there is considerable evidence to support the notion that obesity is a heterogeneous condition, and excessive visceral adiposity for a given BMI may be an indicator of dysfunctional adipose tissue, triggering increased ectopic fat deposition (5–7). Increasingly compelling research has also shown that obesity has pathophysiologic consequences on bone and skeletal muscle health and function (8–11). This infiltration appears as a feature of certain disease processes (e.g., type 2 diabetes) (12) as well as prolonged sedentary behavior (13), which is often characterized with gross morphologic data from older adults by using computed tomography (CT) (8, 14) or localized intramuscular adipose tissue, intramyocellular lipid, or bone marrow adipose tissue with magnetic resonance technologies (10, 15).
Ectopic adipose tissue deposition may occur long before an individual meets the BMI criterion for obesity or is considered at clinical risk of cardiometabolic comorbidities. Previous research has revealed a robust link between intermuscular adipose tissue and elevated concentrations of proinflammatory, adipocyte-derived hormones and cytokines (16, 17), which may also lead to skeletal muscle insulin resistance and impaired muscle and bone quality (18, 19). Thus, in conjunction with pronounced changes in the hormonal and metabolic milieu, excessive ectopic adiposity could yield a general, inhospitable physiologic environment contributing to musculoskeletal fragility. Despite the well-documented trajectory of concurrent metabolic, morphologic, and functional declines in older adults (20, 21), much less is known about the interrelations between obesity, altered fat partitioning, muscle attenuation, and bone density in young to middle-aged adults. It is also unclear if there is an influence of greater general adiposity on bone and muscle health, which is distinct from that caused by variations in localized adipose tissue distribution on adjacent muscle and bone within individuals. With consideration of the mounting evidence that implicates an interrelation in muscle, fat, and bone (22), we hypothesized that the presence of larger ectopic stores of VAT would be associated with a lower attenuation of muscle and bone mineral density (BMD), particularly in the regions representing the greatest stores of VAT. Therefore, the purpose of this study was to examine the independent association between subcutaneous adipose tissue (SAT),VAT, muscle attenuation, and the density of trabecular and cortical bone across a large, heterogeneous cohort of young and middle-aged adults.
SUBJECTS AND METHODS
Subjects
We identified 8833 thoracic or abdominal CT scans from 7230 patients (46.4% women) with complete demographic and anthropometric information at the University of Michigan Health System. All scans took place between 1995 and 2013, and at the time of scans, patients were 18–64.9 y of age. Between the thoracic and lumbar vertebral levels T7 and L 5 studied, the vertebral level T12 was shown in the greatest number (5618) of eligible CT scans, and the T7 level was shown in the least number with 3156 scans. Computations of SAT and VAT areas and trabecular and cortical BMDs were conducted at each vertebral level. At the vertebral level of L4 (with 5381 available scans), psoas muscle areas and densities were also determined.
Analytic morphomic data
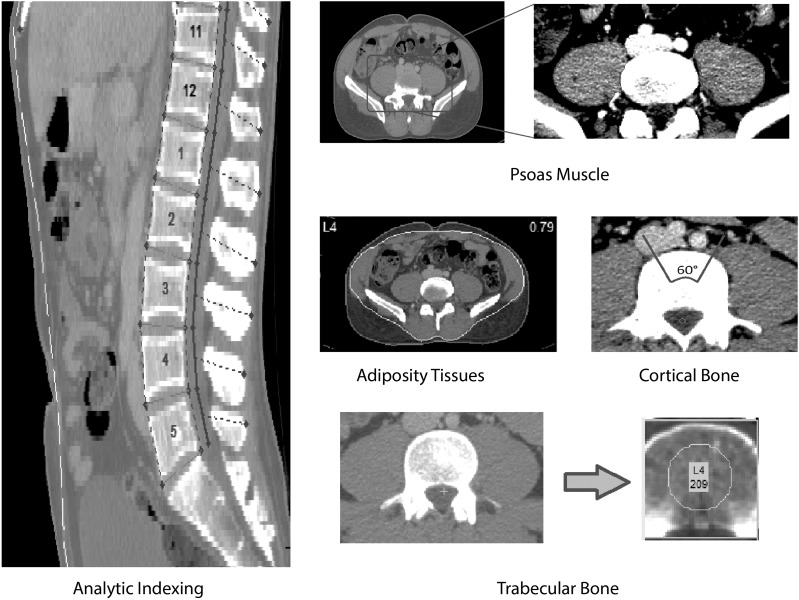
Patient CT scans were processed and analyzed by using a proprietary, semiautomated process developed with MATLAB R2014b (version 8.4; MathWorks Inc.). The process of analytic morphomic processing is illustrated in Figure 1. The process started from confirming the locations of vertebral levels by trained CT processors and was followed by identifying key anatomic landmarks for semiautomated visceral fascial determination at different levels and drawing contours of psoas muscles at the L4 level. The algorithm used these inputs to automatically generate morphologic measurements. Specifically, we measured trabecular bone density as the mean density within a circle that was one-half the size of the vertebral body. Cortical bone density was measured as the mean of the level of half-maximum of the bone signal peak at every angle within a 60 degree wedge. Psoas muscle density was measured as the mean of voxel attenuation in Hounsfield units within psoas contours, the psoas area as the total area inside psoas contours, and lean-psoas as the psoas area multiplied by the normalized psoas density [mapping (−85,85) to (0,1)]. Within psoas contours, indicators of a low-attenuation psoas area (area where Hounsfield units were between 0 and 34) and normal attenuation psoas area (area where Hounsfield units were between 35 and 100) were calculated (23). The VAT area was measured as the total area inside the abdominal fascia meeting fat-density thresholds (−205 to −51 Hounsfield units), and the SAT area was measured as the total area between the abdominal fascia and skin. For a subset of scans (1867 scans in total), additional processing was performed to identify the paraspinal muscle between levels T11 and L3. The study was approved by the University of Michigan Institutional Review Board.
FIGURE 1.
Analytic morphomic processing.
Statistical analysis
Statistical analyses were conducted on the basis of all cohorts as well as stratified by sex. Correlation coefficients were used to assess the association between bone and muscle densities and BMI, VAT area, and SAT area. Because of the concern of potential outliers in measuring densities, we report Spearman's rank correlation coefficient (ρ), which is a nonparametric measure of statistical dependence. Moreover, this method allowed for the detection of both linear and nonlinear associations. To assess correlations between the trabecular bone density and adiposity at each level, we used all CT scans that were available. Cortical bone density, psoas muscle, and paraspinal muscle were likewise assessed. To assess associations between visceral adiposity and bone and muscle densities when controlling the effects from age and BMI, we evaluated partial correlations by treating these as controlling variables. The partial correlation between 2 variables, given a set of controlling variables, is the correlation between residuals from the linear regression of these 2 variables on controlling variables. Because of the concern of the intraclass correlation of multiple scans from the same patient, this analysis was done in the within-cluster resampling (WCR) framework (24, 25). Specifically, one single scan was randomly chosen per patient, and correlations and partial correlations were calculated as described. This process was repeated 100 times. Final estimates and SDs were determined from all 100 estimates on the basis of the WCR framework. R code (version 3.1.1; http://www.r-project.org) is available in the Supplemental Material to illustrate how to implement the WCR framework. This method efficiently used all available data and also addressed the intraclass correlation. Holm's method was used to control the family-wise error rate when considering multiple comparisons at different vertebral levels, which is a uniformly more powerful method than Bonferroni correction.
RESULTS
Descriptive data are presented as means and SEs as well as for comparison of men and women (Table 1). Despite greater SAT, women had significantly less visceral adiposity than men. Lean-psoas and psoas areas were greater in men than women; however, women had higher muscle-attenuation coefficients and higher mean BMDs.
TABLE 1.
Summary of demographics and morphomics at L4 by sex1
| Women |
Men |
||||||
| Scans, n | Patients, n | Mean ± SD | Scans, n | Patients, n | Mean ± SD | P | |
| Age, y | 5989 | 4677 | 43.17 ± 13.04 | 7127 | 5620 | 44.09 ± 15.38 | <0.001 |
| Height, m | 2747 | 2073 | 1.64 ± 0.08 | 3088 | 2530 | 1.78 ± 0.09 | <0.001 |
| Weight, kg | 4228 | 3353 | 75.65 ± 21.26 | 4970 | 4059 | 89.21 ± 24.31 | <0.001 |
| BMI, kg/m2 | 2322 | 1817 | 28.69 ± 10.06 | 2786 | 2347 | 28.40 ± 7.06 | 0.29 |
| Cortical BMD, HU | 3246 | 2717 | 323.28 ± 87.72 | 4002 | 3428 | 303.17 ± 81.09 | <0.001 |
| Trabecular BMD, HU | 3249 | 2720 | 209.45 ± 59.99 | 4006 | 3430 | 197.12 ± 67.89 | <0.001 |
| SAT area, cm2 | 4488 | 3641 | 279.40 ± 137.82 | 5073 | 4085 | 222.83 ± 145.47 | <0.001 |
| VAT area, cm2 | 5009 | 4076 | 100.99 ± 72.40 | 5615 | 4535 | 134.30 ± 103.08 | <0.001 |
| Psoas area, cm2 | 5989 | 4677 | 18.37 ± 5.78 | 7126 | 5619 | 30.08 ± 8.82 | <0.001 |
| Psoas density, HU | 5989 | 4677 | 55.74 ± 8.94 | 7127 | 5620 | 55.10 ± 9.75 | <0.001 |
| Lean psoas, HU × cm2 | 5989 | 4677 | 7.61 ± 2.49 | 7126 | 5619 | 12.43 ± 3.95 | <0.001 |
P values are from a 2-sample t test in the within-cluster resampling framework. BMD, bone mineral density; HU, Hounsfield units; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
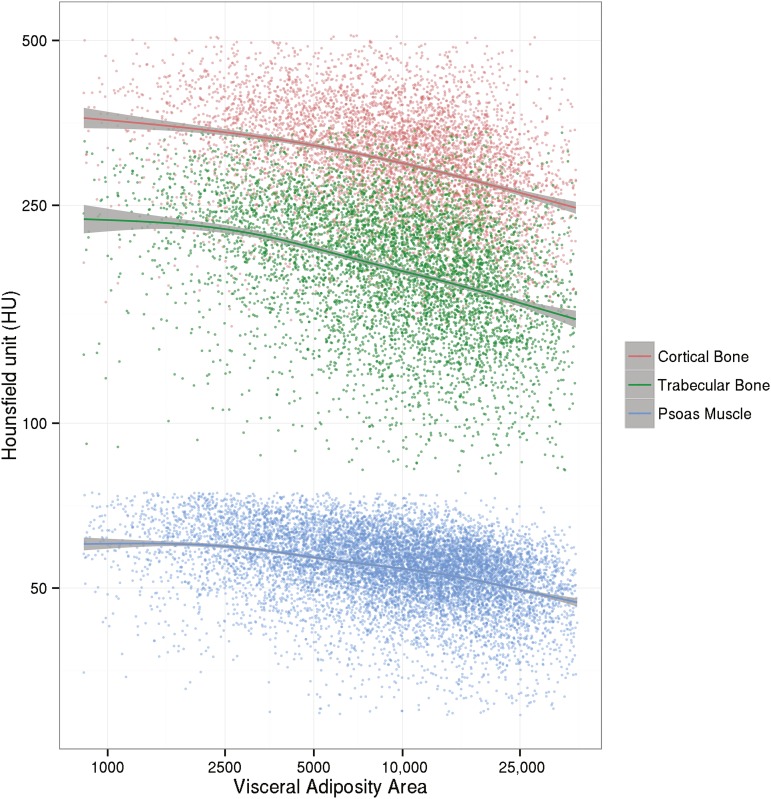
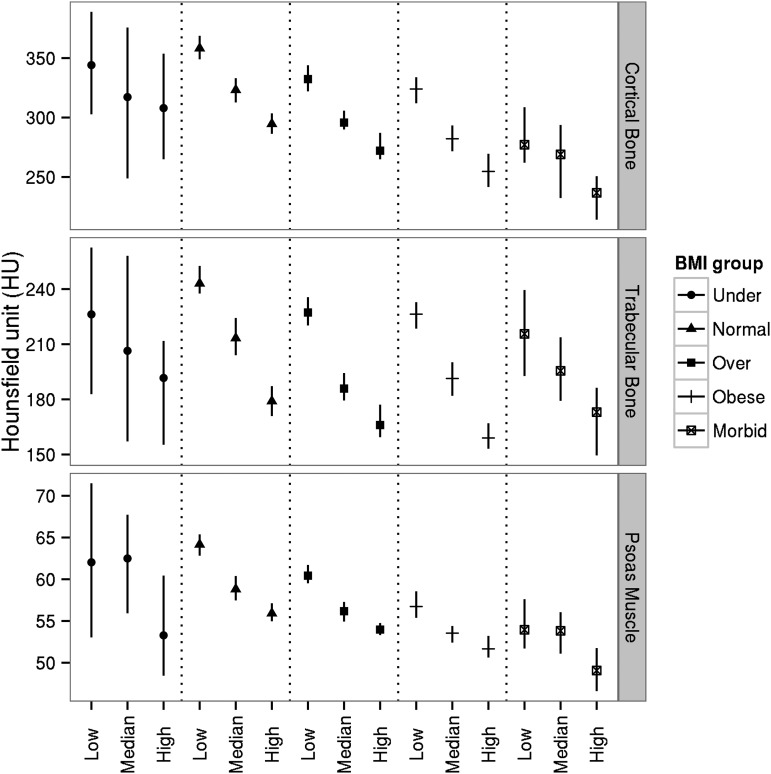
Visceral adiposity was strongly and inversely associated with cortical (ρ = −0.407; P < 0.001) and trabecular (ρ = −0.434; P < 0.001) BMD as well as psoas muscle Hounsfield units (ρ = −0.460; P < 0.001) at the L4 level. Associations of VAT with muscle attenuation and bone densities were much stronger than associations of SAT and BMI with muscle attenuation and bone densities. Even after controlling for age, BMI, and sex, associations between VAT and muscle attenuation and bone densities remained significant and ranged from small to medium effect sizes. These results are summarized in Supplemental Table 1. The 3 curves in Figure 2 depict nonparametric, generalized additive models with a shrinkage version of cubic regression splines. Shaded areas correspond to 95% CIs. There was a nonlinear trend such that curves declined to coincide with greater VAT areas. Furthermore, we conducted a subgroup analysis by dividing patients into 5 groups according to a standard BMI classification (in kg/m2; i.e., <18.5, 18.5–24.9, 25–29.9, 30–39.9, and ≥40). Within each BMI category, patients were further divided into 3 groups according to tertiles of VAT area. For each of these 15 subgroups, we calculated median Hounsfield units of psoas muscle attenuation, cortical BMD, and trabecular BMD and respective 95% CIs (Figure 3). Within almost all BMI categories, each incremental increase in VAT tertile was associated with a decrease in median Hounsfield units for muscle attenuation and BMD. Nonoverlapping CIs reflected statistically significant differences. CIs for the middle 3 BMI categories were smaller because of larger samples.
FIGURE 2.
Scatter plot of bone and muscle densities compared with visceral adiposity area (the y axis is plotted in the log scale).
FIGURE 3.
Medians of muscle attenuation and bone densities for different BMI categories and VAT area tertiles (we divided subjects into 5 groups according to standard BMI cutoffs as follows (in kg/m2): <18.5, 18.5–24.9, 25–29.9, 30–39.9, and ≥40). Within each BMI category, we further divided subjects into 3 groups according to tertiles of VAT areas. In each of these 15 subgroups, we presented median Hounsfield units of psoas muscle attenuation, cortical BMD, and trabecular BMD and respective 95% CIs. Low, median, and high represent 3 VAT-area tertile groups. BMD, bone mineral density; VAT, visceral adipose tissue.
Table 2 provides a summary of correlation and partial correlation coefficients between psoas measures and adiposity measures. Psoas attenuation measures included psoas muscle attenuation and the ratio of the normal attenuation psoas area to low attenuation psoas area. Adiposity assessments included indicators of adiposity (BMI) and direct measures of adiposity (i.e., VAT and SAT areas). Partial correlations were controlled for BMI as well as both BMI and age. Diminished psoas attenuation was more-strongly associated with VAT than BMI. Patterns of associations between BMI and VAT with psoas attenuation measures were similar between men and women, and associations between VAT and psoas attenuation remained significant even after adjustment for both BMI and age.
TABLE 2.
Correlation coefficients between adiposity measures and psoas measures1
| Spearman's correlations/partial correlations |
||||
| Men (2012 scans; 1778 patients) |
Women (1731 scans; 1451 patients) |
|||
| Psoas muscle attenuation | Ratio of normal to low attenuation psoas areas | Psoas muscle attenuation | Ratio of normal to low attenuation psoas areas | |
| BMI | −0.329 | −0.414 | −0.315 | −0.453 |
| SAT area | −0.341 | −0.424 | −0.269 | −0.386 |
| VAT area | −0.485 | −0.544 | −0.439 | −0.544 |
| VAT area with BMI controlled for | −0.377 | −0.395 | −0.322 | −0.352 |
| VAT area with BMI and age controlled for | −0.253 | −0.264 | −0.241 | −0.275 |
All correlation coefficients were significant with P values < 0.001. Correlations for men were not significantly different from those for women. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Supplemental Figure 1 shows associations between the attenuation of paraspinal muscles, BMI, VAT, and SAT between T11 and L3. In women only, VAT was a stronger predictor of lower paraspinal muscle attenuation than was BMI.
Supplemental Figures 2 and 3 show correlations between bone densities and adiposity measures for cortical and trabecular bone, respectively. Correlations are reported at vertebral levels from T7 to L5. Multiple-comparison–adjusted P values were reported. Trabecular BMD had much stronger associations with VAT than was shown for either BMI or SAT. Associations were virtually unchanged after controlling for BMI but decreased when adjusted for both BMI and age, which suggested a greater impact of age on trabecular bone. Likewise, cortical BMD was more-strongly associated with VAT than what was shown for both BMI and SAT. However, these associations dropped significantly when we controlled for both BMI and age, which again suggested the largest influence of age on cortical bone density. In addition, partial correlations between the VAT area and cortical BMD after controlling for BMI and age were only significant at lumbar vertebral levels.
DISCUSSION
The primary finding of this investigation was that VAT was strongly and inversely associated with vertebral trabecular and cortical BMDs and muscle attenuation in adults even after adjustment for effects of BMI and age. These findings are important considering that obesity is often touted as a protective factor for bone health and muscle strength with greater musculoskeletal mechanical and gravitational demands being coincident with the need to lift and maneuver a larger body habitus. Higher BMIs are associated with larger visceral fat stores across sexes and ethnicities (26), and VAT is generally considered to be the underlying driver linking higher BMIs to cardiometabolic chronic diseases (4). Thus, it seems that the pathophysiologic influence of VAT on the quality of vertebral muscle and bone may oppose the seemingly positive influence of a greater mechanical loading with higher body masses. These findings are in agreement with a recent study that showed, at the tissue level, that premenopausal women with more central adiposity had inferior bone quality and stiffness and markedly lower bone formation (19). Thus, in combination with other research that indicated that obesity may be associated with increased risk of fracture (27), these collective findings support the need for additional research to identify mechanisms linking greater adiposity to adverse effects on bone density and muscle attenuation as well as for improvements in clinical screening algorithms to identify individuals at highest risk.
It is well known that sedentary older adults are at significantly increased risks of weakness and sarcopenic obesity (28, 29), which are, in turn, thought to be the primary contributing factors of musculoskeletal fragility (27, 30–32), cardiometabolic abnormalities (33, 34), and early all-cause mortality (35–37). Findings have also indicated that localized adipose tissue within and surrounding the muscle is related to reduced muscle quality (i.e., strength per unit of muscle mass) in obesity and aging (38–40) as well as incident mobility disability (41). Accordingly, our results suggest that, at the levels where VAT areas were largest, associations between VAT, muscle attenuation, and cortical and trabecular BMDs were also large. This finding is interesting because if may support the notion that excess fat in the intra-abdominal region has a negative, pathophysiologic influence on local muscle and bone. Perhaps just as noteworthy if that, after we examined the variability of VAT within standardized BMI categories, we showed that individuals with higher BMIs and lower VAT areas had higher muscle attenuation and BMDs than did persons with lower BMIs and higher VAT areas. Indeed, a so-called cellular crosstalk between muscle and fat tissue may lead to a disruption in muscle growth and decreased functional capacity (42); however, the simultaneous net effect on muscle attenuation and bone density is less well understood. Not surprisingly, nearly all research related to the influence of adiposity to potentiate risk of musculoskeletal fragility has been conducted in elderly populations. Nevertheless, obesity and sedentary behavior are paralleled with significant increases in VAT and skeletal muscle fat infiltration (13, 43–45), the confluence of which represents a robust predictor for muscular weakness (38, 39) and cardiometabolic disease risk (34, 46) throughout adulthood.
Despite a similar trend in associations between VAT, muscle attenuation, and trabecular and cortical BMDs between men and women, we showed a dimorphic pattern of adipose tissue distribution and musculoskeletal densities. Although men had larger total muscle and lean areas than did women, women had higher muscle attenuation and BMDs. This finding was somewhat surprising because other reports have revealed lower muscle attenuation and greater intermuscular adipose tissue in women than men (14, 39). One potential explanation for this discrepancy is that most previous studies examined these factors as age-related phenomena in lower-extremity musculature (39). It is possible that the morphologic differences between men and women, as well as the interrelation between adiposity and musculoskeletal integrity, may be strongly mediated by age and the menopause transition (47). Because of the strong, inverse association between VAT, muscle attenuation, and trabecular and cortical BMDs, as well as the fact that young and middle-aged men tend to have a larger proportion of VAT to total fat than women (48), men may be at earlier risk of diminished spinal musculoskeletal quality. Overall, the negative associations between VAT and psoas attenuation measures were greater than those between VAT and trabecular and cortical bone densities (Table 2, Supplemental Figures 2 and 3). However, because of the proximity of VAT to the psoas muscle in the current investigation, these results may not necessarily be applicable to other muscle and, particularly, to those of the extremities. A recent study showed significant sex- and age-related variability in muscle attenuation even in the “trunk” muscle groups (14), and thus, mechanisms and mediators of this variability should be further explored to better understand changes in functional capacity and risk of cardiometabolic and morphologic deterioration.
Although cross-sectional, our study adds to the growing body of literature by showing a strong, independent association between greater VAT areas, lower muscle attenuation, and diminished BMDs in a large, nonelderly, heterogeneous sample of adults. These findings support another recent investigation that showed that longitudinal increases in BMI and body fat in men were coincident with declines in both muscle and bone mass (32). Whether VAT plays a direct, pathophysiologic role in the quality of local vertebral muscle and bone or the negative associations between VAT and both muscle and BMD quality are merely coincident to aging and reduced metabolic or intrinsic mechanical and gravitational stimuli [i.e., through the combination of greater sedentary behavior and low levels of physical activity (49)], remain to be determined. A better understanding of the cause and temporal sequence of these sequelae would provide much-needed information pertaining to risk screening and stratification and also to identify preventive and treatment options. Regardless, because these are potentially overlapping and modifiable consequences of both overnutrition and insufficient physical activity, there is a dire need for more comparative-effectiveness clinical studies to identify behavioral strategies that may concurrently reduce VAT across BMIs and increase the loading to bone and muscle.
In conclusion, ∼35% of adults in the United States are obese (50). Although primary public health concerns of obesity are related to abnormalities in cardiometabolic health and function, our study suggests a significant negative implication of visceral fat accumulation on musculoskeletal integrity. Visceral adiposity was shown to be inversely associated with lower trabecular and cortical BMDs and muscle attenuation in both men and women even after adjustment for BMI and age. Moreover, young and middle-aged men had greater VAT areas, lower muscle attenuation, and vertebral BMDs. Thus, future studies are needed to identify early preventive and treatment options to simultaneously target the reduction of abdominal adiposity and improvements in musculoskeletal quality.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—PZ and MP: conducted the research; PZ: analyzed data and performed the statistical analysis; GLS: provided critical comments on the manuscript; SCW: had primary responsibility for the final content of the manuscript; and all authors: designed the research and wrote the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BMD, bone mineral density; CT, computed tomography; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; WCR, within-cluster resampling.
REFERENCES
- 1.Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care 2010;13:382–90. [DOI] [PubMed] [Google Scholar]
- 2.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002;51:2005–11. [DOI] [PubMed] [Google Scholar]
- 3.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am 2008;37:841–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. [DOI] [PubMed] [Google Scholar]
- 6.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008;28:1039–49. [DOI] [PubMed] [Google Scholar]
- 7.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301–13. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 2000;89:104–10. [DOI] [PubMed] [Google Scholar]
- 9.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 2009;297:E987–98. [DOI] [PubMed] [Google Scholar]
- 10.Bredella MA, Gill CM, Gerweck AV, Landa MG, Kumar V, Daley SM, Torriani M, Miller KK. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology 2013;269:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gower BA, Casazza K. Divergent effects of obesity on bone health. J Clin Densitom 2013;16:450–4. [DOI] [PMC free article] [PubMed]
- 12.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, Pi-Sunyer FX, Heshka S; MRI Ancillary Study Group of the Look AHEAD Research Group. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 2009;89:807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 2007;85:377–84. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DE, D'Agostino JM, Bruno AG, Demissie S, Kiel DP, Bouxsein ML. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J Gerontol A Biol Sci Med Sci 2013;68:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi A, Zoico E, Goodpaster BH, Sepe A, Di Francesco V, Fantin F, Pizzini F, Corzato F, Vitali A, Micciolo R, et al. Quantification of intermuscular adipose tissue in the erector spinae muscle by MRI: agreement with histological evaluation. Obesity (Silver Spring) 2010;18:2379–84. [DOI] [PMC free article] [PubMed]
- 16.Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17:1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, Fantin F, Bosello O, Cominacini L, Harris TB, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci 2010;65:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 2002;57:M326–32. [DOI] [PubMed] [Google Scholar]
- 19.Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, Müller R, Zhao B, Guo X, Lang T, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab 2013;98:2562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barzilay J, Blaum C, Moore T, Xue Q, Hirsch C, Walston J, Fried L. Insulin resistance and inflammation as precursors of frailty. The Cardiovascular Health Study. Arch Intern Med 2007;167:635–41. [DOI] [PubMed] [Google Scholar]
- 21.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010;5:e10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev 2014;15:51–60. [DOI] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 1999;48:839–47. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman EB, Sen PK, Weinberg CR. Within-cluster resampling. Biometrika 2001;88:1121–34. [Google Scholar]
- 25.Rieger RH, Weinberg CR. Analysis of clustered binary outcomes using within-cluster paired resampling. Biometrics 2002;58:332–41. [DOI] [PubMed] [Google Scholar]
- 26.Nazare JA, Smith J, Borel A, Haffner S, Balkau B, Ross R, Massien C, Alméras N, Després J. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr 2012;96:714–26. [DOI] [PubMed] [Google Scholar]
- 27.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res 2012;27:1–10. [DOI] [PubMed]
- 28.Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci 2008;63:1076–81. [DOI] [PubMed] [Google Scholar]
- 29.Ryu M, Jo J, Lee Y, Chung YS, Kim KM, Baek WC. Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013;42:734–40. [DOI] [PubMed] [Google Scholar]
- 30.Compston JE, Watts N, Chapurlat R, Cooper C, Boonen S, Greenspan S, Pfeilschifter J, Silverman S, Díez-Pérez A, Lindsay R, et al. Obesity is not protective against fracture in postmenopausal women. Am J Med 2011;124:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res 2012;2012:172957. [DOI] [PMC free article] [PubMed]
- 32.Pasco JA, Gould H, Brennan SL, Nicholson GC, Kotowicz MA. Musculoskeletal deterioration in men accompanies increases in body fat. Obesity (Silver Spring) 2014;22:863–7. [DOI] [PubMed]
- 33.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 2007;102:919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balboa-Castillo T, Guallar-Castillon P, Leon-Munoz LM, Graciani A, Lopez-Garcia E, Rodriguez-Artalejo F. Physical activity and mortality related to obesity and functional status in older adults in Spain. Am J Prev Med 2011;40:39–46. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz J, Sui X, Lobelo F, Morrow J, Jackson A, Sjoumlstroumlm M, Blair S. Association between muscular strength and mortality in men: prospective cohort study. BMJ 2008;337:a439. [DOI] [PMC free article] [PubMed]
- 37.Xue QL, Beamer BA, Chaves PH, Guralnik JM, Fried LP. Heterogeneity in rate of decline in grip, hip, and knee strength and the risk of all-cause mortality: the Women's Health and Aging Study II. J Am Geriatr Soc 2010;58:2076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 2009;90:1579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 2001;90:2157–65. [DOI] [PubMed] [Google Scholar]
- 40.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–92. [DOI] [PubMed] [Google Scholar]
- 41.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–33. [DOI] [PubMed] [Google Scholar]
- 42.Rodeheffer MS. Tipping the scale: muscle versus fat. Nat Cell Biol 2010;12:102–4. [DOI] [PubMed] [Google Scholar]
- 43.Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 2002;51:144–51. [DOI] [PubMed] [Google Scholar]
- 44.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1991;54:509–15. [DOI] [PubMed] [Google Scholar]
- 45.Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J 1995;9:273–8. [PubMed] [Google Scholar]
- 46.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008;11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CG, Carr MC, Murdoch SJ, Mitchell E, Woods NF, Wener MH, Chandler WL, Boyko EJ, Brunzell JD. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab 2009;94:1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11–8. [DOI] [PubMed]
- 49.Kohrt WM, Barry D, Schwartz R. Muscle forces or gravity: what predominates mechanical loading on bone? Med Sci Sports Exerc 2009;41:2050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogden C, Carroll M, Kit B, Flegal K. Prevalence of obesity among adults: United States, 2011–2012. Hyattsville, MD: National Center for Health Statistics; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.