Abstract
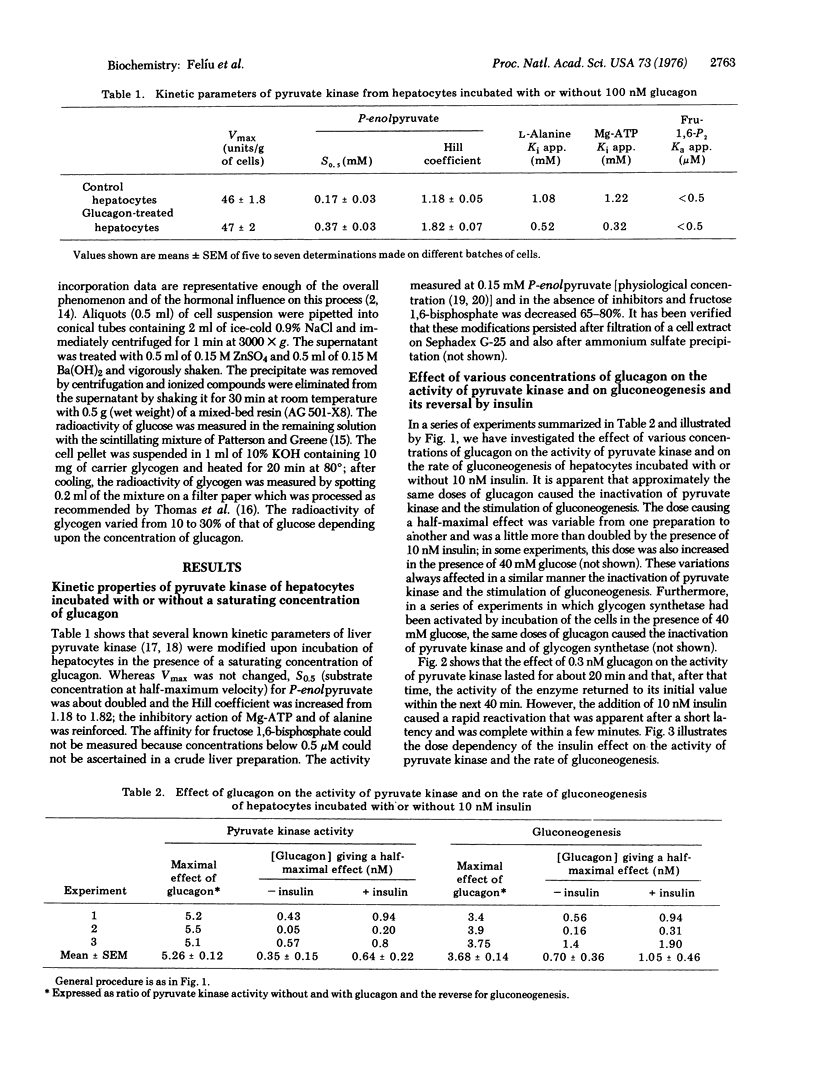
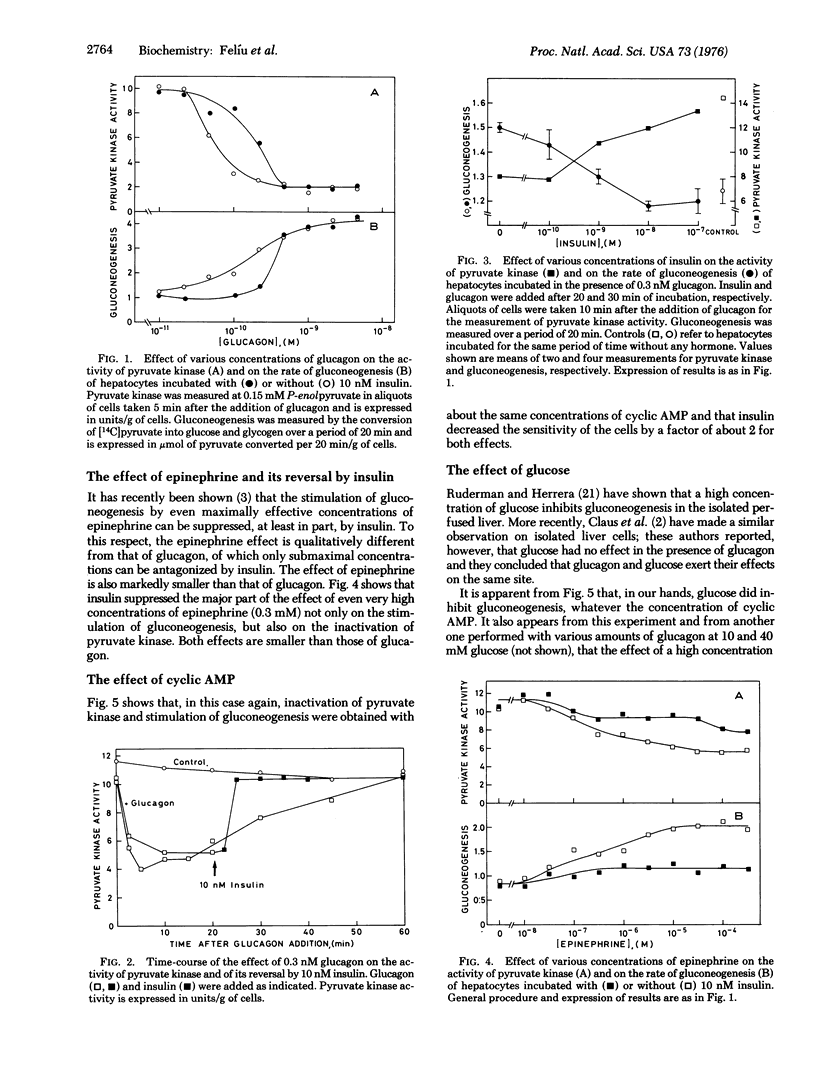
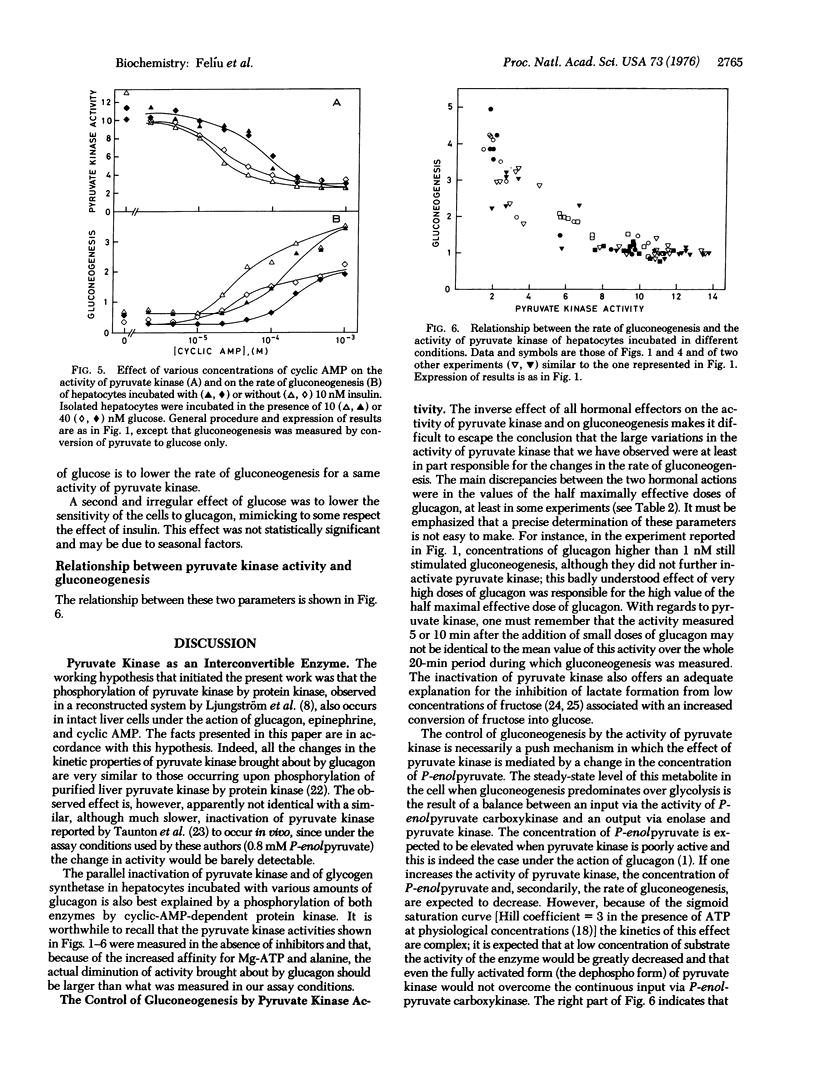
Treatment of isolated rat hepatocytes with saturating concentrations of glucagon caused several modifications properties of pyruvate kinase (ATP:pyruvate 2-O-phosphotransferase, EC 2.7.1.40): S0.5 (substrate concentration at half maximum velocity) for phosphoenolpyruvate was about doubled, whereas Vmax was not changed; the activity measured at 0.15 mM phosphoenolpyruvate (physiological concentration) was reduced 65-80%; and there was also an increase in the Hill coefficient and in the affinity of the enzyme for the inhibitors Mg-ATP and alanine. Glucagon, 3':5'-cyclic AMP, and epinephrine caused an inactivation of pyruvate kinase together with a sitmulation of gluconeogenesis. Insulin (10 nM) antagonized the effect of suboptimal doses of glucagon or cyclic AMP and of even maximal doses of epinephrine, on both pyruvate kinase activity and on gluconeogenesis. These observations can be explained by a phosphorylation of pyruvate kinase by cyclic-AMP-dependent protein kinase, as described by Ljungström et al. [(1974) Biochim. Biophys. Acta 358, 289-298] in a reconstructed system. They offer a molecular explanation for the hormonal control of gluconeogenesis. Glucose caused an inhibition of gluconeogenesis with no corresponding change in pyruvate kinase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carbonell J., Felíu J. E., Marco R., Sols A. Pyruvate kinase. Classes of regulatory isoenzymes in mammalian tissues. Eur J Biochem. 1973 Aug 1;37(1):148–156. doi: 10.1111/j.1432-1033.1973.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- Claus T. H., Pilkis S. J., Park C. R. Stimulation by glucagon of the incorporation of U-14C-labeled substrates into glucose by isolated hepatocytes from fed rats. Biochim Biophys Acta. 1975 Sep 8;404(1):110–123. doi: 10.1016/0304-4165(75)90152-x. [DOI] [PubMed] [Google Scholar]
- Claus T. H., Pilkis S. J. Regulation by insulin of gluconeogenesis in isolated rat hepatocytes. Biochim Biophys Acta. 1976 Feb 24;421(2):246–262. doi: 10.1016/0304-4165(76)90291-9. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Krug F., Cuatrecasas P. Inhibitors of glucagon inactivation. Effect on glucagon--receptor interactions and glucagon-stimulated adenylate cyclase activity in liver cell membranes. Biochim Biophys Acta. 1974 Mar 20;343(1):101–120. doi: 10.1016/0304-4165(74)90242-6. [DOI] [PubMed] [Google Scholar]
- Ekman P., Dahlqvist U., Humble E., Engström L. Comparative kinetic studies on the L-type pyruvate kinase from rat liver and the enzyme phosphorylated by cyclic 3', 5'-AMP-stimulated protein kinase. Biochim Biophys Acta. 1976 Apr 8;429(2):374–382. doi: 10.1016/0005-2744(76)90285-0. [DOI] [PubMed] [Google Scholar]
- Faupel R. P., Seitz H. J., Tarnowski W., Thiemann V., Weiss C. The problem of tissue sampling from experimental animals with respect to freezing technique, anoxia, stress and narcosis. A new method for sampling rat liver tissue and the physiological values of glycolytic intermediates and related compounds. Arch Biochem Biophys. 1972 Feb;148(2):509–522. doi: 10.1016/0003-9861(72)90170-1. [DOI] [PubMed] [Google Scholar]
- Flory W., Peczon B. D., Koeppe R. E., Spivey H. O. Kinetic properties of rat liver pyruvate kinase at cellular concentrations of enzyme, substrates and modifiers. Biochem J. 1974 Jul;141(1):127–131. doi: 10.1042/bj1410127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidmann B., Goodman E. H., Jr, Saunders H. L., Kostos V., Weinhouse S. An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch Biochem Biophys. 1971 Apr;143(2):566–578. doi: 10.1016/0003-9861(71)90241-4. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDaniel H. G. Acute suppression of hepatic gluconeogenesis by glucose in the intact animal. Am J Physiol. 1975 Dec;229(6):1569–1575. doi: 10.1152/ajplegacy.1975.229.6.1569. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Herrera M. G. Glucose regulation of hepatic gluconeogenesis. Am J Physiol. 1968 Jun;214(6):1346–1351. doi: 10.1152/ajplegacy.1968.214.6.1346. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Sue F., Morimura H. Feed-forward activation and feed-back inhibition of pyruvate kinase type L of rat liver. Biochem Biophys Res Commun. 1967 Nov 17;29(3):444–449. doi: 10.1016/0006-291x(67)90477-9. [DOI] [PubMed] [Google Scholar]
- Taunton O. D., Stifel F. B., Greene H. L., Herman R. H. Rapid reciprocal changes in rat hepatic glycolytic enzyme and fructose diphosphatase activities following insulin and glucagon injection. J Biol Chem. 1974 Nov 25;249(22):7228–7239. [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Titanji V. P., Zetterqvist O., Engstroöm L. Regulation in vitro of rat liver pyruvate kinase by phosphorylation-dephosphorylation reactions, catalyzed by cyclic-AMP dependent protein kinases and a histone phosphatase. Biochim Biophys Acta. 1976 Jan 23;422(1):98–108. doi: 10.1016/0005-2744(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Veneziale C. M. Gluconeogenesis from fructose in isolated rat liver. Stimulation by glucagon. Biochemistry. 1971 Aug 31;10(18):3443–3447. doi: 10.1021/bi00794a020. [DOI] [PubMed] [Google Scholar]



