Abstract
Aim
To define the racial differences present after PEA and asystolic IHCA and explore factors that could contribute to this disparity
Methods
We analyzed PEA and asystolic IHCA in the Get-With-The-Guidelines-Resuscitation database. Multilevel conditional fixed effects logistic regression models were used to estimate the relationship between race and survival to discharge and return of spontaneous circulation (ROSC), sequentially controlling for hospital, patient demographics, comorbidities, arrest characteristic, process measures, and interventions in place at time of arrest.
Results
Among the 561 hospitals, there were 76,835 patients who experienced IHCA with an initial rhythm of PEA or asystole (74.8% white, 25.2% black). Unadjusted ROSC rate was 55.1% for white patients and 54.1% for black patients (unadjusted OR: 0.94 [95% CI, 0.90–0.98], p=0.016). Survival to discharge was 12.8% for white patients and 10.4% for black patients (unadjusted OR: 0.83 [95% CI, 0.78–0.87], p<0.001). After adjusting for temporal trends, patient characteristics, hospital, and arrest characteristics, there remained a difference in survival to discharge (OR: 0.85 [95% CI, 0.79–0.92]) and rate of ROSC (OR: 0.88 [95% CI, 0.84–0.92]). Black patients had a worse mental status at discharge after survival. Rates of DNAR placed after survival from were lower in black patients with a rate of 38.3% compared to 44.5% in white patients (p<0.001).
Conclusion
Black patients are less likely to experience ROSC and survival to discharge after PEA or asystole IHCA. Individual patient characteristics, event characteristics, and hospital characteristics don’t fully explain this disparity. It is possible that disease burden and end-of-life preferences contribute to the racial disparity.
Keywords: Heart arrest, Cardiopulmonary resuscitation, Defibrillation, Chest compression, Racial Disparity
Introduction
In the US, African-Americans experience significant health disparities across a range of medical conditions, including cardiac arrest outcomes, both in and out of the hospital.1–5 Out-of-hospital arrest disparities have been explained in part by factors such as increased time to emergency medical services arrival, decreased rate of bystander cardiopulmonary resuscitation (CPR), decreased likelihood of having the arrest be witnessed, and decreased rate of ventricular tachycardia (VT) or ventricular fibrillation (VF).1, 2, 6, 7 For in-hospital cardiac arrests (IHCA), work elucidating racial differences in outcomes has focused on arrests due to ventricular arrhythmias, where hospital-level factors (i.e. racial clustering in hospitals with worse outcomes) were found to be a large contributor.5
The vast majority of IHCA are due to pulseless electrical activity (PEA) or asystole.8, 9 While VT and VF are often due to cardiac etiologies, PEA and asystolic arrests have a multifactorial etiology and lower overall survival.8–12 In addition, intra- and post- resuscitation management differs greatly from arrests due to VF and VT, where the focus tends to be on defibrillation and cardiac catheterization.13 Because of the wider array of reasons which cause PEA and asystolic arrests, there may be additional or alternative factors which cause racial disparities in these rhythms. These factors, which possibly play a role in arrest outcomes, include difference in end-of-life decisions and level of control of chronic medical conditions. We sought to further define the racial differences present after IHCA with initial rhythm of PEA and asystole and explore factors that could be contributing to this disparity. To our knowledge, this is the first study to focus on racial disparities for in-hospital cardiac arrests which are of PEA or asystole.
Methods
We analyzed data from the American Heart Association’s Get With The Guidelines®-Resuscitation (GWTG-R) registry (formerly National Registry of Cardiopulmonary Resuscitation). This is an American Heart Association (AHA) sponsored quality improvement registry database of IHCA which has previously been described.8 Hospitals participate voluntarily and provide information about their facility, staffing, and resuscitation services. Information is collected from patients’ hospital charts, cardiac arrest record sheets, paging system logs, pharmacy records of drugs utilized in resuscitation efforts, and billing charge sheets. All the data collected are entered utilizing Utstein definitions.14 Outcome, A Quintiles Company, is the data collection coordination center for the American Heart Association/American Stroke Association Get With The Guidelines® programs.
The GWTG-R has also been linked to the American Hospital Association’s database, which contains information about 6500 hospitals in the US. From this, we abstracted hospital characteristics, including bed size, geographic location of hospital, presence of residency training program, and whether the hospital was in an urban or rural setting.
In our analysis, we included all IHCA between 2000 and 2009. Our analysis included patients whose initial cardiac arrest rhythm was asystole or PEA. Patients were included if they were identified, through their medical records, as black or white. Cardiac arrests that occurred in procedure suites, operation rooms, ambulatory units, or the emergency department were excluded. Patients with missing or unknown initial rhythm of arrest and race were also excluded (Figure 1).
Figure 1. Study population.
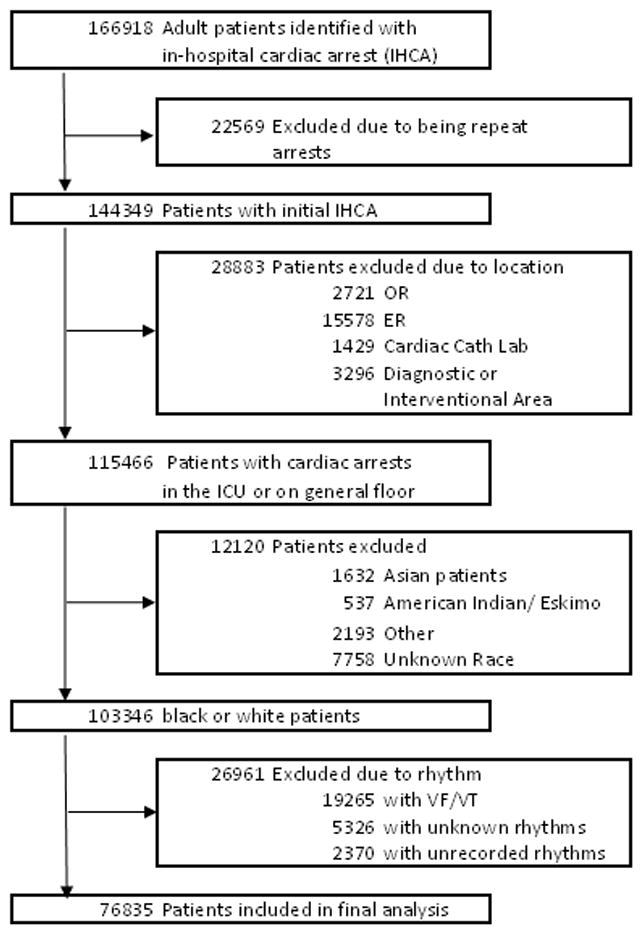
Abbreviations: IHCA, in-hospital cardiac arrest; OR, operating room; ER, emergency room; Cath, catheterization; ICU, intensive care unit; VF, ventricular fibrillation; VT, ventricular tachycardia
Study Outcomes and Variables
The primary outcome was survival to hospital discharge, and the secondary outcome was return of spontaneous circulation (ROSC) for 20 minutes post-resuscitation. Patient race was the primary independent variable. Patient factors included age, sex, initial rhythm, co-morbidities prior to cardiac arrest, and interventions in place at the time of resuscitation. Resuscitation event characteristics included length of cardiac arrest, day of week, and time of day. End-of-life measures including DNAR status, length of time from achieving ROSC to DNAR, and reason resuscitation ended were also extracted, as were pressors used during the resuscitation.
Statistical Analysis
Patient, event, hospital, and end-of-life care characteristics as well as unadjusted outcomes were compared between white and black patients using chi-squared tests for categorical data and t-tests for continuous data. Adjustment for confounders that may account for differences between white and black patients was performed by sequentially controlling for different types of covariates. First, a generalized estimating equation model was used to estimate the average survival difference between black and white patients in the study population. Then, multilevel conditional fixed-effects logistic regression models were used to estimate the relationship between race and survival to discharge, sequentially controlling for: 1) hospital factors and temporal trends, 2) patient age, sex, rhythm type, and event characteristics, 3) patient co-morbidities, and 4) interventions in place at the time of the event. A fixed effects model is akin to adding a dummy variable for each hospital into the model, which allows these hospital variables to be correlated with both the variable of interest and the outcome. Thus, the hospital characteristics (both measured and unmeasured) are controlled for as potential confounders in the model.15 A secondary analysis was performed in the same manner for ROSC. In addition, a subgroup analysis for survival to discharge was conducted based on the type of vasopressors used during the resuscitation.
Statistical analyses were performed using Stata, version 12.0 (StataCorp). All significance tests used a 2-sided p-value <0.05.
Results
Pre-Arrest Characteristics
Our final analysis included 76,835 patients from 561 hospitals who experienced IHCA with an initial rhythm of PEA or asystole. Of these, 57,149 were white (74.8%) and 19,236 were black (25.2%). Table 1 shows baseline sociodemographic and clinical information by race. White patients were older and more likely to be male. Cardiac diagnosis (CHF, MI, or arrhythmias) were found more often in white patients. Black patients were more likely to have renal insufficiency, diabetes, sepsis, and baseline CNS depression. Of patients in a non-intensive care setting, black patients were less likely to be in a telemetry bed.
Table 1.
Individual patient characteristics
| White [n = 57149] | Black [n = 19236] | p | |
|---|---|---|---|
| Age, mean (SD), years | 71 (15.2) | 63(16.5) | <0.001 |
| Male | 33532 (58.8) | 10038 (52.2) | <0.001 |
| Pre-Existing Conditions | |||
| Prior MI | 9154 (17.5) | 1951 (11.5) | <0.001 |
| MI during this admission | 8647(16.5) | 1662 (9.8) | <0.001 |
| Arrhythmias | 17318 (33.1) | 4623 (27.3) | <0.001 |
| Prior CHF | 11364 (21.7) | 3740 (22.1) | 0.38 |
| CHF this admission | 9940 (19.0) | 3010 (17.8) | <0.001 |
| DM | 15680 (30.0) | 6065 (35.8) | <0.001 |
| Hepatic Insufficiency | 4193 (8.0) | 1700 (10.0) | <0.001 |
| Electrolyte Abnormality | 9620 (18.4) | 3683 (21.7) | <0.001 |
| Renal Insufficiency | 17647 (33.8) | 7819 (46.1) | <0.001 |
| PNA | 8403 (16.1) | 3076 (18.1) | <0.001 |
| Hypotension/Hypoperfusion | 15618 (29.9) | 4980 (29.4) | 0.21 |
| Trauma | 2004 (3.8) | 542 (3.2) | <0.001 |
| Sepsis | 8961 (17.1) | 4023 (23.7) | <0.001 |
| Neoplastic process | 7441 (14.2) | 2565 (15.1) | 0.004 |
| Respiratory Insufficiency | 23657 (45.2) | 7733 (45.6) | 0.42 |
| Baseline CNS depression | 6735 (12.9) | 2928 (17.3) | <0.001 |
| Acute Stroke | 1995 (3.8) | 1013 (6.0) | <0.001 |
| Acute non-stroke neurological event | 4186 (8.0) | 1562 (9.2) | <0.001 |
| Prior to Admission Location | <0.001 | ||
| SNF | 4448 (7.8) | 2117 (11.0) | |
| Home | 42145 (73.8) | 14002 (72.8) | |
| Other Hospital | 6503 (11.4) | 1554 (8.1) | |
| Interventions in Place prior to Arrest | |||
| Pacemaker | 4046 (7.1) | 852 (4.4) | <0.001 |
| Intra-aortic balloon pump | 704 (1.3) | 96 (0.5) | <0.001 |
| Continuous Anti-Arrhythmics | 2710 (5.0) | 649 (3.6) | <0.001 |
| PA Catheter | 2177 (4.0) | 465 (2.6) | <0.001 |
| Central Venous Access | 8164 (14.3) | 3330 (17.3) | <0.001 |
| Invasive airway | 16675 (29.2) | 6680 (34.7) | <0.001 |
| Assisted or mechanical ventilation | 17526 (30.7) | 6749 (35.1) | <0.001 |
| Vaso-active medications | 15743 (28.9) | 5487 (30.2) | 0.001 |
| Dialysis or Extracorporeal filtration therapy | 1898 (3.5) | 1005 (5.5) | <0.001 |
| Intra-arterial catheter | 5713 (10.0) | 1920 (10.0) | 0.95 |
All results shown as n (%) unless otherwise indicated. Abbreviations: MI- Myocardial Infarction; CHF- Congestive Heart Failure; PNA- Pneumonia; CNS- Central Nervous System; SNF- Skilled Nursing Facility.
White patients were more likely to have cardiac interventions in place, such as intra-arterial balloon pumps and pulmonary artery catheters, while black patients were more likely to be intubated and receiving vaso-active medications.
e Table 1 shows the differences in hospital characteristics. Hospitals in this study had proportions of black patients that reflect the geographic distribution of blacks within the United States.16 Hospitals that were larger and had approved training programs had a higher percentage of black IHCA patients.
Event Characteristics
Measured variables from the arrest were also analyzed for the two groups (Table 2). White patients were more likely than black patients to have an initial rhythm of asystole (47.8% vs 44.7%; p<0.001). We can also see that non-ICU black patients were more likely to be on the general floor, as opposed to under telemetry monitoring (23.4% vs 21.2%; p<0.001). There was little to no difference in the proportion of weekend arrest (36.2% vs 36%; p=0.72), but there was a difference between night arrests (36.5% for white patients vs 34.4% for black patients; p<0.001) and witnessed arrests (78.8% for white patients vs 77.1% for black patients; p<0.001). Hospital wide resuscitation teams were found at 86.9% of hospitals where white patients experienced IHCA, which was significantly higher than 82.9% of hospitals were black patients experienced IHCA (p<0.001).
Table 2.
Event Characteristics
| White | Black | P | |
|---|---|---|---|
| Type of Initial Rhythm | <0.001 | ||
| Asystole | 27312 (47.8) | 8602 (44.7) | |
| PEA | 29837 (52.2) | 10634 (55.3) | |
| Location at Time of Arrest | <0.001 | ||
| ICU | 32773 (57.3) | 11063 (57.5) | |
| General Floor | 12127 (21.2) | 4494 (23.4) | |
| Telemetry Floor | 12248 (21.4) | 3678 (19.1) | |
| Code duration, median (Q25–75), min | |||
| Overall | 17 (10–27) | 17 (10–27) | 0.20 |
| Achieved ROSC (Q25–75) | 14 (7–26) | 14 (7–25) | 0.54 |
| No ROSC (Q25–75) | 19 (13–28) | 20 (13–28) | 0.003 |
| Asystole (Q25–75) | 16 (10–26) | 17 (10–25) | 0.01 |
| PEA (Q25–75) | 17 (9–28) | 17 (9–27) | 0.57 |
| Epinephrine boluses, median (Q25–75), n | |||
| Overall | 3 (2–4) | 3 (2–4) | <0.001 |
| Achieved ROC | 2 (1–3) | 2 (1–4) | <0.001 |
| No ROC | 3 (2–5) | 3 (3–5) | <0.001 |
| Weekend Arrest | 18552 (36.2) | 6186 (36.0) | 0.72 |
| Night Arrest | 20717(36.5) | 6566(34.4) | <0.001 |
| Witnessed Arrest | 45028(78.8) | 14829(77.1) | <0.001 |
| Hospital Wide Arrest Response Team | 49636(86.9) | 15951(82.9) | <0.001 |
All results shown as n (%) unless otherwise indicated. Abbreviations: PEA- Pulseless Electrical Activity; ICU- Intensive Care Unit; ROSC- Return of Spontaneous Circulation
Arrest Outcomes
The unadjusted ROSC rate after IHCA was 55.1% for white patients and 54.1% for black patients (Table 3). This difference appeared to be mediated by the asystole arrests, in subgroup analysis, rather than PEA, where there was not a significant difference in ROSC rates. The difference appeared more pronounced after adjusting for temporal trends, hospital, patient characteristics, and arrest characteristics (unadjusted OR: 0.94 [95% CI, 0.90–0.98] vs adjusted OR: 0.88 [95% CI, 0.84–0.92]) (Table 4).
Table 3.
Unadjusted outcomes
| White | Black | p | |
|---|---|---|---|
| Return of Spontaneous Circulation for >20 min | 31467 (55.1) | 10398 (54.1) | 0.016 |
| Length of Stay, median (Q25–75), days | 5.1 (1.5–13.2) | 6.1 (1.9–15.8) | <0.001 |
| Survival for 24 hrs | 15591 (27.3) | 4923 (25.6) | <0.001 |
| Survival to discharge | 7277 (12.8) | 1994 (10.4) | <0.001 |
| Discharge Location | 0.03 | ||
| Home | 2550 (35.1) | 641 (32.2) | |
| Hospice | 465 (6.4) | 123 (6.2) | |
| Other hospital | 740 (10.2) | 202 (10.1) | |
| Rehab | 1291 (17.8) | 327 (16.4) | |
| SNF | 1972 (27.1) | 619 (31.0) | |
| ROSC by Rhythm | |||
| Asystole | 13938 (51.0) | 4081 (47.5) | <0.001 |
| PEA | 17529 (58.8) | 6317 (59.4) | 0.24 |
| Survival to discharge by Rhythm | |||
| Asystole | 3301 (12.1) | 735 (8.6) | <0.001 |
| PEA | 3976 (13.34) | 1259 (11.9) | <0.001 |
| Cerebral Perfusion Category at Discharge | <0.001 | ||
| 1 | 2858 (40.0) | 639 (31.9) | |
| 2 | 2074 (28.3) | 544 (27.2) | |
| 3 | 1047 (14.3) | 380 (19.0) | |
| 4 | 299 (4.1) | 164 (8.2) | |
All results shown as n (%) unless otherwise indicated. Abbreviations: ROSC- Return of Spontaneous Circulation; PEA: Pulseless Electrical Activity
Table 4.
Association between race and adjusted arrest outcomes
| OR | 95% Confidence Interval | ||
|---|---|---|---|
| Survival to Discharge | |||
| Unadjusted outcome | 0.84 | 0.79 | 0.89 |
| Adjusted for temporal trends and hospital | 0.86 | 0.81 | 0.92 |
| Adjusted for above plus age, sex, rhythm type, event location, and event characteristicsa | 0.82 | 0.76 | 0.87 |
| Adjusted for above plus co-morbiditiesb | 0.86 | 0.80 | 0.92 |
| Adjusted for above plus interventions in placec | 0.85 | 0.79 | 0.92 |
| PEA, fully adjusted for above variables | 0.92 | 0.83 | 1.00 |
| Asystole, fully adjusted for above variables | 0.77 | 0.68 | 0.86 |
| Return of Spontaneous Circulation | |||
| Unadjusted outcome | 0.94 | 0.90 | 0.98 |
| Adjusted for temporal trends and hospital | 0.93 | 0.90 | 0.97 |
| Adjusted for above plus age, sex, rhythm type, event location, and event characteristicsa | 0.90 | 0.86 | 0.94 |
| Adjusted for above plus co-morbiditiesb | 0.88 | 0.84 | 0.92 |
| Adjusted for above plus interventions in placec | 0.88 | 0.84 | 0.92 |
| PEA, fully adjusted for above variables | 0.91 | 0.85 | 0.97 |
| Asystole, fully adjusted for above variables | 0.84 | 0.78 | 0.90 |
| Survival with good neurologic function | |||
| Unadjusted outcome | 0.70 | 0.61 | 0.79 |
| Adjusted for temporal trends and hospital | 0.67 | 0.59 | 0.77 |
| Adjusted for above plus age, sex, rhythm type, event location, and event characteristicsa | 0.62 | 0.53 | 0.73 |
| Adjusted for above plus co-morbiditiesb | 0.68 | 0.57 | 0.81 |
| Adjusted for above plus interventions in placec | 0.67 | 0.57 | 0.80 |
Abbreviations: OR- odds rato.
Event characteristics adjusted for in the model were weekend, night, witnessed, presence of a hospital-wide arrest response team, and causes of arrest that occurred in >1% of events.
Includes number of co-morbidities, illness category, and pre-existing conditions from Table 1.
Includes number of interventions and all interventions in Table 1 except “assisted or mechanical intervention,” which was omitted due to significant collinearity with invasive airway.
The rate of survival to hospital discharge for black patients was 10.4% versus 12.8% for white patients, with a more pronounced difference noted in asystole (Table 3). Similarly, the disparity was not resolved with adjusting for potential confounders (unadjusted OR: 0.84 [95% CI, 0.79–0.89] vs adjusted OR: 0.85 [95% CI, 0.79–0.92]) (Table 4). Disparities were also noted in discharge location and neurological status on discharge, with white patients being more likely to have a CPC 1 (p<0.001) and be discharged home (p=0.02) (Table 3). The difference in neurological status was apparent even after adjusting for potential confounders (adjusted OR: 0.67 [95% CI 0.57–0.80]).
Do Not Attempt Resuscitation (DNAR) Status
As a possible marker of end-of-life decision making characteristics, we calculated the rates of DNAR orders for patients after survival from IHCA. Unadjusted data shows that black patients had a lower incidence of DNAR orders in place post-arrest (38.3% for black patients vs 44.5% for white patients; p<0.001) (Table 5). These were put in place later compared to those put in place by white patients, with a median difference of almost 5 hours (p<0.001). In addition, for those that did have DNAR orders in place, white patients were more likely to have care withdrawn after an IHCA when compared to black patients (31.4% for white patients vs 24.8% for black patients; p<0.001).
Table 5.
End of Life Care
| White | Black | p | |
|---|---|---|---|
| End of Life Care/Discussion | |||
| Time to DNAR, median (Q25–75), hrs | 7.7 (1.4–56.3) | 12.4 (2.5–74.9) | <0.001 |
| DNAR declared prior to death | 16822 (44.5) | 4852 (38.3) | <0.001 |
| Life support withdrawn if declared DNAR | 8652 (31.4) | 2235 (24.8) | <0.001 |
| Reason Resuscitation Ended if Patient Died | <0.001 | ||
| Deemed futile | 23418 (41.0) | 8413 (43.7) | |
| Advanced Directive | 3995 (7.0) | 1329 (6.9) | |
| Restrictions by Family | 584 (1.0) | 106 (0.6) | |
All results shown as n (%) unless otherwise indicated. Abbreviations: DNAR- Do Not Attempt Resuscitate.
Discussion
We conducted the largest study to date of racial disparities in in-hospital cardiac arrest and found that black patients in PEA and asystole have worse outcomes than their white counterparts. Not only did we find that black patients were significantly more likely to die in the hospital after cardiac arrest, those that survived were more 33% less likely to be discharged with normal mental status. These outcomes support prior results in VF/VT. However, in contrast to that work, the disparities in this analysis persisted after adjusting for known patient and hospital level characteristics, as well as clustering by hospital, suggesting that unmeasured characteristics contribute to the disparities in PEA and asystole to a greater extent than for ventricular arrhythmias. We found no evidence to suggest that black patients were receiving less aggressive resuscitation. In fact, resuscitations in black patients were statistically longer and resulted in increased use of epinephrine. In addition, we found that post-arrest, black patients were less likely to receive a do-not-attempt-resuscitation (DNAR) order and if they did, it was likely to occur later in the process. Also, they were less likely to have care withdrawn once declared DNAR.
If such a pattern were present in the patients preceding cardiac arrest, it could have skewed the sample in favor of white patients for whom resuscitation was attempted, being more “resuscitatable,” because those unlikely to survive were dropped from the sample by virtue of a DNAR order before they arrest. Unfortunately this data set only includes patients who suffer a cardiac arrest and so disparities in pre-arrest DNAR are not knowable. However, with the exception of cardiac interventions, black patients in this cohort were more likely to have intensive interventions in place at the time of arrest, including mechanical ventilation and pressor support, suggesting a more aggressive approach.
While, in general, DNAR orders are often underutilized in patients at high risk of impending death, this is more likely to be the case in black patients, who are more likely to receive life-prolonging measures (such as feeding tubes, mechanical ventilation, ICU admission and CPR) and less likely to receive comfort-directed care or be enrolled in hospice.17–25 National data also shows that black patients are more likely to die in the hospital when compared to white patients.26, 27 In addition, reversal of DNAR is also seen more often in black patients as is revoking hospice.22, 28 Furthermore, while discussions about end-of-life care seem to assist white patients in receiving less life-prolonging care, they do not have a similar effect on black patients.29 Racial differences in DNAR use have been tied to black patients having less access to information about advanced directives, discordance between patient and physician race, and cultural differences.30, 31 Large, academic hospitals, of which black patients in this study were likely to be in, also tend to have lower DNAR rates, but clustering by hospital did not alleviate the disparity in outcomes.32 Another complicating factor may be the age at which patients have their cardiac arrest. In this study, black patients were on average 8 years younger than their white counterparts, which may increase the barrier to addressing end-of-life care.
Aside from end-of-life care, other factors which may play a role in the racial disparity in outcomes are prevalence of chronic medical conditions and control of these conditions, which have been shown to be worse in black patients. Literature shows that not only is incidence of diabetes higher among black individuals, glycemic control tends to be worse also.33–35 And while white individuals tend to have greater prevalence of chronic kidney disease, black individuals have a much higher prevalence of end stage renal disease.36, 37 For neoplastic diseases, black individuals are likely to be diagnosed at higher stages for certain cancers and for others, they’re likely to have higher mortality and co-morbidity burden at the same stage.38, 39 While our analysis controls for presence of chronic condition, the data only provided the variables as dichotomous and so we were unable to control for the severity of the condition, a potential unmeasured confounder. Finally, one other variable that raises the question about potential unmeasured confounders is the disparity in pre-arrest bed allocation with a greater number of black patients arresting in non-telemetry settings. Further work to elucidate the etiology behind this may shed additional light on the disparities in outcomes seen in this study.
Our study contributes to the current body of literature on IHCA by showing that there is racial disparity present in PEA and asystole IHCA. Coupled with other work related to end-of-life care and racial disparities, we postulate that disease burden and end-of-life preferences may contribute to this disparity. The study has several important limitations. First, as this is an observational study, we are only able to show associations. In addition, as the data include only those patients who had a cardiac arrest, we are unable to compare differences in pre-arrest patient cohorts. Finally, although racial disparities in health are closely tied to access to care and economic status, we were unable to control for those variables in our dataset.
Conclusions
We conclude that significant racial disparities exist in outcomes between black and white victims of in-hospital cardiac arrest. However the etiology for these differences remains to be determined. It does not appear to be related to more aggressive resuscitation attempts in white patients but may in fact be a product of inclusion of patients for whom resuscitation was unlikely to be successful and thus may have benefited from more attention to pre-arrest goals of care. While VF/VT arrest outcomes may be improved by focusing on hospital characteristics, these are less likely to have an impact on PEA and asystole IHCA.
Supplementary Material
Acknowledgments
Dr. Edelson is supported by a career development award from the National Heart, Lung, and Blood Institute (K23 HL097157). In addition, she has received research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. Drs. Edelson and Churpek have a patent pending (ARCD. P0535US.P2). Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080).
Footnotes
Previously presented (poster) at the American Heart Association annual meeting in November 2012.
Conflict of Interest Statement
All other authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ebell MH, Smith M, Kruse JA, Drader-Wilcox J, Novak J. Effect of race on survival following in-hospital cardiopulmonary resuscitation. J Fam Pract. 1995;40(6):571–577. [PubMed] [Google Scholar]
- 2.Galea S, Blaney S, Nandi A, et al. Explaining racial disparities in incidence of and survival from out-of-hospital cardiac arrest. Am J Epidemiol. 2007;166(5):534–543. doi: 10.1093/aje/kwm102. [DOI] [PubMed] [Google Scholar]
- 3.Becker LB, Han BH, Meyer PM, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329(9):600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 4.Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: racial differences in outcome in Seattle. Am J Public Health. 1993;83(7):955–959. doi: 10.2105/ajph.83.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302(11):1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookoff D, Kellermann AL, Hackman BB, Somes G, Dobyns P. Do blacks get bystander cardiopulmonary resuscitation as often as whites? Ann Emerg Med. 1994;24(6):1147–1150. doi: 10.1016/s0196-0644(94)70246-2. [DOI] [PubMed] [Google Scholar]
- 7.Shah KS, Shah AS, Bhopal R. Systematic review and meta-analysis of out-of-hospital cardiac arrest and race or ethnicity: black US populations fare worse. Eur J Prev Cardiol. 2014;21(5):619–38. doi: 10.1177/2047487312451815. [DOI] [PubMed] [Google Scholar]
- 8.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 9.Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38(1):101–108. doi: 10.1097/CCM.0b013e3181b43282. [DOI] [PubMed] [Google Scholar]
- 10.Tortolani AJ, Risucci DA, Rosati RJ, Dixon R. In-hospital cardiopulmonary resuscitation: patient, arrest and resuscitation factors associated with survival. Resuscitation. 1990;20(2):115–128. doi: 10.1016/0300-9572(90)90047-i. [DOI] [PubMed] [Google Scholar]
- 11.Skogvoll E, Isern E, Sangolt GK, Gisvold SE. In-hospital cardiopulmonary resuscitation. 5 years’ incidence and survival according to the Utstein template. Acta Anaesthesiol Scand. 1999;43(2):177–184. doi: 10.1034/j.1399-6576.1999.430210.x. [DOI] [PubMed] [Google Scholar]
- 12.Parish DC, Dane FC, Montgomery M, Wynn LJ, Durham MD. Resuscitation in the hospital: differential relationships between age and survival across rhythms. Crit Care Med. 1999;27(10):2137–2141. doi: 10.1097/00003246-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Link MS, Atkins DL, Passman RS, et al. Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S706–719. doi: 10.1161/CIRCULATIONAHA.110.970954. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110(21):3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 15.Allison P. Fixed effects regression models. SAGE Publications, Inc; 2009. [Google Scholar]
- 16.Rastogi S, Johnson TD, Hoeffel EM, MPD . The Black Population: 2010. Washington DC: US Dept of Commerce; 2011. United States Census 2010. [Google Scholar]
- 17.Mitchell SL, Teno JM, Roy J, Kabumoto G, Mor V. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. Jama. 2003;290(1):73–80. doi: 10.1001/jama.290.1.73. [DOI] [PubMed] [Google Scholar]
- 18.Barnato AE, Chang CC, Saynina O, Garber AM. Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med. 2007;22(3):338–345. doi: 10.1007/s11606-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169(5):493–501. doi: 10.1001/archinternmed.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gramelspacher GP, Zhou XH, Hanna MP, Tierney WM. Preferences of physicians and their patients for end-of-life care. J Gen Intern Med. 1997;12(6):346–351. doi: 10.1046/j.1525-1497.1997.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodlin SJ, Zhong Z, Lynn J, et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333–2339. doi: 10.1001/jama.282.24.2333. [DOI] [PubMed] [Google Scholar]
- 22.Borum ML, Lynn J, Zhong Z. The effects of patient race on outcomes in seriously ill patients in SUPPORT: an overview of economic impact, medical intervention, and end-of-life decisions. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(5 Suppl):S194–198. doi: 10.1111/j.1532-5415.2000.tb03132.x. [DOI] [PubMed] [Google Scholar]
- 23.Wenger NS, Pearson ML, Desmond KA, et al. Epidemiology of do-not-resuscitate orders. Disparity by age, diagnosis, gender, race, and functional impairment. Arch Intern Med. 1995;155(19):2056–2062. [PubMed] [Google Scholar]
- 24.Zheng NT, Mukamel DB, Caprio T, Cai S, Temkin-Greener H. Racial disparities in inhospital death and hospice use among nursing home residents at the end of life. Med Care. 2011;49(11):992–998. doi: 10.1097/MLR.0b013e318236384e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepardson LB, Gordon HS, Ibrahim SA, Harper DL, Rosenthal GE. Racial variation in the use of do-not-resuscitate orders. J Gen Intern Med. 1999;14(1):15–20. doi: 10.1046/j.1525-1497.1999.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard RS, Fisher ES, Teno JM, et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc. 1998;46(10):1242–1250. doi: 10.1111/j.1532-5415.1998.tb04540.x. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention, National Center for Health Statistics. . [Jun 28 2012 1 34 :21 AM];Underlying Cause of Death 1999–2009 on CDC WONDER Online Database, released 2012. Data for year 2009 are compiled from the Multiple Cause of Death File 2009, Series 20 No. 2O, 2012, Data for year 2008 are compiled from the Multiple Cause of Death File 2008, Series 20 No. 2N, 2011, data for year 2007 are compiled from Multiple Cause of Death File 2007, Series 20 No. 2M, 2010, data for years 2005–2006 data are compiled from Multiple Cause of Death File 2005–2006, Series 20, No. 2L, 2009, and data for years 1999–2004 are compiled from the Multiple Cause of Death File 1999–2004, Series 20, No. 2J, 2007. Accessed at http://wonder.cdc.gov/ucd-icd10.html on.
- 28.Johnson KS, Kuchibhatla M, Tulsky JA. What explains racial differences in the use of advance directives and attitudes toward hospice care? J Am Geriatr Soc. 2008;56(10):1953–1958. doi: 10.1111/j.1532-5415.2008.01919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mack JW, Paulk ME, Viswanath K, Prigerson HG. Racial disparities in the outcomes of communication on medical care received near death. Arch Intern Med. 2010;170(17):1533–1540. doi: 10.1001/archinternmed.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy ST, Palmer JM, Azen S, Frank G, Michel V, Blackhall LJ. Ethnicity and advance care directives. J Law Med Ethics. 1996;24(2):108–117. doi: 10.1111/j.1748-720x.1996.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 31.Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282(6):583–589. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- 32.Zingmond DS, Wenger NS. Regional and institutional variation in the initiation of early do-not-resuscitate orders. Arch Intern Med. 2005;165(15):1705–1712. doi: 10.1001/archinte.165.15.1705. [DOI] [PubMed] [Google Scholar]
- 33.James GD, Baker P, Badrick E, Mathur R, Hull S, Robson J. Ethnic and social disparity in glycaemic control in type 2 diabetes; cohort study in general practice 2004–9. J R Soc Med. 2012;105(7):300–8. doi: 10.1258/jrsm.2012.110289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 35.Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 37.Kiberd BA, Clase CM. Cumulative risk for developing end-stage renal disease in the US population. J Am Soc Nephrol. 2002;13(6):1635–1644. doi: 10.1097/01.asn.0000014251.87778.01. [DOI] [PubMed] [Google Scholar]
- 38.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee NA, He Y, Keating NL. Racial Differences in Breast Cancer Stage at Diagnosis in the Mammography Era. Am J Public Health. 2013;103(1):170–6. doi: 10.2105/AJPH.2011.300550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


