Abstract
Background
Minimally invasive lung lobectomy and segmentectomy (video-assisted thoracic surgery; VATS) are assumed to result in better quality of life (QOL) and less postoperative pain, compared with standard, open approaches. To date, few prospective studies have compared the two approaches. We performed a prospective cohort study to compare QOL and pain scores during the first 12 months after VATS or open anatomic resection.
Methods
Patients were prospectively enrolled from May 2009 to April 2012. Patients with clinical stage I lung cancer who were scheduled to undergo anatomic lung resection were eligible. The brief pain index (BPI) and SF-36 Health Survey were conducted perioperatively and at four time points during the first 12 months after surgery. Intent-to-treat analyses using mixed-effects models were used to longitudinally assess the effect of treatment on QOL components (physical component summary [PCS] and mental component summary [MCS]) and pain.
Results
In total, 74 patients underwent thoracotomy, and 132 underwent VATS (including 19 patients who were converted to thoracotomy); 40 and 80 patients, respectively, completed the 12-month surveys. Baseline characteristics were similar between the two groups. PCS and BPI scores were similar between the two groups throughout the 12 months of follow-up. MCS, however, was consistently worse in the VATS group.
Conclusions
Patient-reported PCS and pain scores after VATS and thoracotomy were similar during the first 12 months after surgery.
Keywords: VATS, Thoracotomy, Pain, QOL
Introduction
Video-assisted thoracic surgery (VATS) for anatomic resection of lung cancer has gained wide acceptance in the thoracic surgery community. After some initial concerns regarding the oncologic soundness of the approach, compared with open thoracotomy—concerns that have since been mostly dispelled [1-4]—the technique has been enthusiastically adopted, owing to the perceived advantages of minimally invasive approaches. Indeed, studies (primarily retrospective ones) suggest that there are certain physiologic advantages to the VATS approach, including less postoperative pain [5, 6], shorter hospitalization [7], better tolerability in older patients [8, 9], and even lower costs [10, 11]. A significant limitation of most of these studies, however, is their retrospective design, which can introduce significant recollection bias [12]. Furthermore, many of these studies are not analyzed as intent-to-treat, and conversions from VATS to thoracotomy are often inappropriately included in the thoracotomy arm. Last, and perhaps most important, the focus of many studies assessing the benefits of VATS has been “objective” measures of quality of life (QOL) after surgery, with the implication that these serve as a more accurate reflection of the impact of the operation on patients [13]. However, with the mandate for patient-reported measures as a component of quality care, and with future reimbursement of care linked to quality, patient-reported measures of pain and QOL, once marginalized, have gained significantly in importance. We performed a prospective cohort study to compare QOL and pain scores during the first 12 months after VATS or open anatomic resection for early-stage non-small cell lung cancer (NSCLC).
Material and Methods
Eligibility
The study was approved by the institutional review board at Memorial Sloan-Kettering Cancer Center, and all patients gave consent for participation in the study. Patients were eligiblefor this study if they had histologically confirmed or suspected clinical stage I [14] NSCLC (as determined by positron emission tomography, computed tomography, and nonroutine invasive mediastinal and/or hilar staging) and if they were deemed to be medically fit for anatomic lung resection (segmentectomy or lobectomy). Patients were excluded if they had undergone a previous lung resection or had received preoperative chemotherapy and/or radiation. Patients who either had more-advanced-stage disease or did not have NSCLC following surgery were removed from the study. Patients were invited to participate in the study after consenting to their surgical procedure. The choice of surgical approach (thoracotomy vs. VATS, lobectomy vs. segmentectomy) was based on surgeon preference (thoracotomy is the standard preference for three surgeons in the group; VATS, for five). Thoracotomy was defined as a procedure that included any rib spreading, including standard posterolateral thoracotomy and muscle-sparing axillary thoracotomy. VATS was performed via three incisions, with the largest (utility) incision approximately 4 cm in size. Some VATS cases included robotic assistance with the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA). Operations were considered conversions if the initial attempt at resection was performed using VATS but was aborted due to technical difficulties, such as bleeding or adhesions; if the VATS procedure was primarily used for staging, with no true intent to resect, the operation was considered a thoracotomy. Because more surgeons in our group typically perform VATS resections in this cohort of patients, rather than thoracotomies, our accrual target was 80 VATS cases and 40 thoracotomy cases who completed the 12 month follow-up evaluations.
Demographic Data Collected
We prospectively collected data from all patients, including demographic characteristics, comorbidities, lung function test results, pathologic stage, tumor type, any postoperative morbidity, hospital length of stay, and disease status during the 12 months of follow-up.
QOL Measures
We obtained data on health-related QOL using the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36). The SF-36 taps eight health domains: physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. Each of the eight domains is scored on a Likert-like scale (1 to 5), with higher scores indicating better QOL. Factor analyses of the SF-36 provide strong support for a two-factor model of health, with one factor encompassing aspects of physical health and the second factor encompassing aspects of emotional health. Physical component summary (PCS) and mental component summary (MCS) scales from the SF-36 have been standardized to national norms [15]. Reliability estimates for the PCS and MCS exceeded 0.90 [16], and internal consistency reliability estimates for all scales were ≥0.78. Following registration and enrollment, patients filled out the SF-36 survey preoperatively, at the first postoperative visit (approximately two weeks after discharge), and at the 4-month, 8-month, and 12-month postoperative visits. If patients developed a documented recurrence during follow-up, no additional QOL surveys were collected.
Pain Measures
Pain was measured using the brief pain inventory (BPI). The BPI is a pain assessment tool used to measure both pain intensity and pain interference in cancer patients [17, 18]. Patients rate the severity of their pain at its worst and least during the previous week, on average, and “right now” [18]. Patients rate their level of pain interference in seven contexts: (1) work, (2) activity, (3) mood, (4) enjoyment, (5) sleep, (6) walking, and (7) relationships [19]. The BPI also assesses the patient's pain intervention, pain quality, and perception of the cause of pain. Other BPI items include (1) a shade-in of the patient's area of pain on a front and back view of a human figure; (2) rating the amount of relief the patient feels that the current pain treatments provide; (3) rating the duration of the patient's pain relief after taking prescribed pain medications; and (4) assessing the patient's attribution of pain to the disease, the treatment of the disease, or conditions unrelated to the disease. There is no scoring algorithm for the scale, but “worst pain” or the arithmetic mean of the four severity items can be used as measures of pain severity, and the arithmetic mean of the seven interference items can be used as a measure of pain interference [18]. Following registration and enrollment, patients filled out the BPI preoperatively, on postoperative days 2 to 4 (while still hospitalized), at the initial postoperative visit (approximately two weeks after discharge), and at the 4-month, 8-month, and 12-month postoperative visits. If patients developed a documented recurrence during follow-up, no additional BPI surveys were collected.
Pain Management
All patients received an epidural catheter before their operation. Most commonly, the epidural infusion was begun in the operating room, using bupivacaine only, followed postoperatively by an infusion of hydromorphone (8 mc/cc) and bupivacaine (0.05%) at 6 cc/h, with a 6-cc bolus. Additionally, Toradol was used when not contraindicated by renal function. The epidural catheters were managed by the Anesthesia Pain Service, which is present in the hospital 24 hours a day. After the epidural was removed, oral narcotics were prescribed as needed.
Statistical Methods
We compared demographic characteristics between the VATS and thoracotomy cohorts using χ2 statistics for categorical variables and the Wilcoxon rank sum test for continuous variables. QOL analysis investigated two endpoints: physical component summary (PCS) and mental component summary (MCS). Each of these endpoints was analyzed in a longitudinal fashion using linear mixed-effects models, which included all patients who had the baseline assessment and at least one postbaseline (outpatient) assessment. All models included a time effect and adjusted for baseline QOL, as well as for demographic and disease characteristics that were differently distributed in the two cohorts. In addition, an interaction between treatment group and time was included to allow for the possibility that changes in QOL over time are different between the VATS group and the thoracotomy group. On the basis of these models, we calculated adjusted PCS and MCS scores at each time point and compared them using contrasts.
Pain analysis investigated two endpoints: average pain, defined as the mean across the four reported pain scores (worst, least, average, and right now), and average interference, defined as the mean across the seven scores examining interference (general activity, mood, walking ability, normal work, relations, sleep, and life enjoyment). For each endpoint, the inpatient pain scores were log-transformed and modeled using linear mixed-effects models. Outpatient pain scores were categorized as clinically significant (score of 4 or higher [20, 21]) or not (score<4) and analyzed using nonlinear mixed effect models, with the same approach as described above for QOL analyses.
To account for the possibility that missing reports of QOL or pain are related to the actual outcomes (informative missingness), we used a pattern-mixture models approach and stratified all models by the observed pattern of missing data [22].
Statistical analysis was conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina). All significance tests were 2-sided and used a 5% level of significance.
Results
Patients were prospectively enrolled from May 2009 to April 2012. In total, 74 patients underwent thoracotomy (72 of whom completed the baseline surveys), and 132 underwent VATS (19 patients were converted from VATS to thoracotomy and were analyzed in the VATS arm); 40 and 80 patients, respectively, were followed up for 12 months and completed the final survey, representing 59% of all patients who consented to participate in the study (55% in the thoracotomy group versus 61% in the VATS group; p=0.58). The study was closed when the target sample size based on a 12-month follow-up was reached; 45 patients (22%) had less than 12 months of data collected because of study completion. The remaining cases of incomplete data were attributable to dropout (n=19; 9%) or death or disease recurrence (n=18; 9%); additionally, 4 patients received postoperative chemotherapy and were therefore removed from the study (Table 1). Baseline characteristics were similar between the two groups. There was 1 30-day death in the VATS arm and none in the thoracotomy arm; length of stay was similar between the groups. Postoperative complications are listed in Table 2.
Table 1.
Available Surveys at Each Time Point
| Parameter | Thoracotomy Patients, no. (n=74) | VATS Patients, no. (n=132) |
|---|---|---|
| Survey time point | ||
| In-hospital | 72 | 132 |
| Postoperative | 71 | 128 |
| 4 months | 61 | 116 |
| 8 months | 51 | 95 |
| 12 months | 40 | 80 |
| Reason why data <12 months | ||
| Withdrew/lost to follow-up | 5 | 14 |
| Death/recurrence/new cancer | 6 | 12 |
| Study completed and surveys stopped | 22 | 23 |
| Chemotherapy | 1 | 3 |
Table 2.
Demographic Characteristics
| Characteristic | Thoracotomy (N=74) | VATS (N=132) | p Value |
|---|---|---|---|
| Age (median, range) | 66 (22-88) | 69 (28-85) | 0.38 |
| Sex (female) | 37 (50) | 84 (64) | 0.06 |
| Cigarette smoking history | 0.47 | ||
| Current | 6 (8) | 15(11) | |
| Former | 58 (78) | 93 (70) | |
| Never | 10 (14) | 24 (18) | |
| Pack years (median, range) | 30 (0-126) | 28.3 (0-132) | 0.35 |
| FEV1% (median, range) | 91 (42-146) | 93.5 (39-171) | 0.19 |
| DLCO% (median, range) | 86 (43-134) | 85 (52-163) | 0.96 |
| Clinical stage | 0.41 | ||
| Ia | 54 (73) | 103 (78) | |
| Ib | 20 (27) | 29 (22) | |
| Pulmonary comorbidities (none) | 52 (70) | 97 (73) | 0.51 |
| Cardiac comorbidities (none) | 31(42) | 55 (42) | 0.66 |
| Endocrine comorbidities (none) | 60 (81) | 10 (8) | 0.02 |
| Renal comorbidities (none) | 1 (1) | 4 (3) | 0.45 |
| Other cancers (yes) | 25 (35) | 43 (33) | 0.84 |
| Pathology | 0.02 | ||
| Adenocarcinoma | 56 (76) | 117 (89) | |
| Other | 18 (24) | 11 (8) | |
| Complications | |||
| Death (30 days) | 0 | 1 | |
| Prolonged air leak (>7 days) | 2 | 8 | |
| Atrial fibrillation | 6 | 10 | |
| Urinary tract infection | 2 | 5 | |
| Length of stay, days, median (range) | 4 (2-14) | 4(1-25) |
Data are no. (%), unless otherwise noted.
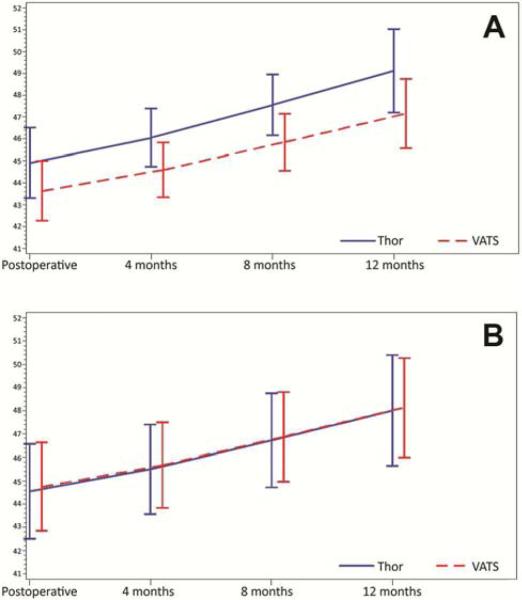
Adjusted PCS scores were similar between the groups throughout the course of follow-up, whereas adjusted MCS scores were consistently better in the thoracotomy group (Figure 1 and Table 3a). For each pain outcome investigated, adjusted pain scores were likewise similar between groups during the postoperative period (days 2-4; Figure 2). There was no difference between groups with respect to the risk of having clinically significant pain at each of the outpatient evaluations (months 4, 8, and 12 postsurgery) (Figure 3 and Table 3b).
Figure 1.
Results of the Medical Outcomes Study 36-item Short-Form Health Survey. Adjusted physical component summary (PCS) scores were similar between groups (A), whereas adjusted mental component summary (MCS) scores were consistently better in the thoracotomy group (B).
Table 3.
a. Adjusted Quality of Life Scores
| Time point | MCS | PCS | ||||
|---|---|---|---|---|---|---|
| VATS | Thoracotomy | p Value | VATS | Thoracotomy | p Value | |
| Baseline | 42.4 | 43.5 | 48.9 | 50.3 | ||
| Postoperative | 43.6 | 44.9 | 0.149 | 45.7 | 44.5 | 0.85 |
| 4 months | 44.6 | 46.1 | 0.036 | 45.7 | 45.5 | 0.86 |
| 8 months | 45.8 | 47.6 | 0.024 | 46.9 | 46.7 | 0.89 |
| 12 months | 47.2 | 49.1 | 0.08 | 48.1 | 48 | 0.93 |
Figure 2.
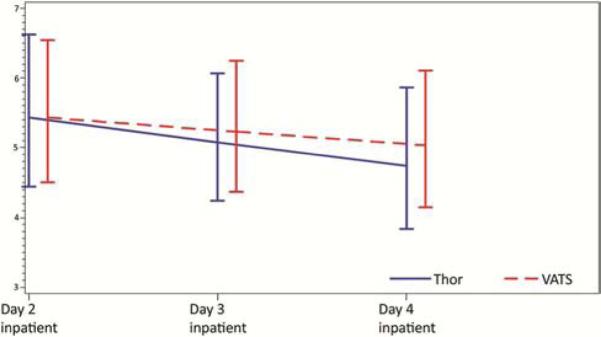
Results of the brief pain index on postoperative days 2-4. Results were reported as pain >4 (on a scale of 1-7); there was no difference in these scores between groups during follow-up.
Figure 3.
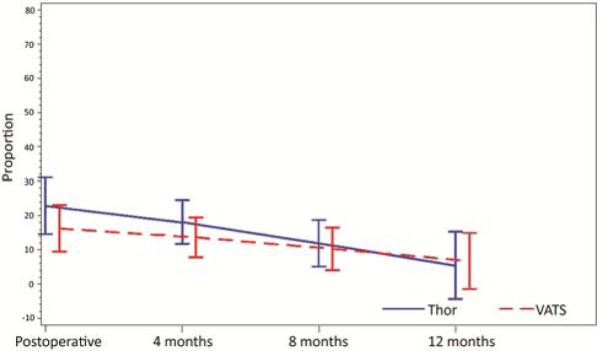
Results of the brief pain index. Results were reported as pain >4 (on a scale of 1-7); there was no difference in these scores between groups during follow-up.
Table 3.
b Adjusted Pain >4
| Time point | VATS | Thoracotomy | p Value |
|---|---|---|---|
| Baseline | 14% | 11% | |
| Postoperative | 16% | 23% | 0.18 |
| 4 months | 14% | 18% | 0.24 |
| 8 months | 10% | 12% | 0.69 |
| 12 months | 7% | 6% | 0.84 |
Comment
The results from this prospective study indicate that, in terms of pain and QOL, there is no quantifiable difference between VATS and thoracotomy for the treatment of stage I NSCLC, with the exception of SF-36 MHS scores, which were consistently higher (ie, better) in the thoracotomy group. In both groups, QOL scores improved throughout the 12 months of follow-up, and pain scores approached baseline levels by 4 months. A similar number of patients between groups reported no longer using narcotics at the first postoperative visit (13% for VATS vs. 6% for thoracotomy; data not shown), and, likewise, by 12 months, there was a similar incidence of reported residual pain at the surgical site (28% for VATS vs. 37% for thoracotomy).
A possible explanation for the similarity in pain scores between the groups is that the pain control provided by the epidural catheter, with the addition of Toradol and the presence of a full-time pain service, was sufficient to mask any potential differences between surgical approaches. Although we did not capture any data between the time of discharge and the first follow-up visit—and, therefore, may have missed differences in pain scores during this period—it is more likely that the two groups achieved similar pain control with narcotics, given the similar narcotic usage between the two groups. A second possible explanation (not supported by our data) is that, owing to the intent-to-treat analysis, patients who were converted to open approaches (14%) had a significant impact on the perceived benefits of VATS; however, when we looked at this group separately, there were no significant differences between the groups (data not shown). Last, it is unclear why the MCS scores were consistently worse in the VATS arm. It is difficult to account for the subjectivity of the surveys, and it is perhaps the case that patient expectations played a large role in the responses to this question—that is, their responses may indicate that they experienced more or less pain than they had expected. Furthermore, although the differences in the MCS scores were statistically significant, this difference is typically not considered clinically meaningful [18]. On the other hand, PCS scores were consistently similar during the study period.
There are several possible limitations of this study. The most obvious is that the patients could not be randomized between the two surgical arms. Given the lack of equipoise among surgeons, a randomized trial is not feasible in this setting. As the patients at our institution are essentially randomized to the surgeon they see, and as the surgical approach performed reflects the standard practice of each surgeon and is generally not influenced by patients’ prognosis, this potential source of selection bias should have been significantly mitigated. Moreover, one could argue that randomizing patients to surgical procedures against their own preferences or biases could influence their responses to subjective surveys. A second possible limitation is that only 59% of patients completed all surveys. In fact, this is a common problem in QOL studies, and it can introduce significant bias, depending on the reason why patients did not complete all surveys. The analysis method that we used (mixture patterns for longitudinal data) is designed to account for this potentially informative dropout. However, if some of the patients dropped out for reasons related to their pain or QOL, it is possible that this may have influenced the results. In this study, the most common reason for incomplete follow-up was administrative and was unrelated to patients’ outcomes: the study was stopped when the planned number of patients completed 12 months of follow-up (22% of consented patients had incomplete data for this reason). In addition, our pattern-mixture models analysis accounts for potential informative missingness associated with 9.3% of the consented subjects who dropped out during the follow-up period for unexplained reasons [23]. Last, we analyzed the data according to an intent-to-treat analysis, therefore including in the VATS group the 19 patients who were intraoperatively converted to thoracotomy. Although it could be argued that their subsequent QOL and pain outcomes are attributable to the more extensive procedure, we feel strongly that the true representation of an operation must include all possible outcomes, including unforeseen complications, which may lead to a change of operation.
In accordance with the underlying belief that VATS is better tolerated by patients, our expectation in designing this trial was that the VATS group would be associated with better QOL and pain scores. This belief is supported by retrospective studies, including one from our institution [9], which suggest that VATS procedures are better tolerated than thoracotomies. Yet, although this belief in VATS may seem intuitive, when these assumptions are tested prospectively, they are frequently shown to be erroneous—as was demonstrated definitively in comparisons between laparoscopic and open colectomies [24]. Ultimately, these QOL and pain surveys reflect patient-reported outcomes, and despite our own prejudices, the choice of operative approach needs to reflect that reality.
Acknowledgments
Financial Support: NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 50th Annual Meeting of the Society of Thoracic Surgeons, Orlando, FL, January 25–29, 2014.
References
- 1.Thomas P, Doddoli C, Yena S, et al. VATS is an adequate oncological operation for stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2002;21:1094–9. doi: 10.1016/s1010-7940(02)00179-3. [DOI] [PubMed] [Google Scholar]
- 2.McKenna RJ, Jr, Wolf RK, Brenner M, Fischel RJ, Wurnig P. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg. 1998;66:1903–8. doi: 10.1016/s0003-4975(98)01166-7. [DOI] [PubMed] [Google Scholar]
- 3.Kaseda S, Hangai N, Yamamoto S, Kitano M. Lobectomy with extended lymph node dissection by video-assisted thoracic surgery for lung cancer. Surg Endosc. 1997;11:703–6. doi: 10.1007/s004649900431. [DOI] [PubMed] [Google Scholar]
- 4.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg. 2000;24:27–30. doi: 10.1007/s002689910006. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 5.Tschernko EM, Hofer S, Bieglmayer C, Wisser W, Haider W. Early postoperative stress: video-assisted wedge resection/lobectomy vs conventional axillary thoracotomy. Chest. 1996;109:1636–42. doi: 10.1378/chest.109.6.1636. [DOI] [PubMed] [Google Scholar]
- 6.Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285–9. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 7.Atkins BZ, Harpole DH, Jr, Mangum JH, Toloza EM, D'Amico TA, Burfeind WR., Jr Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg. 2007;84:1107–12. doi: 10.1016/j.athoracsur.2007.05.013. discussion 1112–3. [DOI] [PubMed] [Google Scholar]
- 8.Demmy TL, Plante AJ, Nwogu CE, Takita H, Anderson TM. Discharge independence with minimally invasive lobectomy. Am J Surg. 2004;188:698–702. doi: 10.1016/j.amjsurg.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–5. doi: 10.1016/j.athoracsur.2007.07.080. discussion 235–6. [DOI] [PubMed] [Google Scholar]
- 10.Ramos R, Masuet C, Gossot D. Lobectomy for early-stage lung carcinoma: a cost analysis of full thoracoscopy versus posterolateral thoracotomy. Surg Endosc. 2012;26:431–7. doi: 10.1007/s00464-011-1891-y. [DOI] [PubMed] [Google Scholar]
- 11.Burfeind WR, Jr, Jaik NP, Villamizar N, Toloza EM, Harpole DH, Jr, D'Amico TA. A cost-minimisation analysis of lobectomy: thoracoscopic versus posterolateral thoracotomy. Eur J Cardiothorac Surg. 2010;37:827–32. doi: 10.1016/j.ejcts.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Handy JR, Jr, Asaph JW, Douville EC, Ott GY, Grunkemeier GL, Wu Y. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg. 2010;37:451–5. doi: 10.1016/j.ejcts.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy—video-assisted thoracic surgery versus muscle-sparing thoracotomy: a randomized trial. J Thorac Cardiovasc Surg. 1995;109:997–1001. doi: 10.1016/S0022-5223(95)70326-8. discussion 1001–2. [DOI] [PubMed] [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M, editors. AJCC Cancer Staging Manual. 6th edition Springer-Verlag; 2002. [Google Scholar]
- 15.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264–79. [PubMed] [Google Scholar]
- 16.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 17.Fillenbaum GG. Screening the elderly. A brief instrumental activities of daily living measure. J Am Geriatr Soc. 1985;33:698–706. doi: 10.1111/j.1532-5415.1985.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 18.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–44. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 20.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–84. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 21.Palos GR, Mendoza TR, Mobley GM, Cantor SB, Cleeland CS. Asking the community about cutpoints used to describe mild, moderate, and severe pain. Pain. 2006;7:49–56. doi: 10.1016/j.jpain.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- 23.Little RJA. Pattern-mixture models for multivariate incomplete data. JAMA. 1993;88:125–34. [PubMed] [Google Scholar]
- 24.Dowson HM, Cowie AS, Ballard K, Gage H, Rockall TA. Systematic review of quality of life following laparoscopic and open colorectal surgery. Colorectal Dis. 2008;10:757–68. doi: 10.1111/j.1463-1318.2008.01603.x. [DOI] [PubMed] [Google Scholar]