Abstract
We report ophthalmic and genetic findings in families with autosomal recessive rod-cone dystrophy (arRCD) and RP1 mutations. Detailed ophthalmic examination was performed in 242 sporadic and arRCD subjects. Genomic DNA was investigated using our customized next generation sequencing panel targeting up to 123 genes implicated in inherited retinal disorders. Stringent filtering coupled with Sanger sequencing and followed by cosegregation analysis was performed to confirm biallelism and the implication of the most likely disease causing variants. Sequencing identified 9 RP1 mutations in 7 index cases. Eight of the mutations were novel, and all cosegregated with severe arRCD phenotype, found associated with additional macular changes. Among the identified mutations, 4 belong to a region, previously associated with arRCD, and 5 others in a region previously associated with adRCD. Our prevalence studies showed that RP1 mutations account for up to 2.5% of arRCD. These results point out for the necessity of sequencing RP1 when genetically investigating sporadic and arRCD. It further highlights the interest of unbiased sequencing technique, which allows investigating the implication of the same gene in different modes of inheritance. Finally, it reports that different regions of RP1 can also lead to arRCD.
1. Introduction
Rod-cone dystrophy (RCD), also known as retinitis pigmentosa, is a heterogeneous group of inherited disorders affecting primary rod photoreceptors in the majority of cases with secondary cone degeneration [1, 2]. Population-based studies showed that 1 in 4,000 individuals is affected around the world [1]. Patients diagnosed with RCD initially complain of night blindness due to rod dysfunction followed by progressive visual field constriction, abnormal color vision, and eventually loss of central vision due to cone photoreceptor involvement [1].
RCD is inherited as a Mendelian trait in most cases [3]. On the basis of its mode of inheritance and prevalence, RCD can be divided into 3 groups: autosomal dominant (ad) (30–40%), autosomal recessive (ar) (50–60%), and X-linked (xl) (5–15%) [3]. To date, mutations in at least 53 genes were reported to cause nonsyndromic RCD (till 25 June 2014, https://sph.uth.edu/retnet/). Prevalence studies revealed rhodopsin (RHO), retinitis pigmentosa GTPase regulator (RPGR), and usherin (USH2A) as being the most frequently mutated genes in adRCD [4, 5], xlRCD [4], and arRCD, respectively [6]. Of note is that many other genes with lower prevalence are also implicated in the genetic etiology of RCD [7, 8]. Mutations in RP1 were first shown to cause adRCD [9–11]; however, since 2005, articles have shed light on its implication in arRCD etiology [12–20]. RP1 mutations were shown to account for ≈5.5% and ≈1% of adRCD and arRCD cases, respectively [8–20]. Interestingly, Avila-Fernandez et al. [12] reported that a founder nonsense mutation in the Spanish population p.Ser542* is responsible for 4.5% of arRCD cases suggesting that RP1 mutations are more prevalent in arRCD than previously thought [12].
Retinitis pigmentosa 1 (RP1) is a photoreceptor-specific gene encoding a protein regulated by oxygen [10]. RP1 protein is required for correct orientation and higher-order stacking of outer segment disks [21] and was shown to be part of the photoreceptor axoneme [22]. RP1 localizes to the connecting cilia of photoreceptors and may assist in maintenance of ciliary structure or transport down the photoreceptor [22]. Like many retinal degeneration genes, the mechanism by which mutations in RP1 lead to photoreceptor cell death is still unclear.
We developed an unbiased and time-efficient retinal gene next generation sequencing array (NGS), which was further revised and improved to target more than 120 genes implicated in inherited retinal diseases (IRDs) (list available upon request) [23]. Using this NGS panel, we screened a total of 242 subjects with sporadic and recessive RCD in order to detect disease causing mutations and to report the prevalence of pathogenic mutations in RP1 causing arRCD.
2. Methods
2.1. Ethics Statement and Clinical Diagnosis of Rod-Cone Dystrophy
The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the local Ethics Committee (CPP, Ile de France V). Informed written consent was obtained from each study participant. Index patients underwent full ophthalmic examination as previously described [23].
2.2. Targeted Next Generation Sequencing
A cohort of 242 subjects affected with sporadic and arRCD was investigated in the present study. Prior to NGS screening, molecular genetic analysis with microarray (Asper Ophthalmics, Tartu, Estonia), followed by direct Sanger sequencing of EYS and C2orf71 (major and minor genes implicated in RCD, newly discovered at the beginning of our study), was performed in 201 index subjects (82%) [2, 24]. As RPGR exon ORF15 (MIM 312610) is not targeted by existing NGS panels, we excluded mutations in this “hot spot” by Sanger sequencing. Although our NGS panel was selected from the SureSelect Human All Exon Kits Version 4 (Agilent, Massy, France), this design was improved after analyzing the first 83 subjects with sporadic and arRCD. More precisely, a total of ≈300 Kb regions were added in order to cover all the previously nontargeted regions. Thus, whereas the first design covered the exons and the flanking intronic regions of 120 genes implicated in IRDs, the second covered 123 genes in total. The eArray web-based probe design tool was used for this purpose (https://earray.chem.agilent.com/earray). All probes were designed and synthesized by Agilent Technologies (Santa Clara, CA, USA). Sequence capture, enrichment, and elution were performed according to Agilent's instructions. The complete details were described elsewhere [23].
2.3. Assembly and Variant Calling
Sequence reads were aligned to the reference human genome (UCSC hg19) using CASAVA1.7 software (Illumina) and the ELANDv2 alignment algorithm. Sequence variation annotation was performed using the IntegraGen in-house pipeline, which consisted of gene annotation (RefSeq), detection of known single nucleotide polymorphisms (dbSNP 135) followed by mutation characterization (missense, intronic, synonymous, nonsense, splice site, and insertions/deletions).
2.4. Quality Control and Coverage Assessment
The first NGS retinal panel harbored 120 IRDs genes, encompassing 321,240 kb length per sample. However, after improvement, the same panel contained ≈600 Kb and covered 123 IRD genes. The depth of coverage was calculated by counting the number of sequenced bases mapping to the target regions. Mean depth of coverage was calculated per base pair for all samples; however, only the results of subjects having RP1 mutations were shown.
2.5. Discrete Filtering of Annotated Variants
In order to identify disease causing mutations among nonpathogenic single nucleotide polymorphisms, we used a filtering approach against a set of polymorphisms that are available in the public databases: dbSNP 137, 1000 Genomes Project [25], HapMap [26], and Exome Variant Server [27] with removal of variants with a minor allele frequency (MAF) ≥ 0.005 in case of presumed autosomal recessive mode of inheritance.
2.6. Pathogenicity Assessment
We stratified candidate mutations based on their functional class by giving a priority to frameshifts, stop codons, and disruptions of canonical splice sites variants [28]. For missense changes, amino acid conservation across 46 different species was studied using the UCSC Genome Browser [29]. If no amino acid change was found, then the residue was considered as “highly conserved.” If a different change was seen in less than four species and not in the primates, then it was considered as “moderately conserved” and if a change was present in 5–7, it was considered as “marginally conserved”; otherwise, the amino acid residue was considered as “not conserved.” Pathogenic prediction was performed using two software programs: Polyphen2 [30] and SIFT [31], based on species/homologue conservation, putative structural domains, and 3D structures (if available). Analysis of potential splice site variant consequences when relevant was done using human splicing finder [32].
2.7. Known Genotype-Phenotype Correlations
The search for previous genotype-phenotype associations was done by searching numerous literature databases, including Online Mendelian Inheritance in Man (http://omim.org/), Human Gene Mutation Database [33], Leiden Open Variation Database [34], and RetNet (https://sph.uth.edu/retnet/).
2.8. Validation of the Identified Variants and Cosegregation Analyses
Sanger sequencing was performed to validate disease causing mutations in RP1. The respective primer information can be communicated on request. In addition, blood samples were collected from additional family members when possible and cosegregation analyses on extracted DNA were performed as previously described [35, 36].
3. Results
3.1. Clinical Data
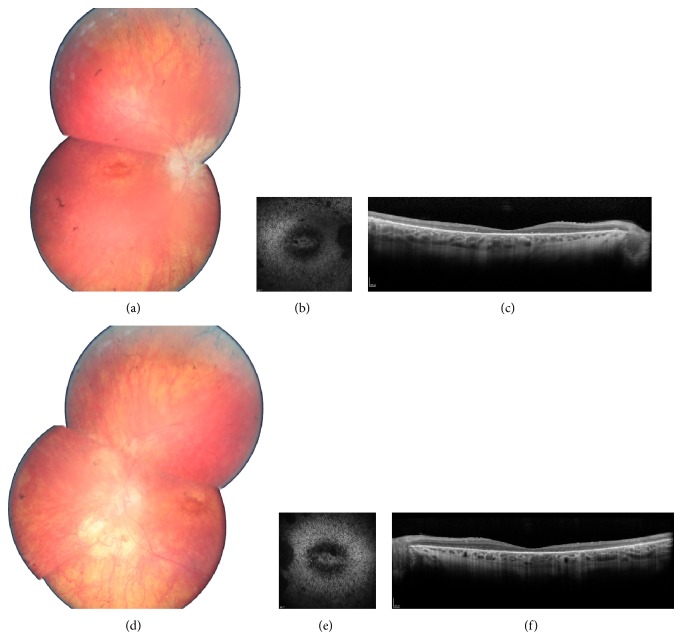
Clinical data are summarized in Table 1. Among identified patients, 5 were females, 2 were male, and ages at time of examination ranged from 25 to 42. All patients were diagnosed before age 20 mostly based on night blindness from early childhood and secondary central vision loss. They all showed severe RCD with constricted visual fields, no detectable responses on full field electroretinogram, and both peripheral involvement and macular involvement (Figure 1 presents fundus pictures of patient II.1 (CIC01245) in family F752 as an example). Comparing visual acuity and visual fields for these arRCD patients with those of adRCD cases published by Audo and coworkers [8], we noticed a more severe phenotype in recessive cases. However, more cases with RP1 mutations would be needed to draw statistical conclusion.
Table 1.
Clinical data of the 7 index patients with RP1 recessive mutations.
| Patient | Age at time of testing | Age of onset | Sex | Family history | Symptoms at time of diagnosis | BCVA OD/OS With refraction |
Color vision (15 desaturated Hue) |
Binocular kinetic visual field (III4e stimulus) |
FF and mfERG | Fundus examination | FAF | Sd-OCT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F303: II.1 (CIC00445) | 42 | 6 | F | No other affected FM, from France. | Night blindness | Hand motion in both eyes | Impossible due to low vision | Reduced to peripheral islands of perception | Both undetectable | Pale optic disc narrowed blood vessels, macular atrophic changes, and optic nerve drusen | Hypoautofluorescence in the macular region | Thinning of outer retina in the macular region |
|
| ||||||||||||
| F335: III.1 (CIC00491) | 36 | 3 | M | Two other brothers affected; parents first cousins | Night blindness and rapid decreased vision | LP in both eyes | Impossible due to low vision | Impossible due to low vision | Both undetectable | Widespread RPE changes and retinal atrophy in both the periphery and the macular area | Widespread loss of FAF | Widespread thinning of outer retina |
|
| ||||||||||||
| F674: III.6 (CIC01106) | 25 | 19 | F | Parents first cousins from Turkey, one female and male cousins affected also from a consanguineous union | Night blindness and decreased vision | HM −3 (−1) 0° 20/160 −3 (−0.50) 0° |
Dyschromatopsia with no specific axis | Reduced to 5 central degrees | Both undetectable | Well-colored optic disc and no narrowing of retinal vessels; RPE changes in the periphery and macular atrophic changes | Hypoautofluorescence in the macular region and outside the vascular arcades | Thinning of outer retina in the macular region |
|
| ||||||||||||
| F752: II.1 (CIC01245) | 31 | Early teens | F | Two sisters affected | Night blindness | 20/63 plano (−3) 180° 20/50 plano (−1.75) 180° |
Deutan defect on both eyes | Reduced to 10° × 20° | Both undetectable | Pale optic disc head, narrowed retinal vessels, and RPE changes in the periphery with some macular atrophic changes | Hypoautofluorescence in the macular region and outside the vascular arcades | Thinning of outer retina in the macular region |
|
| ||||||||||||
| F782 II.5 (CIC01300) | 27 | 9 | M | Parents from Algeria, first cousins | Night blindness and decreased vision | 20/50 −9.25 (−2.50) 15° 20/50 −9 (−1.75) 100° |
— | Reduced to the 10 central degrees | Both undetectable | Mild optic disc pallor, atrophic macular changes, and peripheral pigment deposits | Hypoautofluorescence in the macular region | Thinning of outer retina in the macular |
|
| ||||||||||||
| F1941: III.1 (CIC04130) | 30 | childhood | F | Parents from Algeria, first cousins | Night blindness | 20/100 −4.25 (−1.25) 150° 20/80 −4.25 (−1.25) 150° |
Normal at the saturated test | Reduced to the 10 central degrees | Both undetectable | Well-colored optic disc but narrowed retinal vessels; RPE changes in the periphery and macular atrophic changes | Hypoautofluorescence in the macular region and outside the vascular arcades | Thinning of outer retina in the macular region |
|
| ||||||||||||
| F3110: III.5 (CIC05941) | 27 | 5 | F | One cousin on mother side may have RCD | Night blindness and decreased vision | 20/125 +2 (−2) 95° 20/125 +1.75 (−2) 70°c |
Dyschromatopsia with no specific axis | Reduced to the 10 central degrees | Both undetectable | Pale optic disc, narrowed retinal vessels, and RPE changes in the periphery with some macular atrophic changes | Hypoautofluorescence in the macular region and outside the vascular arcades | Thinning of outer retina in the macular region |
F: female, FM: family member, M: male, BCVA: best corrected visual acuity; OD: ocula dextra (right eye); OS: ocula sinistra (left eye); FF and mfERG: full-field and multifocal ERG; FAF: fundus autofluorescence; Sd-OCT: spectral domain optical coherence tomography; RPE: retinal pigment epithelium; LP: light perception; HM: hand motion.
Figure 1.
Ophthalmic features of family F752: II.1 (CIC01245): fundus color photographs ((a) and (d) for right and left eye resp.), autofluorescence ((b) and (e) for right and left eye resp.), and spectral domain optical coherence tomography horizontal macula scans ((c) and (f) for right and left eye resp.), showing severe rod-cone dystrophy signs with macular involvement.
3.2. Sequencing Statistics
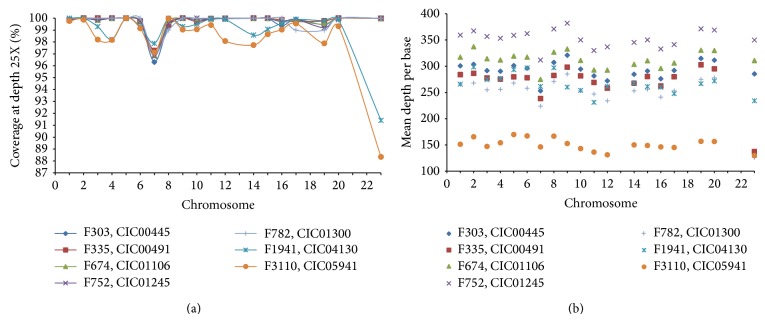
In index patients, the overall sequencing coverage of the target regions was ≥88% for a 25X depth of coverage in each of the chromosomes (Figure 2(a)), resulting a mean sequencing depth of 299 times per base. Mean sequencing results per base in each target chromosome gene regions were shown in Figure 2(b). It is of importance to mention that <1% of target regions were not covered at all. These were fragments of 120 bp belonging in 66% of the cases only to a fraction of an exon. The remaining uncovered targets corresponded each to an entire exon in genes such as CHM, PDZD7, RP9, CC2D2A, IMPDH1, CNGA1, and EYS.
Figure 2.
Sequencing statistics in index patients. (a) The overall sequencing coverage of the target regions at 25X depth of coverage is shown in each of the chromosomes. No values were indicated for chromosomes 13, 18, 21, and 22 as they were not targeted. The term chromosome 23 was used to designate the X chromosome. F1941: III.1 (CIC04130) and F3110: III.5 (CIC05941) showed the lowest coverage results. (b) The average mean depth per base pair is shown for each of the chromosomes. Most targets showed coverage around 300 times.
3.3. Detection of Disease Causing Mutations in RP1 Gene
After data filtering, the total number of putative disease causing variants was reduced by 99.3%. Thus, in total, filtering enriched the percentage of putative disease causing mutations from 0.7% (25/3339 variants) to 33.3% (9/25 variants) in the 7 subjects presented here (Table 2). These subjects exhibit RP1 mutations in the last exon 4 that are predicted to lead to a premature stop codon. We found 9 pathogenic mutations in RP1 among which one (p.Ser542* in CIC00445) was already reported by Avila-Fernandez et al. [12] as a founder nonsense mutation in the Spanish population, responsible for 4.5% of arRCD. Although F303 is from French origin, we cannot exclude the possibility of a founder effect of p.Ser542* in our cohort.
Table 2.
List of mutations detected by next generation sequencing after applying relevant filters.
| Patient | Gene | Exon | Allele state | Nucleotide exchange | Protein effect | rs ID | Conservation | Polyohen 2 | SIFT | Pathogenicity | Note |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F303: II.1 (CIC00445) |
NPHP4 | 12 | HTZ | A>G | p.Ser481Asn | no | N C | B | T | Neutral | |
| RP1 | 4 | HTZ | c.1625C>G | p.Ser542 * | — | — | — | — | Disease causing | R M [12] | |
| RP1 | 4 | HTZ | c.4587_4590delTAAG | p.Ser1529Argfs * 9 | — | — | — | — | Disease causing | N M | |
|
| |||||||||||
| F335: III.1 (CIC00491) |
PROM1 | 4 | HTZ | T>C | p.Ile178Val | — | N C | B | T | Neutral | |
| GPR98 | 29 | HTZ | G>A | p.Arg2128Gln | rs149390094 | N C | B | T | Neutral | ||
| RP1 | 4 | HMZ | c.4089_4092delAAGA | p.Arg1364Valfs * 8 | — | — | — | — | Disease causing | N M | |
|
| |||||||||||
| F674: III.6 (CIC01106) |
USH2A | 39 | HTZ | T>G | p.Ser2450Arg | No | H C | P D | D | Probably disease causing | |
| RP1 | 4 | HMZ | c.1205delG | p.Gly402Alafs * 7 | — | — | — | — | Disease causing | N M | |
|
| |||||||||||
| F752: II.1 (CIC01245) |
USH1C | 17 | HTZ | G>A | p.Arg477Trp | TMP_ESP_11_17532053 | H C | P D | D | Probably disease causing | |
| PDE6B | 10 | HTZ | T>C | p.Thr432Ile | — | H C | B | T | Uncertain pathogenicity | ||
| RP1 | 4 | HTZ | c.2025dupA | p.Ser676Ilefs * 22 | — | — | — | — | Disease causing | N M | |
| RP1 | 4 | HTZ | c.2377delA | p.Arg793Glufs * 55 | — | — | — | — | Disease causing | N M | |
|
| |||||||||||
| F782: II.5 (CIC01300) | RP1 | 4 | HMZ | c.1719_1723delCTCAA | p.Ser574Cysfs * 7 | — | — | — | — | Disease causing | N M |
| TULP1 | 5 | HMZ | c.395_418dup | p.Asp124_Glu131del | rs63749128 | — | — | — | Neutral | ||
|
| |||||||||||
| F1941: III.1 (CIC04130) | PCDH15 | 33 | HTZ | C>T | p.Arg1889His | rs145851144 | N C | B | T | Neutral | |
| C2orf71 | 1 | HTZ | C>A | p.Arg656Ser | rs201980758 | N C | B | T | Neutral | ||
| CACNA2D4 | Exon37-Intron 37 | HTZ | C>T | — | rs80092457 | N C | — | — | Neutral | ||
| RP1 | 4 | HMZ | c.1329delG | p.Lys443Asnfs * 12 | — | — | — | — | Disease causing | N M | |
|
| |||||||||||
| F3110: III.5 (CIC05941) |
EYS | 6 | HTZ | C>T | p.Ser326Asn | rs112822256 | N C | B | T | Neutral | |
| MERTK | 8 | HTZ | C>G | p.Arg421Trp | rs138908058 | N C | B | D | Neutral | ||
| PRPF6 | 21 | HTZ | A>G | p.Val915Met | rs139778757 | M C | P D | D | Uncertain pathogenicity | ||
| TULP1 | 14 | HTZ | G>A | p.Ala496Thr | rs141980901 | M C | B | D | Neutral | ||
| EYS | 26 | HTZ | G>A | p.Lys1365Glu | rs16895519 | N C | B | D | Neutral | ||
| MERTK | 18 | HTZ | G>C | p.Glu823Gln | rs55924349 | M C | B | D | Neutral | ||
| RP1 | 4 | HMZ | c.2391_2392delAA | p.Asp799 * | — | — | — | — | Disease causing | N M | |
Probably disease causing mutations are highlighted in bold.
B: benign, HMZ: homozygous, HTZ: heterozygous, M C: marginally conserved, N C: not conserved, N M: novel mutation, R M: recurrent mutation, T: tolerated, P.D: possibly damaging.
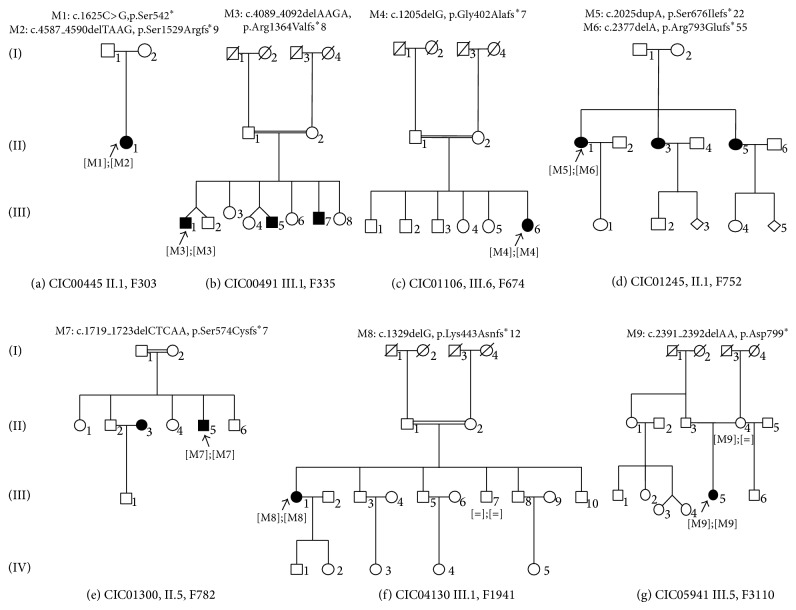
Patient family F303: II.1 (CIC00445) was found to carry compound heterozygous variants: a nonsense mutation c.1625C>G, leading to a predicted premature stop (p.Ser542*) and a deletion c.4587_4590delTAAG leading to a frameshift and a premature termination codon (p.Ser1529Argfs*9) (Table 2, Figure 3). Patient family F752: II.1 (CIC01245) was also found to carry compound heterozygous variants: a 1 bp duplication c.2025dupA leading to p.Ser676Ilefs*22 and a 1 bp deletion c.2377delA leading to p.Arg793Glufs*55 (Table 2).
Figure 3.
Pedigrees of seven families with RP1 mutations underlying autosomal recessive rod-cone dystrophy. Affected and unaffected individuals are represented by shapes filled with black and white colors, respectively. Men and women are indicated by squares and circles, respectively. Index subjects are marked by ↖. Consanguinity is marked by a double horizontal line.
Patients from family F335: III.1 (CIC00491), family F674: III.6 (CIC01106), family F782: II.5 (CIC01300), family F1941: III.1 (CIC04130), and family F3110: III.5 (CIC05941) were found to carry homozygous deletions c.4089_4092delAAGA leading to p.Arg1364Valfs*8; c.1205delG leading to p.Gly402Alafs*7; c.1719_1723delCTCAA leading to p.Ser574Cysfs*7; c.1329delG leading to p.Lys443Asnfs*12; and c.2391_2392delAA leading to p.Asp799*, resp.) (Table 2 and Figure 3). It is important to note that consanguinity was reported in families F335, F674, F782 and F1941.
All RP1 mutations detected by NGS were further validated by Sanger sequencing. All variants cosegregated with the phenotype in available family members. Based on the previous findings, the measured prevalence of RP1-associated arRCD in this cohort is ≈2.5%.
4. Discussion
The current study further demonstrates the usefulness of NGS as a comprehensive genetic diagnostic tool for IRDs with further impact on patients counseling and participation for potential therapeutic trials. Our study applied to a large cohort of sporadic and autosomal recessive cases of RCD identifies 8 novel mutations in a gene not classically screened in arRCD by other methods such as Sanger sequencing or microarray analysis, outlining the interest of this massive parallel sequencing method. Consequently, a prevalence of RP1 mutation in 2.5% of sporadic or arRCD cases in the European population is herein reported.
RP1 is a 15 kb single copy gene clustering the small arm of the chromosome 8 (8q12.1). It encodes a 2506 amino acid protein having a molecular weight of 241 kDa containing a Drosophila melanogaster bifocal (BIF) (amino acid 486–635) and two doublecortin domains. Whereas the BIF domain helps to maintain the photoreceptor morphogenesis, doublecortin domains bind microtubules and regulate their polymerization [22]. Along with RP1L1 (Retinitis Pigmentosa 1-like 1, another retinal-specific protein), RP1 plays essential and synergistic roles in outer segment morphogenesis of rod photoreceptors [22].
To date, at least 50 mutations in RP1 were identified in RCD [8, 12–20], the majority of which are located in its last exon (exon 4) and shown to be transmitted in an autosomal dominant mode of inheritance. Most of RP1 disease causing variants represent nonsense mutations, deletions, or insertions. In mammalian genes, nonsense mutations lead to unstable mRNAs that are degraded by nonsense-mediated decay (NMD). However, exceptions might arise when premature stop codons occur in the last exon [37]. These variants are thought to abolish RP1 function by resulting in a truncated protein lacking important functional domains although still able to interact with some of its protein partner(s) [21]. The latter observation is supported by finding that RP1 mutant mRNA is expressed in a human cell line carrying a homozygous p.Arg677* mutation [21].
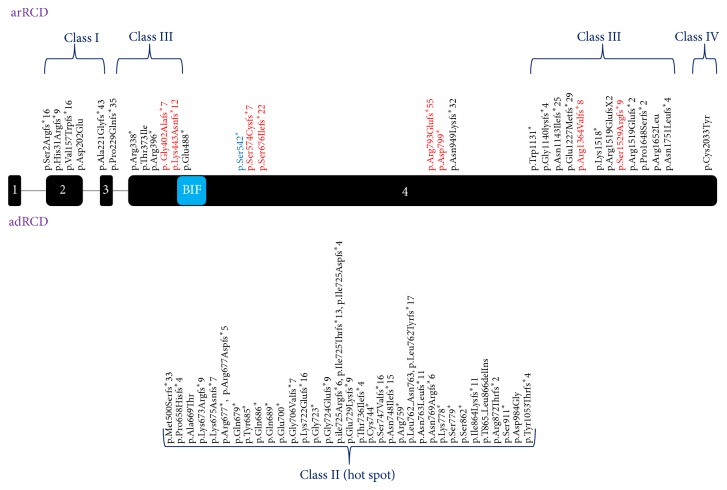
Based on Chen et al. [13], RP1 truncating mutations leading to arRCD or adRCD can be divided into four distinct groups. Class I is composed of truncating mutations located in exons 2 and 3. These variants are sensitive to NMD and thus are considered as true loss-of-function alleles (Figure 4) [13]. Class II involves truncating mutations that are located in a spot between codons 500 and 1053 in exon 4 [13], the so called “RP1 hot spot.” The “hot spot” variants tend to be insensitive to NMD process and thus result in a protein with a potential dominant negative effect leading to adRCD (Figure 4) [13]. Class III includes truncating mutations insensible to NMD located between codons 264 and 499 and between codons 1054 to 1751 in exon 4. These truncating proteins result in a loss of function leading to arRCD (Figure 4) [13]. Finally, class IV includes protein-truncating mutations near the 3′ end of the fourth exon (Figure 4) [13]. Most likely, the resulting proteins display only a minor loss of their C-terminal portion, preserving the majority of functional domains and keeping a residual activity. According to the classification of Chen et al. [13], p.Gly402Alafs*7, p.Lys443Asnfs*12, p.Arg1364Valfs*8, and p.Ser1529Argfs*9 belong to class III (Figure 4).
Figure 4.
Schematic presentation of RP1 disease causing mutations. Disease causing mutations were represented based on the classification by Chen and coworkers [13]. Mutations responsible for recessive arRCD were shown in the upper half, whereas mutations causing adRCD were shown in the lower half. p.Gly402Alafs*7, p.Lys443Asnfs*12, p.Arg1364Valfs*8, and p.Ser1529Argfs*9 belong to class III. Although p.Ser574Cysfs*7, p.Ser676Ilefs*22, p.Arg793Glufs*55, and p.Asp799* are class II mutations, these variants do not cause adRCD but arRCD instead. Amino acid modifications shown in red and blue represent novel frameshift or nonsense mutations and the recurrent p.Ser542* mutation respectively. Protein localization of p.Ser542* was highlighted in blue as it marked a recurrent mutation. adRCD: autosomal dominant: rod-cone dystrophy, arRCD: autosomal recessive rod-cone dystrophy, BIF: drosophila melanogaster bifocal.
The predicted physiopathology for p.Ser542*, p.Ser574Cysfs*7, p.Ser676Ilefs*22, p.Arg793Glufs*55, and p.Asp799* is more complex. According to Chen's classification, these frameshift deletions and nonsense mutations should belong to class II, previously only associated with adRCD. However, herein, they are causing presumably arRCD (Figure 4). To further confirm these findings, clinical and genetic testing of the reported unaffected parents should be done.
Based on the previous findings, we speculate that the classification by Chen and coworkers does not hold true for all mutations. Supporting this statement, Avila-Fernandez et al. [12] reported the same nonsense mutation (p.Ser542*) found in (F303: II.1 (CIC00445)) and located at the 5′ end of the “hot spot” to cause arRP in the Spanish population [12]. These observations are of interest as they point out for an implication of hot spot region for adRCD-RP1 mutation also in case of arRCD. Future studies will need to clarify why some class II mutations lead to adRCD and others to arRCD.
Patients with arRCD and RP1 mutations show a more severe disease than adRCD-RP1 mutant patients with macular atrophy in all our cases. This was first outlined by Lafont et al. [17]. When patients are presenting with late, severe disease, the diagnostic distinction between RCD, with initial rod involvement, and cone-rod dystrophy (CRD) with initial cone involvement is difficult. Of note is that one of the patients (CIC01300) in the present study was initially classified as possibly having severe CRD and his diagnosis was actually revisited after NGS results. This also outlines the interest of unbiased massive parallel sequencing methods for a more precise clinical diagnostic in case of end stage disease. This point will most likely become even more critical with the perspective of therapeutic trials.
4.1. Strength and Limitations
We estimate that 1% of our target regions were not covered. Partially uncovered exons are a real common issue when capturing the DNA sequences using commercially available probes; this bias might imply a loss of some candidate variants. However, we found that rate of 1% is very reasonable when compared with other NGS panels. In addition, in order to exclude the possibility of finding other candidate variants, we have sequenced by Sanger method the majority of these regions. Five of our patients carried homozygous RP1 mutations. For four of the subjects carrying homozygous variants, namely CIC00491, F335; CIC01106, F674; CIC01300, F782 and CIC04130, F1941; co-segregation analysis needs to be done to confirm autosomal recessive inheritance but we do not have access to parent's DNA. CIC05941 was the only one not to report clear consanguinity in the family, and we cannot exclude the possibility of a large deletion on the second allele of RP1 gene. Again, DNA of the father, not available for us, would be helpful to prove autosomal recessive inheritance and the homozygous state of the mutation.
In conclusion, we have reported 9 mutations in RP1 of which 8 were novel causing arRCD [8, 12–20]. Interestingly, a prevalence of ≈2.5% points out for the necessity of sequencing RP1 in sporadic and recessive cases of RCD. Further functional studies are needed to understand the impact of RP1 structure on its function at the molecular level; such a step would strengthen our knowledge in the physiology of retinal photoreceptors.
Acknowledgments
The authors express their sincere gratitude to the families who participated in this study and to the clinical staff for their help in clinical data and DNA collection. This work was supported by Foundation Voir et Entendre (CZ), Prix Dalloz for “la recherche en ophtalmologie” (CZ), Foundation Fighting Blindness (FFB) [CD-CL-0808-0466-CHNO] (IA) and FFB center (FFB grantC-GE-0912-0601-INSERM02), Prix de la Fondation de l'Œil (IA), Ville de Paris and Region Ile de France, and the French State program “Investissements d'Avenir” managed by the Agence Nationale de la Recherche [LIFESENSES: ANR-10-LABX-65].
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Isabelle Audo and Christina Zeitz contributed equally to this work.
References
- 1.Hartong D. T., Berson E. L., Dryja T. P. Retinitis pigmentosa. The Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Audo I., Lancelot M., Mohand-Saïd S., et al. Novel C2orf71 mutations account for approximately ~1% of cases in a large French arRP cohort. Human Mutation. 2011;32(4):E2091–E2103. doi: 10.1002/humu.21460. [DOI] [PubMed] [Google Scholar]
- 3.Anasagasti A., Irigoyen C., Barandika O., López de Munain A., Ruiz-Ederra J. Current mutation discovery approaches in Retinitis Pigmentosa. Vision Research. 2012;75:117–129. doi: 10.1016/j.visres.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan L. S., Bowne S. J., Birch D. G., et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Investigative Ophthalmology and Visual Science. 2006;47(7):3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyedahmadi B. J., Rivolta C., Keene J. A., Berson E. L., Dryja T. P. Comprehensive screening of the USH2A gene in Usher syndrome type II and non-syndromic recessive retinitis pigmentosa. Experimental Eye Research. 2004;79(2):167–173. doi: 10.1016/j.exer.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Ávila-Fernández A., Cantalapiedra D., Aller E., et al. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Molecular Vision. 2010;16:2550–2558. [PMC free article] [PubMed] [Google Scholar]
- 7.El Shamieh S., Neuille M., Terray A., et al. Whole-exome sequencing identifies KIZ as a ciliary gene associated with autosomal-recessive rod-cone dystrophy. American Journal of Human Genetics. 2014;94(4):625–633. doi: 10.1016/j.ajhg.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Audo I., Mohand-Saïd S., Dhaenens C. M., et al. RP1 and autosomal dominant rod-cone dystrophy: novel mutations, a review of published variants, and genotype-phenotype correlation. Human Mutation. 2012;33(1):73–80. doi: 10.1002/humu.21640. [DOI] [PubMed] [Google Scholar]
- 9.Guillonneau X., Piriev N. I., Danciger M., et al. A nonsense mutation in a novel gene is associated with retinitis pigmentosa in a family linked to the RP1 locus. Human Molecular Genetics. 1999;8(8):1541–1546. doi: 10.1093/hmg/8.8.1541. [DOI] [PubMed] [Google Scholar]
- 10.Pierce E. A., Quinn T., Meehan T., McGee T. L., Berson E. L., Dryja T. P. Mutations in a gene encoding a new oxygen-regulated photoreceptor protein cause dominant retinitis pigmentosa. Nature Genetics. 1999;22(3):248–254. doi: 10.1038/10305. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan L. S., Heckenlively J. R., Bowne S. J., et al. Mutations in a novel retina-specific gene cause autosomal dominant retinitis pigmentosa. Nature Genetics. 1999;22(3):255–259. doi: 10.1038/10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avila-Fernandez A., Corton M., Nishiguchi K. M., et al. Identification of an RP1 prevalent founder mutation and related phenotype in Spanish patients with early-onset autosomal recessive retinitis. Ophthalmology. 2012;119(12):2616–2621. doi: 10.1016/j.ophtha.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Chen L. J., Lai T. Y. Y., Tam P. O. S., et al. Compound heterozygosity of two novel truncation mutations in RP1 causing autosomal recessive retinitis pigmentosa. Investigative Ophthalmology and Visual Science. 2010;51(4):2236–2242. doi: 10.1167/iovs.09-4437. [DOI] [PubMed] [Google Scholar]
- 14.Khaliq S., Abid A., Ismail M., et al. Novel association of RP1 gene mutations with autosomal recessive retinitis pigmentosa. Journal of Medical Genetics. 2005;42(5):436–438. doi: 10.1136/jmg.2004.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siemiatkowska A. M., Astuti G. D. N., Arimadyo K., et al. Identification of a novel nonsense mutation in RP1 that causes autosomal recessive retinitis pigmentosa in an Indonesian family. Molecular Vision. 2012;18:2411–2419. [PMC free article] [PubMed] [Google Scholar]
- 16.Bocquet B., Marzouka N. A., Hebrard M., et al. Homozygosity mapping in autosomal recessive retinitis pigmentosa families detects novel mutations. Molecular Vision. 2013;19:2487–2500. [PMC free article] [PubMed] [Google Scholar]
- 17.Lafont E. M., Manes G., Sénéchal G., et al. Patients with retinitis pigmentosa due to RP1 mutations show greater severity in recessive than in dominant cases. Journal of Clinical & Experimental Ophthalmology. 2011;2, article 194 doi: 10.4172/2155-9570.1000194. [DOI] [Google Scholar]
- 18.Al-Rashed M., Abu Safieh L., Alkuraya H., et al. RP1 and retinitis pigmentosa: report of novel mutations and insight into mutational mechanism. British Journal of Ophthalmology. 2012;96(7):1018–1022. doi: 10.1136/bjophthalmol-2011-301134. [DOI] [PubMed] [Google Scholar]
- 19.Riazuddin S. A., Shahzadi A., Zeitz C., et al. A mutation in SLC24A1 implicated in autosomal-recessive congenital stationary night blindness. The American Journal of Human Genetics. 2010;87(4):523–531. doi: 10.1016/j.ajhg.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh H. P., Jalali S., Narayanan R., Kannabiran C. Genetic analysis of indian families with autosomal recessive retinitis pigmentosa by homozygosity screening. Investigative Ophthalmology and Visual Science. 2009;50(9):4065–4071. doi: 10.1167/iovs.09-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q., Lyubarsky A., Skalet J. H., Pugh E. N., Jr., Pierce E. A. RP1 is required for the correct stacking of outer segment discs. Investigative Ophthalmology & Visual Science. 2003;44(10):4171–4183. doi: 10.1167/iovs.03-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q., Zuo J., Pierce E. A. The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. Journal of Neuroscience. 2004;24(29):6427–6436. doi: 10.1523/JNEUROSCI.1335-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Audo I., Bujakowska K. M., Léveillard T., et al. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet Journal of Rare Diseases. 2012;7(1, article 8) doi: 10.1186/1750-1172-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Audo I., Sahel J. A., Mohand-Saïd S., et al. EYS is a major gene for rod-cone dystrophies in France. Human Mutation. 2010;31(5):E1406–E1435. doi: 10.1002/humu.21249. [DOI] [PubMed] [Google Scholar]
- 25.Consortium T. G. P. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altshuler D. M., Gibbs R. A., Peltonen L., et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467(7311):52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tennessen J. A., Bigham A. W., O'Connor T. D., et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337(6090):64–69. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bamshad M. J., Ng S. B., Bigham A. W., et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nature Reviews Genetics. 2011;12(11):745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 29.Kent W. J., Sugnet C. W., Furey T. S., et al. The human genome browser at UCSC. Genome Research. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adzhubei I. A., Schmidt S., Peshkin L., et al. A method and server for predicting damaging missense mutations. Nature Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P., Henikoff S., Ng P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature Protocols. 2009;4(7):1073–1082. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 32.Desmet F. O., Hamroun D., Lalande M., Collod-Bëroud G., Claustres M., Béroud C. Human splicing finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Research. 2009;37(9, article no. e67) doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenson P. D., Ball E. V., Mort M., Phillips A. D., Shaw K., Cooper D. N. Current Protocols in Bioinformatics. chapter 1–13. 2012. The Human Gene Mutation Database (HGMD) and its exploitation in the fields of personalized genomics and molecular evolution. [DOI] [PubMed] [Google Scholar]
- 34.Fokkema I. F. A. C., Den Dunnen J. T., Taschner P. E. M. LOVD: easy creation of a locus-specific sequence variation database using an “LSDB-in-a-Box” approach. Human Mutation. 2005;26(2):63–68. doi: 10.1002/humu.20201. [DOI] [PubMed] [Google Scholar]
- 35.Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16(3):p. 1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeitz C., Kloeckener-Gruissem B., Forster U., et al. Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. American Journal of Human Genetics. 2006;79(4):657–667. doi: 10.1086/508067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riazuddin S. A., Zulfiqar F., Zhang Q., et al. Autosomal recessive retinitis pigmentosa is associated with mutations in RP1 in three consanguineous Pakistani families. Investigative Ophthalmology and Visual Science. 2005;46(7):2264–2270. doi: 10.1167/iovs.04-1280. [DOI] [PubMed] [Google Scholar]