Abstract
Cortical function is disrupted in neuroinflammatory disorders, including HIV-associated neurocognitive disorders (HAND). Astrocyte dysfunction includes retraction of foot processes from the blood-brain barrier and decreased removal of neurotransmitters from synaptic clefts. Mechanisms of astrocyte activation, including innate immune function and the fine neuroanatomy of astrocytes, however, remain to be investigated. We quantified the number of GFAP-labeled astrocytes per mm2 and the proportion of astrocytes immunopositive for Toll-like receptor 2 (TLR2) to examine innate immune activation in astrocytes. We also performed detailed morphometric analyses of grey and white matter astrocytes in the frontal and parietal lobes of rhesus macaques infected with simian immunodeficiency virus (SIV), both with and without encephalitis, an established model of AIDS neuropathogenesis. Protoplasmic astrocytes (grey matter) and fibrous astrocytes (deep white matter) were imaged, and morphometric features analyzed using Neurolucida. Grey matter and white matter astrocytes showed no change in cell body size in animals infected with SIV regardless of encephalitic status. In SIV-infected macaques, both grey and white matter astrocytes had shorter, less ramified processes, resulting in decreased cell arbor compared with controls. SIV-infected macaques with encephalitis showed decreases in arbor length in white matter astrocytes and reduced complexity in grey matter astrocytes compared to controls. These results provide the first evidence that innate immune activation of astrocytes is linked to altered cortical astrocyte morphology in SIV/HIV infection. Here, we demonstrate astrocyte remodeling is correlated with infection. Perturbed neuron-glia signaling may be a driving factor in the development of HAND.
Keywords: astrocyte, morphology, encephalitis, toll-like receptor, HIV
Introduction
HIV-associated neurocognitive disorders (HAND) affect over half of patients infected with HIV [14,48,17]. This collection of disorders exists on a spectrum of clinical symptoms and pathologies ranging from asymptomatic neurocognitive impairment with no evidence of encephalitis to AIDS dementia complex and HIV-induced encephalitis (HIVE). While the incidence of HIVE has decreased in the combined anti-retroviral therapy era, more subtle neuropathological alterations are becoming common as patients are living longer with the disease [9]. Thus, as the prevalence of HAND increases, it becomes imperative to understand the underlying mechanisms of HIV neuropathogenesis.
Since HIV infection is rarely seen in neurons [3], indirect mechanisms for HIV-associated neuronal cell damage are being considered including innate immune activation of glial cells [7,35]. Astrocytes exposed to the HIV protein, Tat, show increased TLR2 responsiveness to subsequent activation [13]. Additionally, priming with Tat protein induces a decrease in astrocyte expression of TLR9, which is integral to the suppression of viral infection [39,7].
In the search for other biological factors underlying HAND, neuroanatomical investigations have included the formation of multinucleated giant cells, myelin pallor, astrogliosis, and neuronal and glial cell death [30]. Astrocytes provide crucial metabolic support to neuronal networks in the CNS. While there is no clear consensus of how form and function are correlated [24], concurrent changes in astrocyte morphology and function have been demonstrated in hormonal, neuropathological, and environmental conditions [46,34,40]. Astrocytic hypertrophy and gliosis have been demonstrated in the setting of brain injury or neurodegeneration [10,46]. Conversely, decreased GFAP immunoreactivity and astrocytic atrophy have been reported in chronic disease [18]. Thus, structural changes in astrocyte morphology could result in impaired function [42,6] causing neuronal dysfunction through excitotoxicity [21,8,40], homeostatic imbalances [12,29], and damage to synapses [5,33].
To date, there has been little research to examine morphological and immunological changes in astrocytes, especially in response to HIV infection. Using the nonhuman primate model of HIV infection we observed increased TLR2 expression in both white and grey matter, and altered astrocyte cell numbers that were dependent on encephalitic status. When we compared changes in astrocyte morphology in SIV-infected animals with and without encephalitis, we found dramatically decreased overall process length and process complexity in animals infected with SIV, regardless of encephalitic status. Combined, these alterations could be expected to contribute to the decreased neural function observed in HIV-infected individuals through loss of BBB integrity and neurotransmitter uptake [40,43] at the synaptic cleft.
Materials and Methods
Animals, tissues and virus
Tissues from a total of twenty Indian-origin rhesus macaques (Macaca mulatta) were used for this study (Table 1). These twenty animals included fourteen SIV-infected animals and six uninfected controls. Nine of the SIV-infected animals had SIV encephalitis (SIVE) while five did not have encephalitis (SIV noE). All of the animals with SIV were infected intravenously with 50 ng p27 of either SIVmac251 or SIVmacDeltaB670, as specified in Table 1. The criteria for encephalitic status (SIVE vs. SIV noE) were based on previously published criteria [31].
Table 1.
| Animal | Age (years) | Sex | Inoculum | Major Neuropathologic Diagnoses | Days Post-Infection | Lesions/mm2 | |
|---|---|---|---|---|---|---|---|
| SIV encephalitis | AH20 | 5.71 | male | SIVmac251 | encephalitis, moderate | 112 | 0.03 |
| CP28 | 5.12 | male | SIVmac251 | encephalitis, moderate | 183 | 0.161 | |
| DG84 | 5.45 | male | SIVmac251 | encephalitis, moderate | 102 | 0.288 | |
| M722 | 3.60 | female | SIVB670 | encephalitis, minimal to moderate | 271 | 0.324 | |
| N152 | 5.33 | female | SIVB670 | encephalitis, minimal | 83 | 0 | |
| FV53 | 5.34 | male | SIVmac251 | encephalitis, mild | 65 | 0.058 | |
| GI51 | 4.59 | male | SIVmac251 | encephalitis, moderate | 133 | 0.027 | |
| CG31 | 4.59 | male | SIVmac251 | encephalitis, minimal encephalomalacia, modearate | 171 | 0.020 | |
| GM59 | 4.38 | male | SIVmac251 | encephalitis, mild | 99 | 0.048 | |
| SIV, no encephalitis | EI69 | 5.26 | male | SIVmac251 | cerebral hemorrhage | 89 | 0.007 |
| GP19 | 4.64 | male | SIVmac251 | NSL | 225 | 0 | |
| EI70 | 5.17 | male | SIVmac251 | NSL | 57 | 0 | |
| CC47 | 8.70 | male | SIVmac251 | NSL | 680 | 0 | |
| DE57 | 8.90 | male | SIVmac251 | NSL | 245 | 0 | |
| Uninfected | EI93 | 5.31 | female | n/a | NSL | n/a | 0 |
| HT22 | 2.91 | male | n/a | NSL | n/a | 0 | |
| HM63 | 3.04 | male | n/a | NSL | n/a | 0 | |
| HN64 | 3.03 | male | n/a | NSL | n/a | 0 | |
| HP24 | 3.03 | male | n/a | NSL | n/a | 0 | |
| EB20 | 3.82 | female | n/a | NSL | n/a | 0 |
All animals were housed at the Tulane National Primate Research Center in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Research Council, National Academic Press, Washington, DC. The Tulane Institutional Animal Care and Use Committee approved all studies. All animals were humanely euthanized with an intravenous overdose of pentobarbital and tissues collected at necropsy.
Immunofluorescence
Formalin-fixed, paraffin-embedded tissues were sectioned at 6 μm and mounted onto positively charged glass slides. Sections were baked for 1 hour at 60°C, deparaffinized in xylene, and then rehydrated in graded concentrations of ethanol. Antigen retrieval was carried out for 20 minutes using a microwave on high power and a citrate-based antigen unmasking solution (Vector Labs, Burlingame, CA). Tissues were blocked in a 10% normal goat serum solution (Invitrogen, Carlsbad, CA) for one hour at room temperature before antibodies were applied. Tissues were incubated with GFAP primary antibody (clone GA-5, Sigma, 1:250 dilution) and TLR2 (Abcam ab24192, rabbit polyclonal, 1:50 dilution) overnight at 4°C, washed three times with PBS with 2% bovine serum albumin (Santa Cruz, CA, PBS/BSA), and then incubated in the dark for 60 minutes at room temperature with secondary antibodies directly conjugated with Alexa 488 (green) or Alexa 568 (red) (Molecular Probes/Invitrogen, Carlsbad, CA). Sections were washed three times in PBS/FSG, cover-slipped (CrystalCruz, Santa Cruz) with Prolong Gold with DAPI (Molecular Probes/Invitrogen), and imaged on a Nikon Eclipse TE2000-U microscope.
Quantification of GFAP and TLR2 expression
Images of double-labeled (GFAP and TLR2) sections in non-overlapping fields of cortical grey and white matter were captured by fluorescence microscopy (Nikon Eclipse TE2000-U). An average of five fields of astrocytes were imaged separately for both white and grey matter at 20X, and the number of GFAP and GFAP/TLR2 double-labeled astrocytes was counted and expressed as a percentage of the total number of GFAP-labeled cells.
Quantification of Astrocyte Morphology
All samples were coded and analyzed randomly by a researcher blinded to animal number and condition. Images of non-overlapping fields in frontal and/or parietal lobe sections were captured by fluorescence microscopy using a 40X objective (Nikon Eclipse TE2000-U) and analyzed using Neurolucida software (MBF Bioscience). An average of 10 astrocytes from each animal with well-defined cell bodies and processes in both grey and white matter were chosen for analysis. The cells chosen were fully intact and did not have processes that touched the edges of the field. For our purposes, we classified astrocytes into two main categories: protoplasmic astrocytes, which are abundantly found in grey matter and characterized by highly complex processes, and fibrous white matter astrocytes, which have well-defined processes and moderate branching [24]. The resulting files generated by 2D tracing were analyzed with Neurolucida Explorer (MBF Bioscience), generating data of morphological measurements including cell area, branching points (bifurcations), arbor length and volume as previously described [20,19,37] and represented as a cartoon 1.
Sholl Analyses
Sholl analysis was performed on the data by placing concentric circles around the cell starting from the center of the cell body and radiating outward at increasing radial increments of 10μm. Ten individual astrocytes of both gray and white matter from each animal were imaged and averaged for analysis. Intersections are determined as points where the astrocytic processes cross a concentric ring. Branching points (nodes) are expressed as a quantity per concentric ring area.
Statistical Analyses
Statistics were performed using GraphPad Prism (version 5, GraphPad Software). For analyses of GFAP and TLR2 expression, comparisons were made by one-way ANOVA with Tukey’s post-hoc multiple comparison test to determine significance between groups. Results are expressed as mean ± SD. Significance was set at p < 0.05.
For morphological studies, ten grey or white matter astrocytes were averaged per each animal for analysis. Normality was assessed by Kolmogorov-Smirnov test, and data that passed normality were analyzed by one-way ANOVA. Data that were not distributed normally were assessed by Kruskal-Wallis test with Dunn’s post-hoc multiple comparison test to determine significance between groups. Results are expressed as mean ± SEM. For all analyses, significance was set at p < 0.05.
Results
SIV infection in brain upregulates TLR2 expression on astrocytes
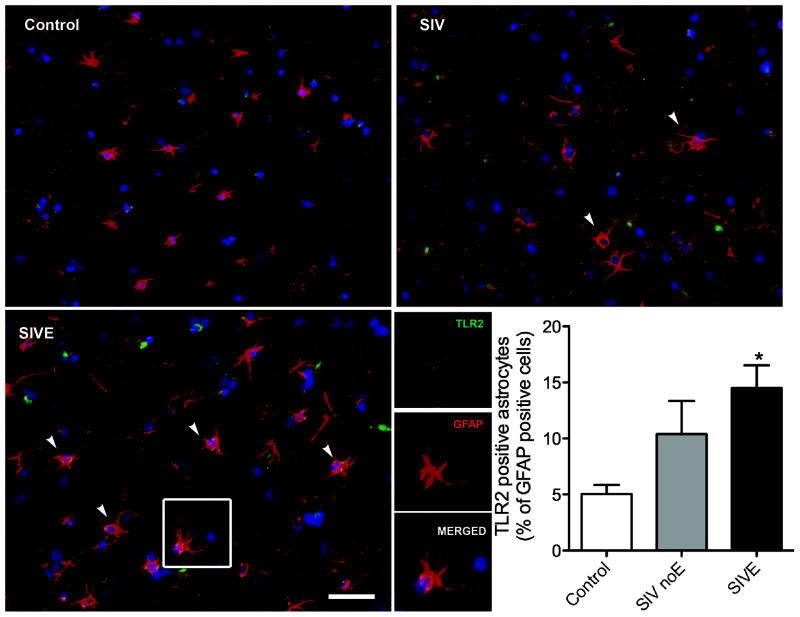
Based on in vitro studies [7], we hypothesized that there would be increased TLR2 expression on astrocytes in brains of macaques with active SIV infection. To address this question, we counted the proportion of astrocytes double immunopositive for TLR2 and GFAP. Expression of TLR2 was limited or absent in grey matter of control macaques (Figure 1A). SIV noE macaques had increased expression of TLR2 on grey matter astrocytes (Figure 1B). Macaques with SIVE, however, had a greater increase in TLR2 expression on grey matter astrocytes compared with both control and SIV noE macaques (Figure 1C and graph). Single channel images are indicated in the SIVE macaque.
Figure 1. TLR2 expression is increased in grey matter of frontal lobes of primates with SIV-induced encephalitis.
In grey matter of control brain, astrocytes (GFAP, red) were generally immunonegative for TLR2 (green). The proportion of astrocytes expressing for TLR2 was significantly increased in animals infected with SIV with encephalitis (SIVE). Scale bar = 25 μm.
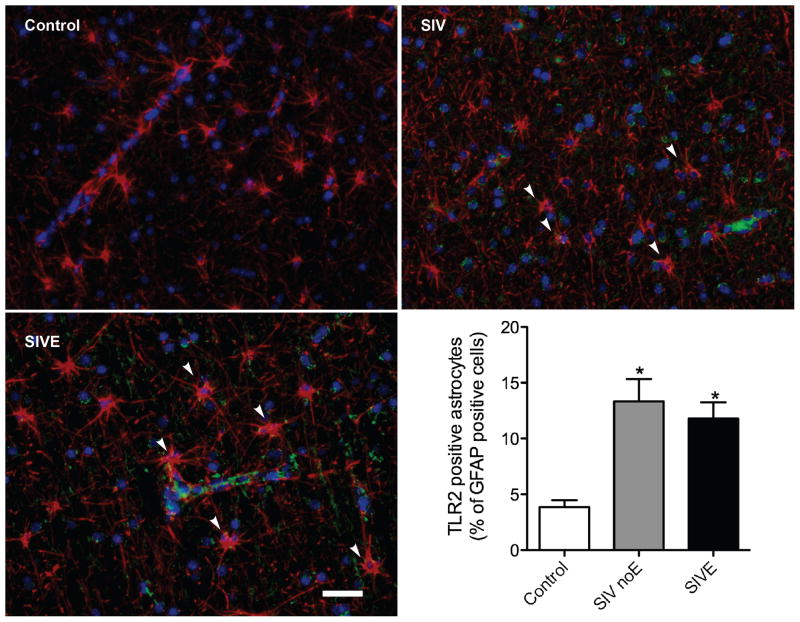
Similarly, white matter astrocytes in control macaques were rarely TLR2 positive (Figure 2A). In macaques infected with SIV, there was a slight increase in the TLR2 expression on white matter astrocytes, both without (Figure 2B) and with (Figure 2C) encephalitis.
Figure 2. TLR2 expression is increased in white matter of frontal lobes of primates with SIV-induced encephalitis.
In white matter of control brain, astrocytes (GFAP, red) were generally immunonegative for TLR2 (green). The proportion of astrocytes expressing TLR2 was significantly increased in animals infected with SIV either without (SIV) or with encephalitis (SIVE). GFAP immunonegative cells, morphologically consistent with endothelial or perivascular cells, were also immunopositive for TLR2 in SIVE macaques. Scale bar = 25 μm.
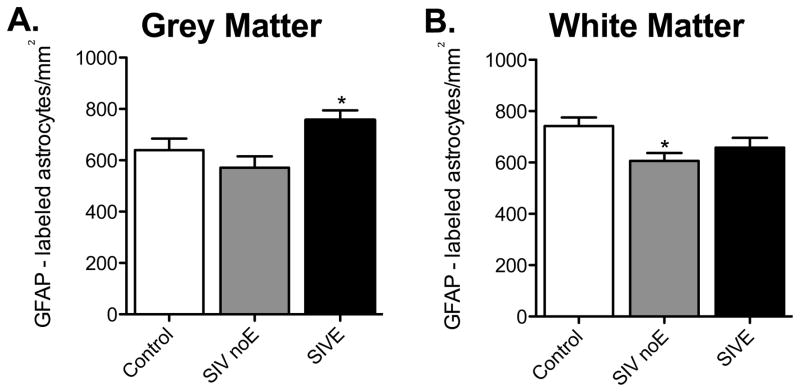
To determine if there were altered numbers of astrocytes in frontal or parietal lobes of macaques infected with SIV, we counted the number of GFAP positive cells per unit area. Macaques with SIVE had increased numbers of grey matter astrocytes per unit area compared with SIV infected animals without encephalitis (p < 0.001, Figure 3A), but not with control animals. In contrast, there was a significant decrease in the number of astrocytes per unit area in white matter of macaques infected with SIV, but without encephalitis compared with control animals (p < 0.05, Figure 3B). There was no significant change in the numbers of white matter GFAP-immunopositive astrocytes in macaques with encephalitis compared to either controls or animals without encephalitis. Thus, there was increased innate immune activation irrespective of altered numbers of astrocytes per unit area. However, when GFAP positive cells from grey and white matter were combined, there was no significant difference between treatment groups (p=0.1122, data not shown). Similarly, there was no significant difference in mean fluorescent intensity for vimentin expression between the groups (p=0.0997, data not shown).
Figure 3. SIV infection alters the number of GFAP-immunopositive astrocytes.
In grey matter, there was increased GFAP immunoreactivity in SIVE astrocytes compared with infected animals without encephalitis, but not with control animals (A). There was a decrease in GFAP-labeled white matter astrocytes in SIV noE animals compared with controls (B). Results are shown as mean ± SD (asterisk (*) indicates p <0.05).
SIV infection does not induce altered cell body size
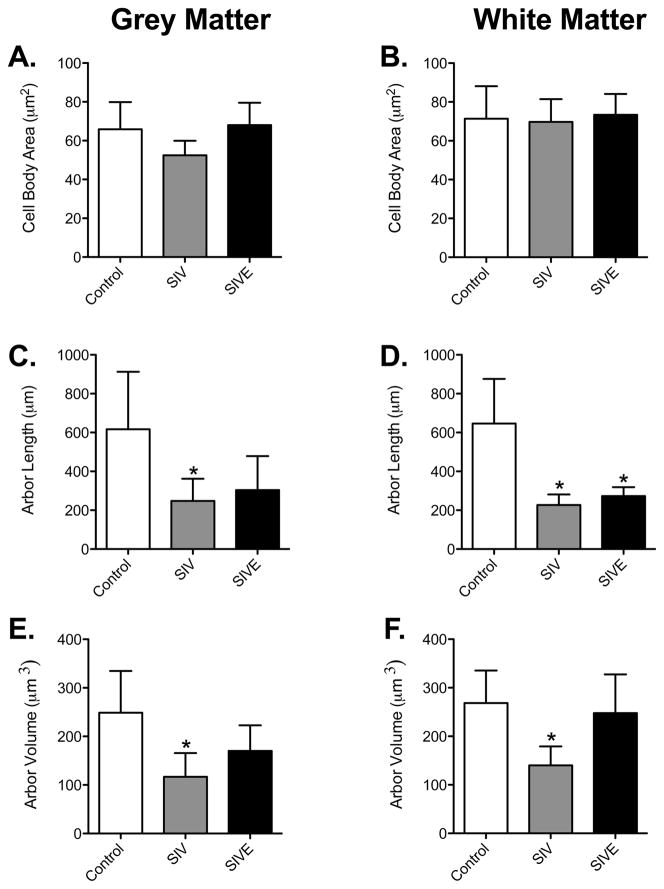
Astrogliosis is often associated with hypertrophy. Therefore, we postulated that there would be astrocyte hypertrophy associated with SIVE. To assess atrophy or hypertrophy, we first measured the area of the cell body of astrocytes. The cell bodies in control grey matter astrocytes averaged 65.86 ± 14.04 μm2 (n = 6, Figure 4A). In SIV-infected macaques without encephalitis there were no significant decrease in cell body area in grey matter astrocytes (52.45 ± 7.497 μm2, n = 5), with a smaller, insignificant decrease in grey matter astrocytes in macaques with SIVE (68.00 ± 11.53 μm2, n = 9). The size of the cell body of white matter fibrous astrocytes did not significantly change in SIV noE (69.74 ± 11.74 μm2, n = 5) or SIVE (73.88 ± 10.74 μm2, n = 9) animals compared to controls (71.35 ± 16.74 μm2, n = 6; Figure 4B). Overall, no astrocyte cell body hypertrophy or atrophy was observed in grey or white matter astrocytes of macaques infected with SIV.
Figure 4. Decreased astrocytic arbor length and volume of animals infected with SIV.
There was no significant difference in cell body size among control, SIV noE, and SIVE animals in both grey and white matter astrocytes (A and B). In grey matter astrocytes, cell arbor was significantly decreased in SIV noE animals (C). In white matter astrocytes (D), however, cell arbors were significantly longer in astrocytes of control animals compared with astrocytes in animals infected with SIV, regardless of encephalitic status. Arbor volume was significantly decreased in astrocytes of SIV noE macaques compared with control animals. This was observed in both grey (E) and white (F) matter. Results are shown as mean ± SD (asterisk (*) indicates p <0.05 compared to controls).
Astrocytic processes are shortened following SIV infection
To determine if there were changes in the distinctive processes that emerge from the astrocyte cell bodies, we measured the total length and volume of the arbor of each astrocyte imaged. Measuring from the cell body to the end of each process, we calculated the length of the astrocyte arbor, which is represented as “arbor length” (Figure 4C–D). In grey matter astrocytes, total cell arbor was decreased significantly in animals infected with SIV, but not in those with encephalitis (Control: 617.2 ± 295.5 μm vs SIV noE: 247.9 ± 113.8 μm and SIVE: 303.6 ± 174.9 μm, Figure 4C). There were significant reductions in white matter astrocyte cell arbor in SIV-infected animals regardless of encephalitic status (Control: 646.4 ± 229.5 μm vs SIV noE: 225.9 ± 54.16 μm and SIVE: 272.9 ± 45.60 μm, Figure 4D). No differences in cell arbor were observed between SIV noE and SIVE grey and white matter astrocytes.
Using the diameter of the astrocyte processes, frusta were created in Neurolucida to determine the arbor volume of the astrocyte processes. The total volume of the astrocytic processes was decreased significantly in SIV noE grey and white matter astrocytes compared with controls (Grey matter, Control: 249.0 ± 85.71 μm3 vs SIV noE: 117.0 ± 48.73 μm3; White matter, Control: 247.7 ± 79.98 μm3 vs SIV noE: 140.2 ± 38.97 μm3, Figure 4E–F). SIV noE animals showed an overall atrophy in astrocyte arbor. Grey and white matter astrocytes of SIVE animals, however, showed no reduction in overall volume even though total arbor length had decreased (Grey matter, SIVE: 170.3 ± 52.39 μm3; White matter, SIVE: 247.7 ± 79.98 μm3, Figure 4E–F). This indicates a general thickening of astrocyte processes in these animals.
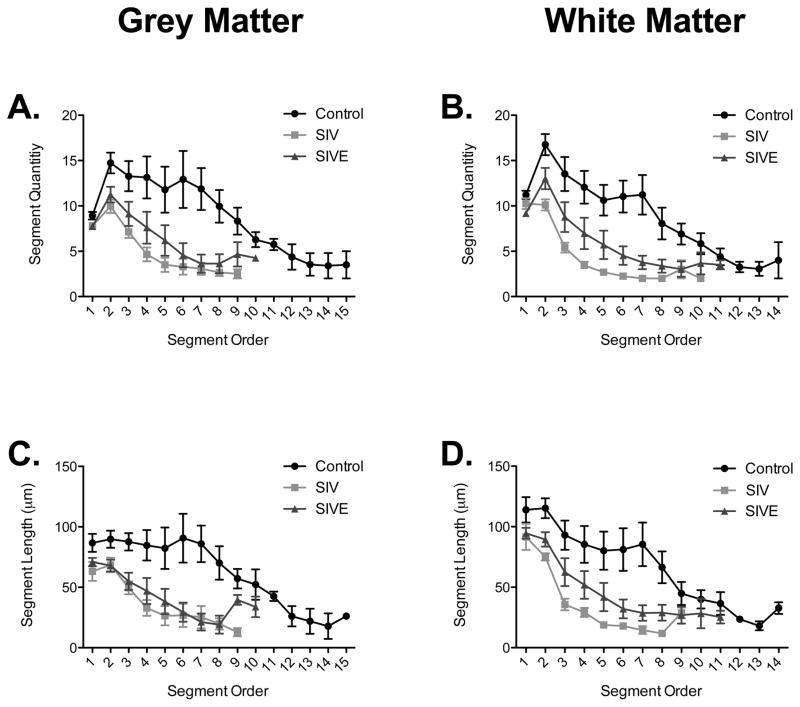
In order to investigate changes in the number of process segments, we separated each process by segment order (Figure 5A–B). We then quantified the number of processes leaving the cell body (shown as first order segment quantity; Supplementary Figure 1A–B). There was a significant decrease in the number of processes emanating from the cell body in both grey and white matter astrocytes in the SIVE group compared with controls (Supplementary Figure 1A–B). Decreases in the number of secondary and tertiary processes, however, were observed only in SIV noE animals compared to control animals (Supplementary Figure 1C–F). To localize the changes observed in astrocytic process length, we separated the total length measurements by branch order (Figure 5C–D). There was a significant decrease in the length of secondary and tertiary branches of white matter astrocytes in SIV noE animals compared to controls (Supplementary Figure 1J and L). Reductions in segment length were observed in tertiary segments of SIV noE and SIVE grey matter astrocytes (Supplementary Figure 1K). Overall, shortening of processes occurred in more distal segments of the processes in animals infected with SIV.
Figure 5. Decreased length and number of segments is consistent across branch orders.
Arrangement of segment quantity by branch order in grey (A) and white (B) matter astrocytes showed a significant decrease in process quantity in SIV noE/SIVE infected astrocytes compared with control astrocytes. Overall arrangements of process segment length by branch order showed a clear decrease in process length in SIV noE/SIVE astrocytes compared with control astrocytes in both grey (C) and white (D) matter. Results are shown as mean ± SEM.
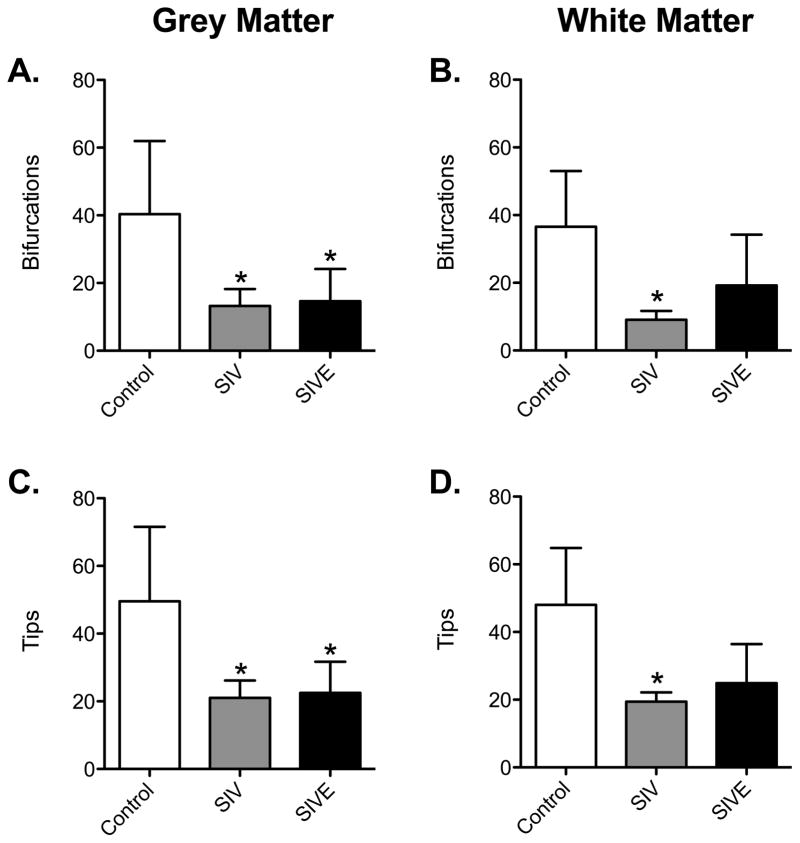
The number of bifurcations decreased significantly in SIV noE and SIVE grey matter astrocytes compared to controls (Control: 40.32 ± 21.60 vs SIV noE: 13.25 ± 5.005 and SIVE: 14.65 ± 9.516, p <0.01, Figure 6A). Additionally, there was a reduction in the number of process end points (tip quantity) in SIVE and SIV noE grey matter astrocytes (Control: 49.57 ± 21.97 vs SIV noE: 21.03 ± 5.151 and SIVE: 22.47 ± 9.254, p < 0.05, Figure 6C). In fibrous white matter astrocytes, decreases in the number of bifurcations (Control: 36.56 ± 16.48 vs SIV: 9.100 ± 2.586 and SIVE: 19.25 ± 14.97) and end points (Control: 48.03 ± 16.82 vs SIV: 19.42 ± 2.720 and SIVE: 24.95 ± 11.51) were significant in SIV noE but not in SIVE macaques compared to controls (Figure 6B and D, p < 0.05).
Figure 6. Astrocytes in SIV-infected animals have less complex morphology.
The quantity of branching points and end points shown by astrocytic processes in grey matter were significantly decreased in astrocytes of animals infected with SIV regardless of encephalitic status (A and C). In white matter astrocytes, only animals with SIV without evidence of encephalitis showed significant decreases in the number of branching points and ending segments (B and D). Results are shown as mean ± SD (asterisk (*) indicates significance from Control group, p <0.05).
Astrocytes in SIV-infected macaques have less complex morphology
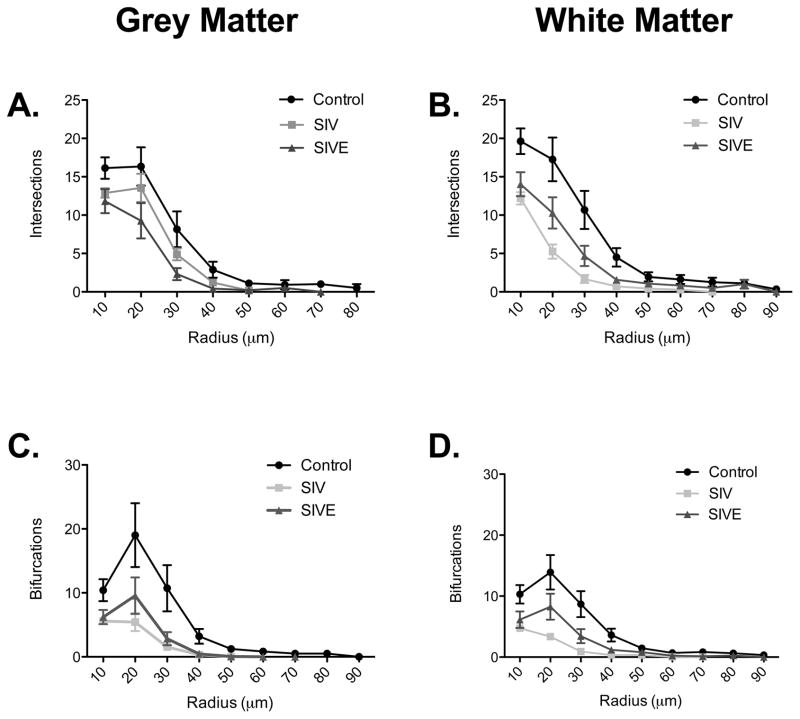
Using modified Sholl analysis to further examine the complexity of astrocytes, we quantified the number of times a process segment crossed over a concentric ring (intersections) and nodes per 10μm increase in radius (Figure 7). No significant changes were seen in the number of intersections in grey or white matter astrocytes in the first 10 μm radius around the cell body of SIVE and SIV noE macaques (Supplementary Figure 2A–B). Decreases in the number of intersections in SIV noE grey and white matter astrocytes, however, were observed 20 μm away from the cell body (Supplementary Figure 2C–D) and again 30 μm from cell body in grey matter astrocytes (Supplementary Figure 2E). Similarly, the number of bifurcations (nodes) were significantly decreased at 20 μm and 30 μm away from the cell body of SIV noE grey (Figure 7C) and white matter astrocytes (Figure 7D). There were no changes in the number of intersections or bifurcations observed in SIVE grey and white matter astrocytes compared to SIV noE or control macaques. By 40 μm and beyond, there was no significant difference between control, SIV noE or SIVE astrocytes (Data not shown).
Figure 7. Sholl analysis of intersections and branching points of white matter and grey matter astrocytes.
Sholl analysis revealed that the branching patterns of grey (A) and white (B) matter astrocytes are more complex in control astrocytes than in SIV noE and SIVE astrocytes, with significantly more intersections found in all concentric radii within 30 μm around the cell body. Sholl analysis of branching points in grey (C) and white (D) matter astrocytes revealed a higher number of branching points in control astrocytes compared with astrocytes in macaques infected with SIV. Results are shown as mean ± SEM.
Overall, we observed a decrease in process length in grey and white matter astrocytes in macaques infected with SIV, with and without encephalitis. Sholl analysis showed a decrease in the complexity of SIV noE grey and white matter astrocytes. Furthermore, grey matter astrocytes from SIV-infected animals with or without SIVE showed a decrease in tip quantity: a further indication of decreased complexity in SIV neuropathogenesis. These changes are distinct from those we have recently described in other neuropathological disorders including globoid cell leukodystrophy [37], and nonhuman primate models for depression [20], and neurobrucellosis [19].
Discussion
This is the first postmortem study to combine studies of innate immune activation and examination of fine neuroanatomical changes in astrocyte morphology in SIV infection of rhesus macaques. We found that cortical grey and white matter astrocytes showed marked atrophy in process length, number, volume, and complexity regardless of the presence of SIVE. In addition, we found increased TLR2 expression on astrocytes in SIV-infected animals indicating both structural and innate immunological dysfunction in SIV neuropathogenesis.
Astrocytes display dynamic plasticity in their distal processes in response to changes in their extracellular environment [44,32,20,19,37]. We showed that, in the setting of SIV infection, grey and white matter astrocytes had fewer tips and branches as part of an overall decreased total arbor. Furthermore, the number of primary processes leaving the soma decreased in the presence of SIVE, reducing the number of processes available for endothelial and neuronal connection. Thus, changes occurred in both the distal and proximal (first, second, and third order) processes. Regardless of the site of retraction, these structural changes would decrease the number of astrocyte processes contacting endothelial cells and neurons. Loss of astrocyte processes is directly linked to leakage of the endothelial cells through downregulation of tight junction proteins [49,50], as is observed in HIVE / SIVE [23,45,15].
Increasing or decreasing the numbers and sizes of astrocytes would impact the volume and alter the composition of the space between astrocytes [36]. As a consequence of this, there would be neuronal dysfunction through excitotoxicity [21,8], homeostatic imbalances [12,29], damage to synapses [5,33], and potentially leading to decreased NAA/Cr ratios observed in clinically asymptomatic HIV-infected patients [4].
Differences between grey and white matter astrocytes were observed along the disease spectrum in SIV-infected macaques. The number of bifurcations decreased significantly for SIV noE and SIVE astrocytes in grey and white matter compared with control astrocytes, indicating a decrease in the complexity of the arbor in these cells. In addition, a reduction in astrocyte length and radius of the arbor in both grey and white matter astrocytes following SIV infection, regardless of encephalitic status was observed. However, the total volume of the arbor was only decreased in SIV-infected animals without encephalitis. Changes in the fine neuroanatomy of astrocytes have been observed in humans with depression [46] or macaques with self-injurious behavior [20], suggesting that astrocyte hypertrophy could be an upstream mechanism for the depression observed in HAND. As GFAP is known to bind glutamate transporters [40], removing astrocyte processes from around synapses could also have a role in glutamate-mediated excitotoxicity.
It is not certain whether these changes in astrocyte morphology are an early pathological event or a consequence of SIV-induced disease. Astrocytes react to various CNS insults through cell body and process hypertrophy, upregulation of intermediate filament expression, and glial scar formation [41]. However, although glial scarring is rarely observed following HIV and SIV infection, it is possible that astrocytes may be “permanently” altered by an initial exposure to SIV [7,13]. It would therefore be very interesting to examine astrocyte activation during the early stages of infection to determine if the effects observed in this study might be even more robust during peak viremia. Alternatively, changes in astrocyte morphology and activation could occur over time with repeated insult [32]. It could also be of interest to examine if older animals have a less robust glial response than do younger animals, as deficits have been observed in microgliosis as a consequence of aging.
HIV and SIV-associated lesions are more likely to occur in white matter [3,9,14,30,48], and hypertrophic astrocytes may reflect local immune activation in white matter [46]. That TLR2 was significantly upregulated on astrocytes in white matter regardless of encephalitic status requires further investigation, but may be a result of the increased ability of cytokines to diffuse in white matter tracts [16] leading to astrocyte activation without local inflammatory cell infiltrates. We have shown that TNF-α is upregulated in brains of macaques infected with SIV [27], and it is established that TNF-α will induce TLR2 expression on astrocytes [28]. The expression of TLRs is rapidly altered in response to pathogens, cytokines, and environmental stressors [1], and stimulation of TLR2 on astrocytes induces neuronal killing through reactive oxygen species [22], and is implicated in reducing synaptic stability [11].
Furthermore HIV-associated proteins have been shown to increase TLR2 expression in astrocytes [7], which, in turn acts in synergy with TLR9 to stimulate innate antiviral activity in the brain [38]. As outlined above, there is also evidence from in vitro studies that HIV proteins downregulate TLR9 expression in astrocytes combined with increased TLR2 expression [7]. Studies of expression levels of TLR9 in rhesus macaque brain tissues were not suitable for publication (not shown).
Limitations of the current study include the use of GFAP immunohistochemical staining to image astrocytes in 6μm sections of brain tissue. Using sections of this thickness limits the morphological analysis of the astrocyte and may not be entirely representative of the entire cell. Primate astrocytes can extend processes up to 100 μm [24], and thus, very thick tissue sections, which are not practical in formalin-fixed paraffin embedded tissues, would be required for complete confocal studies. Our recent studies [20,19,37], and those of others [46,2,47], have used considerably thinner sections with acceptable results. However, studies have shown GFAP expression can be used to visualize much of the arbor [26,32], although by no means all of it [25]. This retrospective study examined astrocytes from frontal and parietal lobes of animals infected with SIV both with and without evidence of encephalitis to obtain a general understanding of the immunological and morphological changes occurring in the brain in response to SIV infection. Future studies would involve Golgi staining of thicker sections, which would allow us to also visualize changes at the astrocytic spine level [46]. These studies could also be extended to examine innate immune activation and astrocyte morphology in other encephalitides, including that associated with Lyme disease. Despite these limitations, we found that grey and white matter astrocytes in SIV-infected animals with encephalitis showed greater decreases in complexity compared with SIV-infection with no encephalitis. We also observed increases in TLR2 expression in both grey and white matter astrocytes in SIV noE and SIVE animals. Taken together, these data show increased innate immune activation combined with a complex change in the fine neuroanatomy and in response to SIV-infection regardless of encephalitic status.
Supplementary Material
There were significantly fewer primary segments in grey and white matter astrocytes in SIVE macaques compared to controls (Supplemental Figure 1A–B). Secondary and tertiary processes in SIV noE grey and white matter astrocytes were reduced compared to controls (Supplemental Figure 1C–F). No changes in length were observed in first order segments in grey and white matter astrocytes (Supplemental Figure 1G–H). Decreases in segment length were observed in second and third order SIV noE white matter astrocytes (Supplemental Figure 1J and L) and again in tertiary SIV noE and SIVE grey and white matter astrocytes (Supplemental Figure 1K). Results are shown as mean ± SD.
There was a significant decrease in the number of intersections at 20μm and 30μm away from the cell body in grey matter astrocytes of SIV noE macaques compared to controls (Supplemental Figure 2C and E). These reductions were only seen at 20 μm from the cell body in white matter astrocytes of SIV noE animals (Supplemental Figure 2D). Reduced numbers of nodes were evident at 20 μm and 30 μm in grey matter and white matter astrocytes in astrocytes in SIV-infected macaques (Supplemental Figure I-L). No changes the number of intersections and nodes were observed in grey and white matter astrocytes of SIVE macaques. Results are shown as mean ± SD.
Acknowledgments
This work was supported by PHS grants OD11104, RR00164, MH077544 (AGM); Non PHS support included Tulane Committee on Research Summer Fellowship (AGM), Tulane University Bridge funding (AGM), and Tulane Neuroscience Program funding (AGM, KBC).
Footnotes
Disclosure
All of the authors have seen and approved this manuscript. AGM oversaw all aspects of the study, and assisted with writing the manuscript. KML is the primary author of the manuscript. She identified brain regions and cell types, conducted IHCs and imaged slides, and performed statistical analysis. PJD completed lesion analysis on all animals listed in Table 1. HAS assisted with all lab procedures. NAR provided the concept for the project, trained KBC in Neurolucida and assisted with writing the manuscript. KBC performed all Neurolucida analyses and assisted with manuscript writing and editing.
Conflict of interest
The authors declare no conflict of interest.
The authors have no conflict of interest in the publication of this manuscript.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. S0092-8674(06)00190-5 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Bailey AR, Hou H, Song M, Obregon DF, Portis S, Barger S, Shytle D, Stock S, Mori T, Sanberg PG, Murphy T, Tan J. GFAP expression and social deficits in transgenic mice overexpressing human sAPPalpha. Glia. 2013;61(9):1556–1569. doi: 10.1002/glia.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissel SJ, Wiley CA. Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain Pathol. 2004;14(1):97–108. doi: 10.1111/j.1750-3639.2004.tb00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bladowska J, Zimny A, Kołtowska A, Szewczyk P, Knysz B, Gąsiorowski J, Furdal M, Sąsiadek MJ. Evaluation of metabolic changes within the normal appearing gray and white matters in neurologically asymptomatic HIV-1-positive and HCV-positive patients: Magnetic resonance spectroscopy and immunologic correlation. Eur J Radiol. 2013;82(4):686–692. doi: 10.1016/j.ejrad.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10(5):392–406. doi: 10.2174/157016212802138832. CHIVR-EPUB-20120511-1[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drewes LR. Making connexons in the neurovascular unit. J Cereb Blood Flow Metab. 2012;32(8):1455–1456. doi: 10.1038/jcbfm.2012.44jcbfm201244. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Hage N, Podhaizer EM, Sturgill J, Hauser KF. Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest. 2011;40(5):498–522. doi: 10.3109/08820139.2011.561904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. Journal of magnetic resonance imaging: JMRI. 2010;32(5):1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8(1–2):51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–2155. doi: 10.1523/JNEUROSCI.3547-03.200424/9/2143. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freria CM, Velloso LA, Oliveira AL. Opposing effects of Toll-like receptors 2 and 4 on synaptic stability in the spinal cord after peripheral nerve injury. J Neuroinflammation. 2012;9:240. doi: 10.1186/1742-2094-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14(7):1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- 13.Henn A, Kirner S, Leist M. TLR2 hypersensitivity of astrocytes as functional consequence of previous inflammatory episodes. Journal of immunology. 2011;186(5):3237–3247. doi: 10.4049/jimmunol.1002787. [DOI] [PubMed] [Google Scholar]
- 14.Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. Int Rev Psychiatry. 2008;20(1):3–13. doi: 10.1080/09540260701862086790246101. [pii] [DOI] [PubMed] [Google Scholar]
- 15.Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27(1):123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konsman JP, Tridon V, Dantzer R. Diffusion and action of intracerebroventricularly injected interleukin-1 in the CNS. Neuroscience. 2000;101(4):957–967. doi: 10.1016/s0306-4522(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 17.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiology of disease. 2010;37(3):542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechuga-Sancho AM, Arroba AI, Frago LM, Garcia-Caceres C, de Celix AD, Argente J, Chowen JA. Reduction in the number of astrocytes and their projections is associated with increased synaptic protein density in the hypothalamus of poorly controlled diabetic rats. Endocrinology. 2006;147(11):5314–5324. doi: 10.1210/en.2006-0766. en.2006-0766 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Lee KM, Chiu KB, Sansing HA, Didier PJ, Ficht TA, Arenas-Gamboa AM, Roy CJ, Maclean AG. Aerosol-induced brucellosis increases TLR-2 expression and increased complexity in the microanatomy of astroglia in rhesus macaques. Frontiers in cellular and infection microbiology. 2013;3:86. doi: 10.3389/fcimb.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KM, Chiu KB, Sansing HA, Inglis FM, Baker KC, Maclean AG. Astrocyte atrophy and immune dysfunction in self-harming macaques. PLoS One. 2013;8(7):e69980. doi: 10.1371/journal.pone.0069980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MC, Ting KK, Adams S, Brew BJ, Chung R, Guillemin GJ. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS One. 2010;5(11):e14123. doi: 10.1371/journal.pone.0014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma D, Jin S, Li E, Doi Y, Parajuli B, Noda M, Sonobe Y, Mizuno T, Suzumura A. The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J Neuroimmunol. 2013;254(1–2):10–18. doi: 10.1016/j.jneuroim.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 23.MacLean AG, Belenchia GE, Bieniemy DN, Moroney-Rasmussen TA, Lackner AA. Simian immunodeficiency virus disrupts extended lengths of the blood--brain barrier. J Med Primatol. 2005;34(5–6):237–242. doi: 10.1111/j.1600-0684.2005.00121.x. JMP121 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010;63(1–2):2–10. doi: 10.1016/j.brainresrev.2009.12.001. S0165-0173(09)00129-5 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(10):3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. 29/10/3276 [pii] [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28(13):3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orandle MS, MacLean AG, Sasseville VG, Alvarez X, Lackner AA. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol. 2002;76(11):5797–5802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phulwani NK, Esen N, Syed MM, Kielian T. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol. 2008;181(6):3841–3849. doi: 10.4049/jimmunol.181.6.3841. 181/6/3841 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierozan P, Zamoner A, Soska AK, de Lima BO, Reis KP, Zamboni F, Wajner M, Pessoa-Pureur R. Signaling mechanisms downstream of quinolinic acid targeting the cytoskeleton of rat striatal neurons and astrocytes. Experimental neurology. 2012;233(1):391–399. doi: 10.1016/j.expneurol.2011.11.005. S0014-4886(11)00409-2 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Renner NA, Lackner AA, MacLean AG. Blood-Brain Barrier Disruption and Encephalitis in Animal Models of AIDS. In: Tkachev S, editor. Non-Flavivirus Encephalitis. In Tech; 2011. pp. 87–102. [Google Scholar]
- 31.Renner NA, Redmann RK, Moroney-Rasmussen T, Sansing HA, Aye PP, Didier PJ, Lackner AA, Maclean AG. S100beta as a novel and accessible indicator for the presence of monocyte-driven encephalitis in AIDS. Neuropathol Appl Neurobiol. 2012;38(2):162–174. doi: 10.1111/j.1365-2990.2011.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renner NA, Sansing HA, Inglis FM, Mehra S, Kaushal D, Lackner AA, Maclean AG. Transient acidification and subsequent proinflammatory cytokine stimulation of astrocytes induce distinct activation phenotypes. J Cell Physiol. 2013;228(6):1284–1294. doi: 10.1002/jcp.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi DJ. Astrocytes join the plasticity party. Nature neuroscience. 2012;15(5):649–651. doi: 10.1038/nn.3095. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safavi-Abbasi S, Wolff JR, Missler M. Rapid morphological changes in astrocytes are accompanied by redistribution but not by quantitative changes of cytoskeletal proteins. Glia. 2001;36(1):102–115. doi: 10.1002/glia.1099. [pii] [DOI] [PubMed] [Google Scholar]
- 35.Salaria S, Badkoobehi H, Rockenstein E, Crews L, Chana G, Masliah E, Everall IP. Toll-like receptor pathway gene expression is associated with human immunodeficiency virus-associated neurodegeneration. J Neurovirol. 2007;13(6):496–503. doi: 10.1080/13550280701558616. 788722851 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Shao Y, Enkvist MO, McCarthy KD. Glutamate blocks astroglial stellation: effect of glutamate uptake and volume changes. Glia. 1994;11(1):1–10. doi: 10.1002/glia.440110103. [DOI] [PubMed] [Google Scholar]
- 37.Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, Maclean AG, Peterson KE, Lackner AA, Bunnell BA. Innate Immune Activation in the Pathogenesis of a Murine Model of Globoid Cell Leukodystrophy. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181(12):8604–8612. doi: 10.4049/jimmunol.181.12.8604. 181/12/8604 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Suh HS, Brosnan CF, Lee SC. Toll-like receptors in CNS viral infections. Curr Top Microbiol Immunol. 2009;336:63–81. doi: 10.1007/978-3-642-00549-7_4. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan SM, Lee A, Bjorkman ST, Miller SM, Sullivan RK, Poronnik P, Colditz PB, Pow DV. Cytoskeletal anchoring of GLAST determines susceptibility to brain damage: an identified role for GFAP. J Biol Chem. 2007;282(40):29414–29423. doi: 10.1074/jbc.M704152200. [DOI] [PubMed] [Google Scholar]
- 41.Sun D, Lye-Barthel M, Masland RH, Jakobs TC. Structural remodeling of fibrous astrocytes after axonal injury. J Neurosci. 2010;30(42):14008–14019. doi: 10.1523/JNEUROSCI.3605-10.2010. 30/42/14008 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37(5):1305–1320. doi: 10.1038/npp.2011.319npp2011319. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tasker JG, Oliet SH, Bains JS, Brown CH, Stern JE. Glial regulation of neuronal function: from synapse to systems physiology. J Neuroendocrinol. 2012;24(4):566–576. doi: 10.1111/j.1365-2826.2011.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88(3):983–1008. doi: 10.1152/physrev.00036.2007. 88/3/983 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Toborek M, Lee YW, Flora G, Pu H, Andras IE, Wylegala E, Hennig B, Nath A. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol. 2005;25(1):181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres-Platas SG, Hercher C, Davoli MA, Maussion G, Labonte B, Turecki G, Mechawar N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology. 2011;36(13):2650–2658. doi: 10.1038/npp.2011.154npp2011154. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126(1):75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- 48.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Current HIV/AIDS reports. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia. 2004;48(1):1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- 50.Willis CL, Nolan CC, Reith SN, Lister T, Prior MJ, Guerin CJ, Mavroudis G, Ray DE. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45(4):325–337. doi: 10.1002/glia.10333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
There were significantly fewer primary segments in grey and white matter astrocytes in SIVE macaques compared to controls (Supplemental Figure 1A–B). Secondary and tertiary processes in SIV noE grey and white matter astrocytes were reduced compared to controls (Supplemental Figure 1C–F). No changes in length were observed in first order segments in grey and white matter astrocytes (Supplemental Figure 1G–H). Decreases in segment length were observed in second and third order SIV noE white matter astrocytes (Supplemental Figure 1J and L) and again in tertiary SIV noE and SIVE grey and white matter astrocytes (Supplemental Figure 1K). Results are shown as mean ± SD.
There was a significant decrease in the number of intersections at 20μm and 30μm away from the cell body in grey matter astrocytes of SIV noE macaques compared to controls (Supplemental Figure 2C and E). These reductions were only seen at 20 μm from the cell body in white matter astrocytes of SIV noE animals (Supplemental Figure 2D). Reduced numbers of nodes were evident at 20 μm and 30 μm in grey matter and white matter astrocytes in astrocytes in SIV-infected macaques (Supplemental Figure I-L). No changes the number of intersections and nodes were observed in grey and white matter astrocytes of SIVE macaques. Results are shown as mean ± SD.